Hydroxamic Acid Isolated from Maize Roots Exhibits Potent Antimicrobial Activity Against Pathogenic Escherichia coli in Broiler Chickens
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Cultivation of Maize Varieties
2.3. Extraction and Quantification of Hydroxamic Acids (HAs)
2.4. Isolation and Identification of the Prevalent E. coli Strain in Birds
2.5. In Vitro Antimicrobial Bioassay of Hydroxamic Acid Against E. coli Isolate
2.6. Animals and Experimental Layout
2.7. Experimental Design
2.8. Inoculation of Experimental Birds with an Isolated E. coli Strain
2.9. Growth Performance of Experimental Birds
2.10. Serum Parameters of Experimental Birds
2.11. Hematological Parameters of Experimental Birds
2.12. Statistical Analysis
3. Results
3.1. Quantification and Chromatographic Analysis of HA in Maize Varieties
3.2. Isolation, Biochemical Identification, and Molecular Confirmation of E. coli
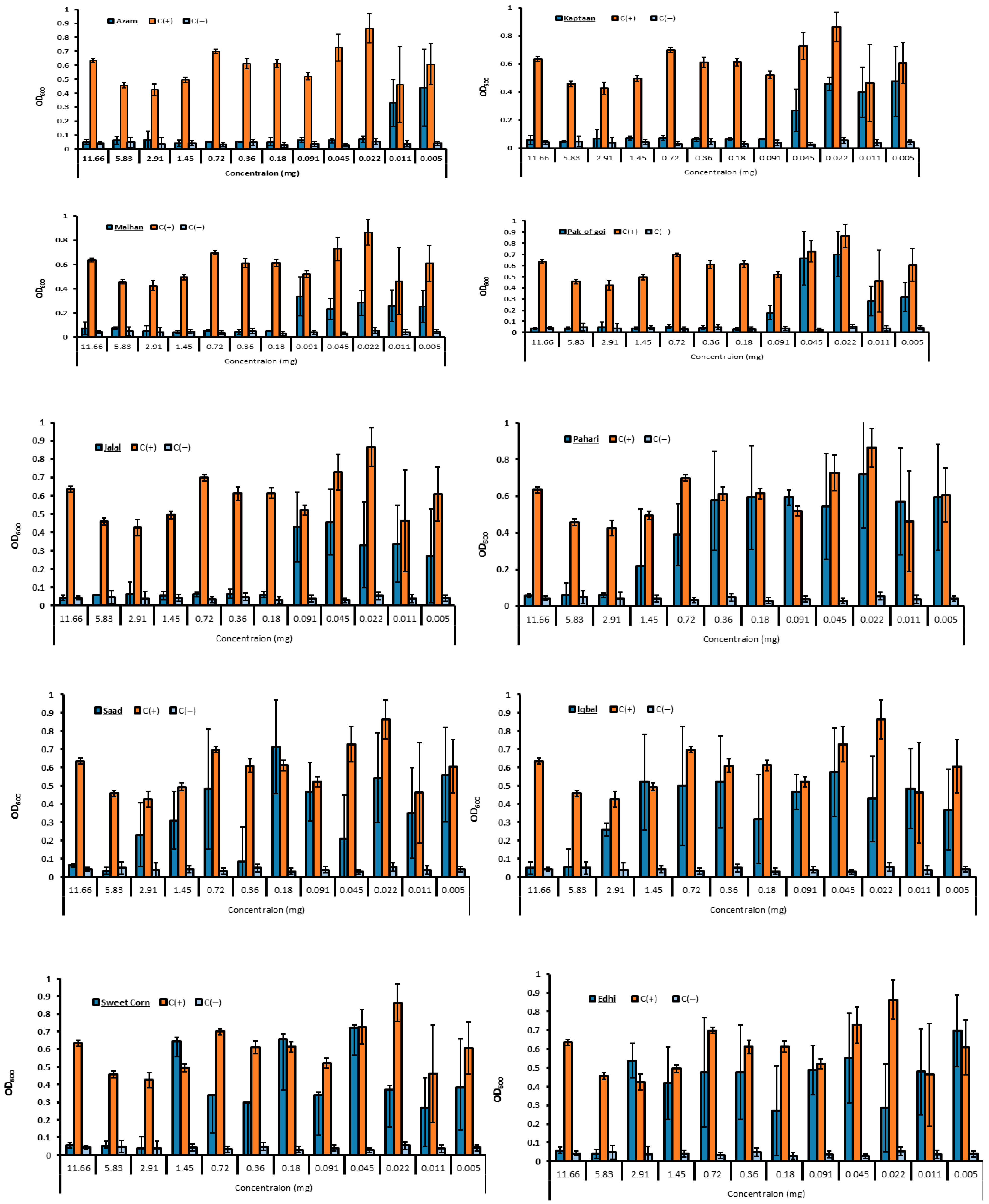
3.3. In Vitro Antibacterial Bioassay Determined the MIC of Hydroxamic Acid for E. coli
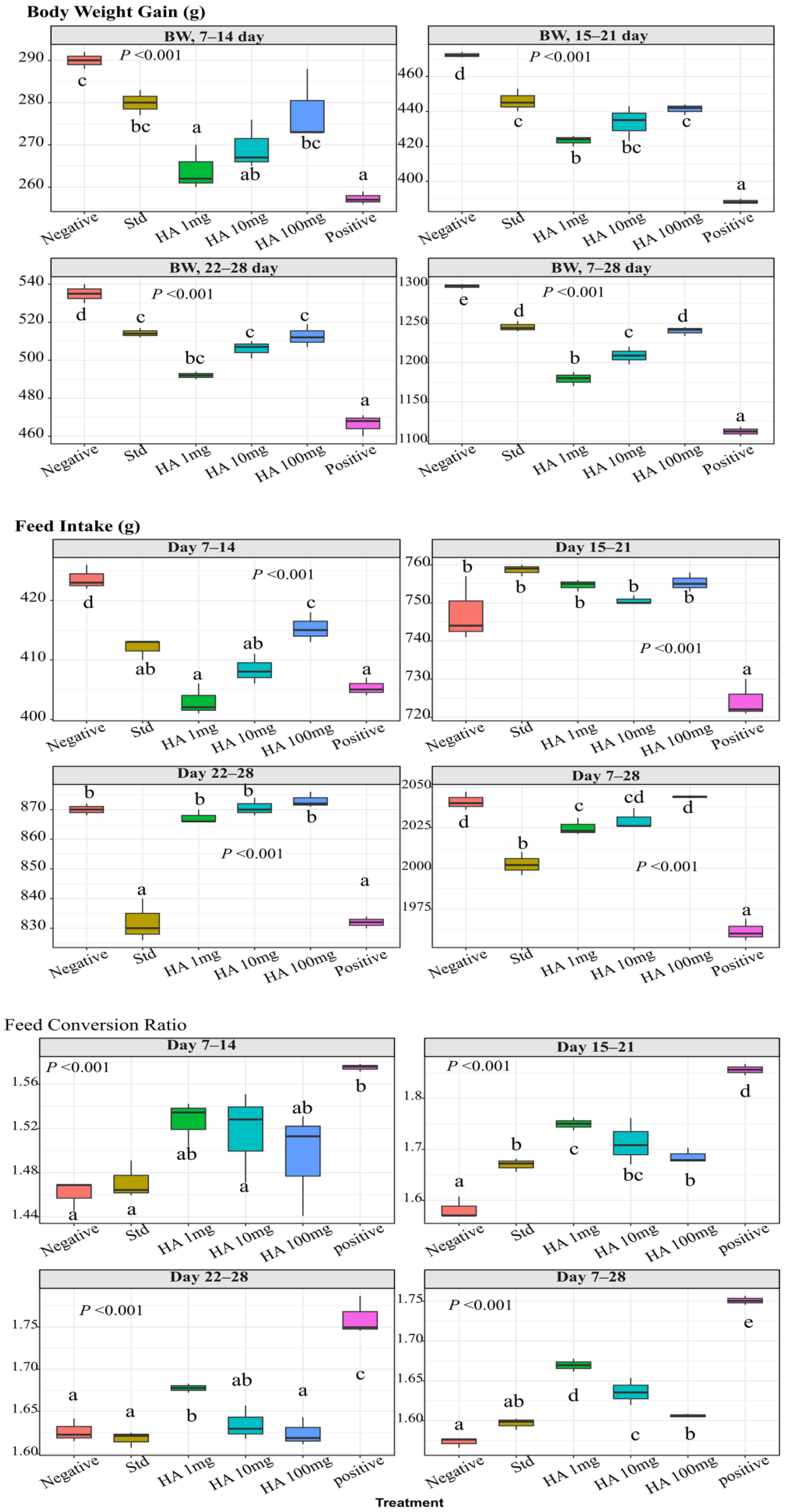
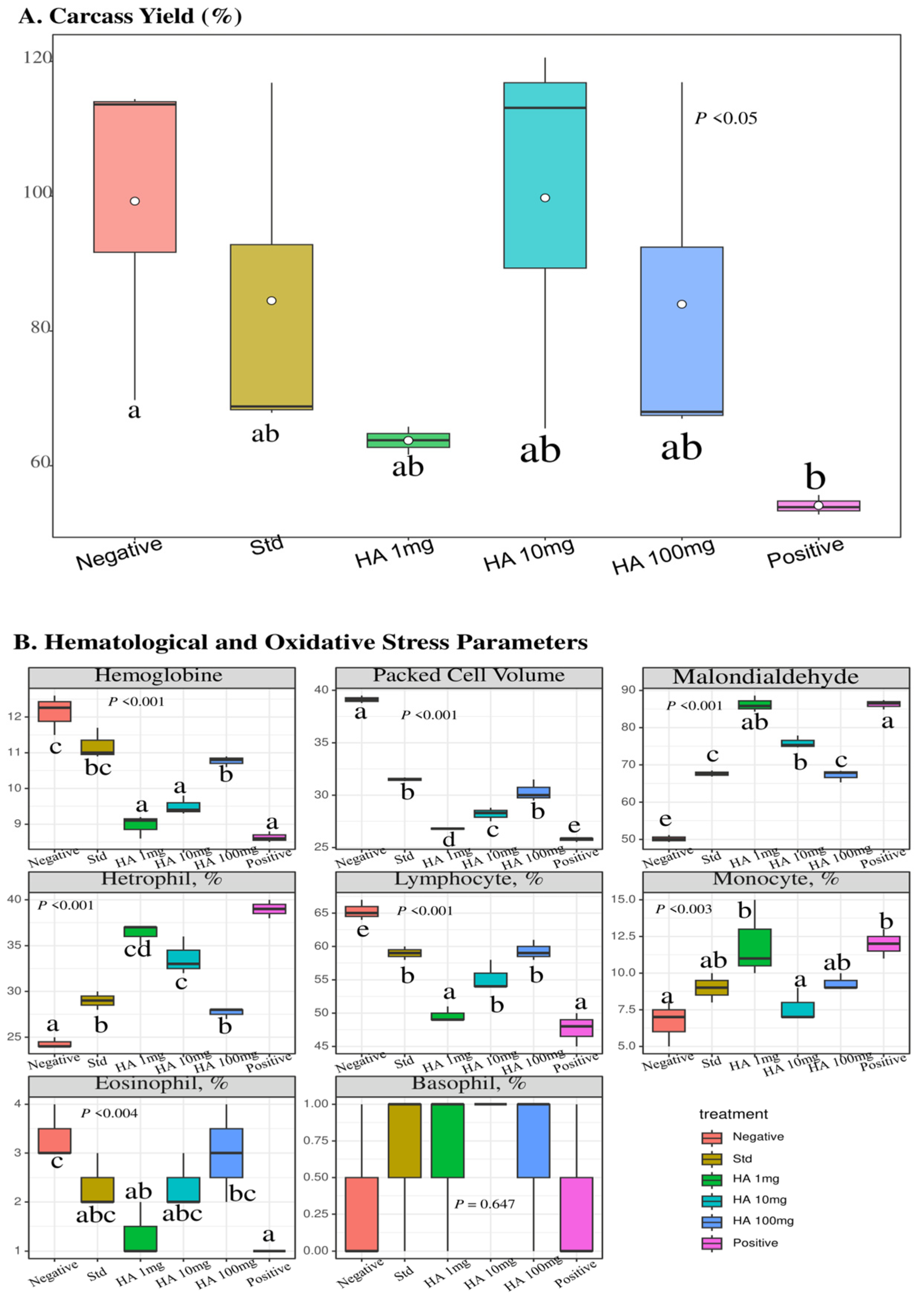
3.4. Hydroxamic Acid Extract Improved the Growth Performance of E. coli-Infected Birds
3.5. Hydroaxamic Acid Enhanced the Blood Parameters Linked with Oxidative Stress and Inflammatory Responses in E. coli-Infected Birds
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HA | Hydroxamic acid |
| AMR | Antimicrobial resistance |
| bp | Base pair |
| BW | Body weight |
| Cfu | Colony-forming unit |
| dpi | Day post-infection |
| E. coli | Escherichia coli |
| FCR | Feed conversion ratio |
| FI | Feed intake |
| GIT | Gastrointestinal tract |
| HPLC | High-performance liquid chromatography |
| Hb | Hemoglobin |
| MDA | Malondialdehyde |
| MIC | Minimum inhibitory concentration |
| nM | Nanomole |
| PCV | Packed cell volume |
| PFA | Phytogenic feed additive |
| PCR | Polymerase chain reaction |
| Std | Standard |
| SDS | Sodium dodecyl sulfate |
| SEM | Standard error of the mean |
| TLC | Total leukocyte count |
| TSI | Triple sugar iron |
| UoA | The University of Agriculture |
References
- McKenzie, F.C.; Williams, J. Sustainable food production: Constraints, challenges and choices by 2050. Food Secur. 2015, 7, 221–233. [Google Scholar] [CrossRef]
- Webb, R.; Buratini, J. Global challenges for the 21st century: The role and strategy of the agri-food sector. Anim. Reprod. (AR) 2018, 13, 133–142. [Google Scholar] [CrossRef]
- Sani, M.A.; Zhang, W.; Abedini, A.; Khezerlou, A.; Shariatifar, N.; Assadpour, E.; Zhang, F.; Jafari, S.M. Intelligent packaging systems for the quality and safety monitoring of meat products: From lab scale to industrialization. Food Control 2024, 160, 110359. [Google Scholar] [CrossRef]
- Gadde, U.; Kim, W.; Oh, S.; Lillehoj, H.S. Alternatives to antibiotics for maximizing growth performance and feed efficiency in poultry: A review. Anim. Health Res. Rev. 2017, 18, 26–45. [Google Scholar] [CrossRef] [PubMed]
- Wickramasuriya, S.S.; Ault, J.; Ritchie, S.; Gay, C.G.; Lillehoj, H.S. Alternatives to antibiotic growth promoters for poultry: A bibliometric analysis of the research journals. Poult. Sci. 2024, 103, 103987. [Google Scholar] [CrossRef] [PubMed]
- Apostolakos, I.; Laconi, A.; Mughini-Gras, L.; Yapicier, Ö.Ş.; Piccirillo, A. Occurrence of colibacillosis in broilers and its relationship with avian pathogenic Escherichia coli (APEC) population structure and molecular characteristics. Front. Vet. Sci. 2021, 8, 737720. [Google Scholar] [CrossRef]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, types, sources, mechanisms, and clinical applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef]
- Cross, D.; McDevitt, R.; Hillman, K.; Acamovic, T. The effect of herbs and their associated essential oils on performance, dietary digestibility and gut microflora in chickens from 7 to 28 days of age. Br. Poult. Sci. 2007, 48, 496–506. [Google Scholar] [CrossRef]
- Biswas, S.; Kim, I. A thorough review of phytogenic feed additives in non-ruminant nutrition: Production, gut health, and environmental concerns. J. Anim. Sci. Technol. 2025, 67, 497–519. [Google Scholar] [CrossRef]
- Brenes, A.; Roura, E. Essential oils in poultry nutrition: Main effects and modes of action. Anim. Feed Sci. Technol. 2010, 158, 1–14. [Google Scholar] [CrossRef]
- Navarro, M.; Stanley, R.; Cusack, A.; Sultanbawa, Y. Combinations of plant-derived compounds against campylobacter in vitro. J. Appl. Poult. Res. 2015, 24, 352–363. [Google Scholar] [CrossRef]
- Hashemipour, H.; Kermanshahi, H.; Golian, A.; Veldkamp, T. Effect of thymol and carvacrol feed supplementation on performance, antioxidant enzyme activities, fatty acid composition, digestive enzyme activities, and immune response in broiler chickens. Poult. Sci. 2013, 92, 2059–2069. [Google Scholar] [CrossRef] [PubMed]
- Mohebodini, H.; Jazi, V.; Bakhshalinejad, R.; Shabani, A.; Ashayerizadeh, A. Effect of dietary resveratrol supplementation on growth performance, immune response, serum biochemical indices, cecal microflora, and intestinal morphology of broiler chickens challenged with Escherichia coli. Livest. Sci. 2019, 229, 13–21. [Google Scholar] [CrossRef]
- Rahman, M.R.T.; Fliss, I.; Biron, E. Insights in the development and uses of alternatives to antibiotic growth promoters in poultry and swine production. Antibiotics 2022, 11, 766. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, D.A.; González, A.; Roupar, D.; Teixeira, J.A.; Nobre, C. How prebiotics have been produced from agro-industrial waste: An overview of the enzymatic technologies applied and the models used to validate their health claims. Trends Food Sci. Technol. 2023, 135, 74–92. [Google Scholar] [CrossRef]
- Yang, C.; Chowdhury, M.K.; Hou, Y.; Gong, J. Phytogenic compounds as alternatives to in-feed antibiotics: Potentials and challenges in application. Pathogens 2015, 4, 137–156. [Google Scholar] [CrossRef]
- Guo, B.; Zhang, Y.; Li, S.; Lai, T.; Yang, L.; Chen, J.; Ding, W. Extract from maize (Zea mays L.): Antibacterial activity of dimboa and its derivatives against ralstonia solanacearum. Molecules 2016, 21, 1397. [Google Scholar] [CrossRef]
- Al Shaer, D.; Al Musaimi, O.; de la Torre, B.G.; Albericio, F. Hydroxamate siderophores: Natural occurrence, chemical synthesis, iron binding affinity and use as trojan horses against pathogens. Eur. J. Med. Chem. 2020, 208, 112791. [Google Scholar] [CrossRef]
- Syed, Z.; Sonu, K.; Dongre, A.; Sharma, G.; Sogani, M. A review on hydroxamic acids: Widespectrum chemotherapeutic agents. Int. J. Biol. Biomed. Eng. 2020, 14, 75–88. [Google Scholar] [CrossRef]
- Ahmad, S.; Veyrat, N.; Gordon-Weeks, R.; Zhang, Y.; Martin, J.; Smart, L.; Glauser, G.; Erb, M.; Flors, V.; Frey, M. Benzoxazinoid metabolites regulate innate immunity against aphids and fungi in maize. Plant Physiol. 2011, 157, 317–327. [Google Scholar] [CrossRef]
- Fesseha, H.; Mathewos, M.; Aliye, S.; Mekonnen, E. Isolation and antibiogram of Escherichia coli O157: H7 from diarrhoeic calves in urban and peri-urban dairy farms of Hawassa town. Vet. Med. Sci. 2022, 8, 864–876. [Google Scholar] [CrossRef]
- Neal, A.L.; Ahmad, S.; Gordon-Weeks, R.; Ton, J. Benzoxazinoids in root exudates of maize attract pseudomonas putida to the rhizosphere. PLoS ONE 2012, 7, e35498. [Google Scholar] [CrossRef] [PubMed]
- Zeb, A.; Ullah, F. A Simple Spectrophotometric Method for the Determination of Thiobarbituric Acid Reactive Substances in Fried Fast Foods. J. Anal. Methods Chem. 2016, 2016, 9412767. [Google Scholar] [CrossRef] [PubMed]
- Hartady, T.; Syamsunarno, M.R.A.; Priosoeryanto, B.P.; Jasni, S.; Balia, R.L. Review of herbal medicine works in the avian species. Vet. World 2021, 14, 2889. [Google Scholar] [CrossRef]
- Xie, A.; Gao, M.; Du, H.; Pan, X. Next-generation probiotics delivery: Innovations and applications of single-cell encapsulation. Curr. Opin. Food Sci. 2025, 61, 101234. [Google Scholar] [CrossRef]
- Morse, S.; Wratten, S.; Edwards, P.; Niemeyer, H. Changes in the hydroxamic acid content of maize leaves with time and after artificial damage; implications for insect attack. Ann. Appl. Biol. 1991, 119, 239–249. [Google Scholar] [CrossRef]
- Davis, C.S.; Ni, X.; Quisenberry, S.S.; Foster, J.E. Identification and quantification of hydroxamic acids in maize seedling root tissue and impact on western corn rootworm (coleoptera: Chrysomelidae) larval development. J. Econ. Entomol. 2000, 93, 989–992. [Google Scholar] [CrossRef]
- Gleńsk, M.; Gajda, B.; Franiczek, R.; Krzyżanowska, B.; Biskup, I.; Włodarczyk, M. In Vitro evaluation of the antioxidant and antimicrobial activity of dimboa [2,4-dihydroxy-7-methoxy-2 h-1,4-benzoxazin-3 (4 h)-one]. Nat. Prod. Res. 2016, 30, 1305–1308. [Google Scholar] [CrossRef]
- Abdelli, N.; Solà-Oriol, D.; Pérez, J.F. Phytogenic feed additives in poultry: Achievements, prospective and challenges. Animals 2021, 11, 3471. [Google Scholar] [CrossRef]
- Paraskeuas, V.; Fegeros, K.; Palamidi, I.; Hunger, C.; Mountzouris, K.C. Growth performance, nutrient digestibility, antioxidant capacity, blood biochemical biomarkers and cytokines expression in broiler chickens fed different phytogenic levels. Anim. Nutr. 2017, 3, 114–120. [Google Scholar] [CrossRef]
- Hafeez, A.; Männer, K.; Schieder, C.; Zentek, J. Effect of supplementation of phytogenic feed additives (powdered vs. Encapsulated) on performance and nutrient digestibility in broiler chickens. Poult. Sci. 2016, 95, 622–629. [Google Scholar] [CrossRef]
- Liu, S.; Song, M.; Yun, W.; Lee, J.; Lee, C.; Kwak, W.; Han, N.; Kim, H.; Cho, J. Effects of oral administration of different dosages of carvacrol essential oils on intestinal barrier function in broilers. J. Anim. Physiol. Anim. Nutr. 2018, 102, 1257–1265. [Google Scholar] [CrossRef]
- Hang, T.; Molee, W.; Khempaka, S.; Paraksa, N. Supplementation with curcuminoids and tuna oil influenced skin yellowness, carcass composition, oxidation status, and meat fatty acids of slow-growing chickens. Poult. Sci. 2018, 97, 901–909. [Google Scholar] [CrossRef]
- Nm, J.; Joseph, A.; Maliakel, B.; Im, K. Dietary addition of a standardized extract of turmeric (turmafeed tm) improves growth performance and carcass quality of broilers. J. Anim. Sci. Technol. 2018, 60, 1–9. [Google Scholar] [CrossRef]
- Yu, C.; Wei, J.; Yang, C.; Yang, Z.; Yang, W.; Jiang, S. Effects of star anise (Illicium verum hook.F.) essential oil on laying performance and antioxidant status of laying hens. Poult. Sci. 2018, 97, 3957–3966. [Google Scholar] [CrossRef]
- Abdel-Wareth, A.; Lohakare, J. Productive performance, egg quality, nutrients digestibility, and physiological response of bovans brown hens fed various dietary inclusion levels of peppermint oil. Anim. Feed Sci. Technol. 2020, 267, 114554. [Google Scholar] [CrossRef]
- Ayalew, H.; Zhang, H.; Wang, J.; Wu, S.; Qiu, K.; Qi, G.; Tekeste, A.; Wassie, T.; Chanie, D. Potential feed additives as antibiotic alternatives in broiler production. Front. Vet. Sci. 2022, 9, 916473. [Google Scholar] [CrossRef] [PubMed]
- Pham, V.H.; Abbas, W.; Huang, J.; Guo, F.; Zhang, K.; Kong, L.; Zhen, W.; Guo, Y.; Wang, Z. Dietary coated essential oil and organic acid mixture supplementation improves health of broilers infected with avian pathogenic Escherichia coli. Anim. Nutr. 2023, 12, 245–262. [Google Scholar] [CrossRef]
- Obianwuna, U.E.; Chang, X.; Oleforuh-Okoleh, V.U.; Onu, P.N.; Zhang, H.; Qiu, K.; Wu, S. Phytobiotics in poultry: Revolutionizing broiler chicken nutrition with plant-derived gut health enhancers. J. Anim. Sci. Biotechnol. 2024, 15, 169. [Google Scholar] [CrossRef] [PubMed]

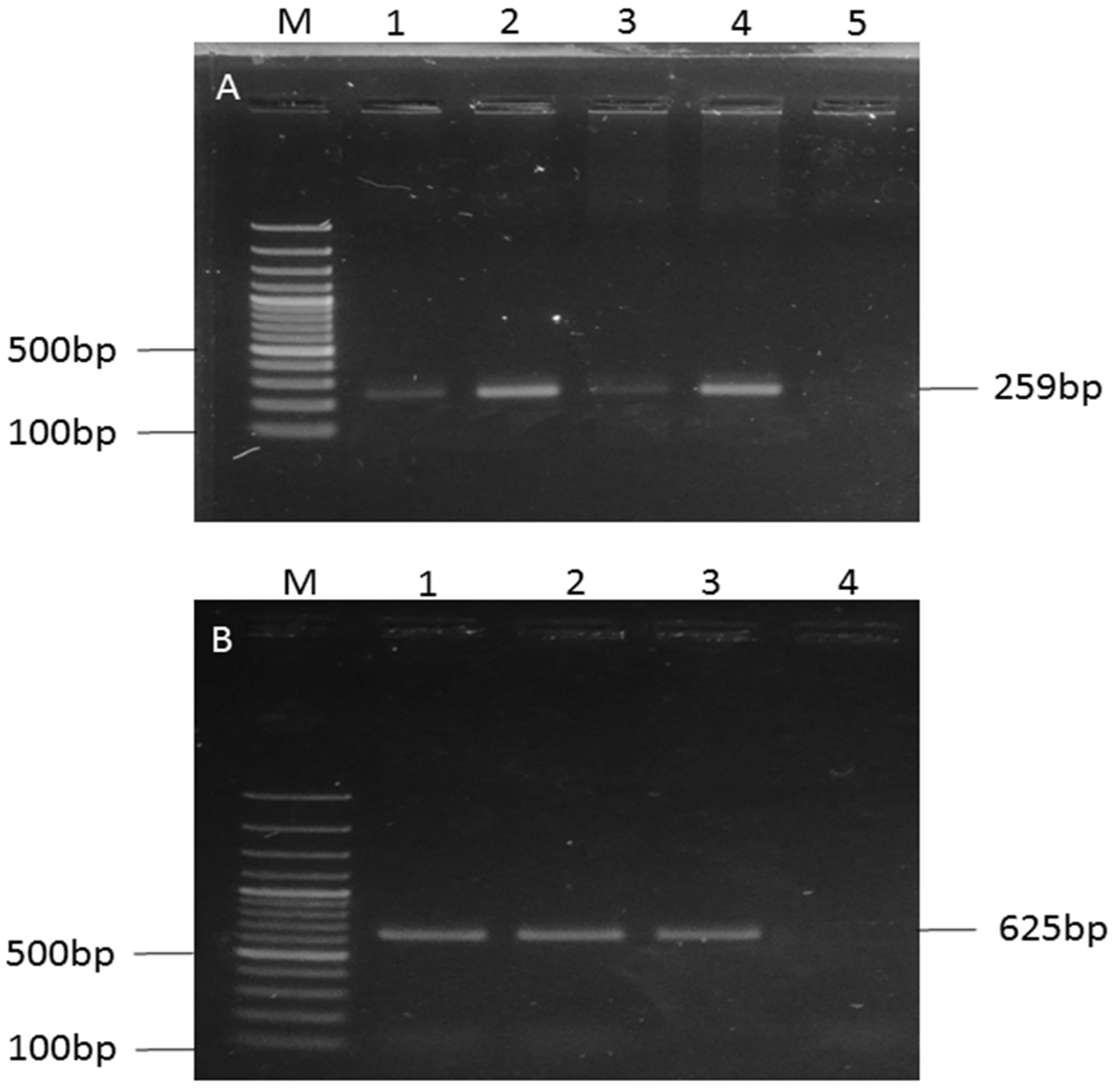
| Gene | Primers Sequence (5′-3′) | Amplicon Size (bp) |
|---|---|---|
| O157 | F: CGGACATCCATGTGATATGG | 259 |
| R: TTGCCTATGTACAGCTAATCC | ||
| H7 | F: GCGCTGTCGAGTTCTATCGAGC | 625 |
| R: CAACGGTGACTTTATCGCCATTCC |
| Ingredients Composition (%) | Starter (0–14 day) | Grower (15–28 day) |
|---|---|---|
| Corn | 54.90 | 58.89 |
| Soybean meal (44%) | 37.40 | 32.90 |
| DL-methionine | 0.45 | 0.45 |
| Dicalcium phosphate | 1.79 | 1.79 |
| Limestone | 1.27 | 1.27 |
| L-lysine | 0.40 | 0.21 |
| Salt | 0.34 | 0.34 |
| Threonine | 0.15 | 0.15 |
| Vegetable oil | 2.30 | 3.0 |
| Vitamin–premix | 0.50 | 0.50 |
| mineral premix | 0.50 | 0.50 |
| Total | 100 | 100 |
| Calculated analysis | ||
| Dry Matter | 88.53 | 89.30 |
| Metabolizable energy Kcal/kg | 2978 | 3076 |
| Crude protein | 21.41 | 20.10 |
| Crude fat | 11.63 | 11.75 |
| Crude fiber | 2.94 | 2.94 |
| Calcium | 0.95 | 0.92 |
| Phosphorus available | 0.45 | 0.43 |
| Methionine | 0.65 | 0.60 |
| Cysteine | 0.30 | 0.29 |
| Lysine | 1.26 | 1.19 |
| Leucine | 1.70 | 1.63 |
| S. No. | Maize Variety | HA Concentration (µg/g Roots) |
|---|---|---|
| 01 | Azam | 35 ± 7 |
| 02 | Pak of goi | 20 ± 5 |
| 03 | Sweet corn | 12 ± 5 |
| 04 | Kaptan | 23 ± 4 |
| 05 | Jalal | 15 ± 5 |
| 06 | Iqbal | 8 ± 5 |
| 07 | Malhan | 14 ± 4 |
| 08 | Edhi | 5 ± 2 |
| 09 | Saad | 10 ± 7 |
| 10 | Pahari | 11 ± 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ullah, Q.; Ahmad, S.; Khan, S.; Ahmad, I.; Khan, S.; Khan, R.; Khan, F.A. Hydroxamic Acid Isolated from Maize Roots Exhibits Potent Antimicrobial Activity Against Pathogenic Escherichia coli in Broiler Chickens. Microbiol. Res. 2025, 16, 222. https://doi.org/10.3390/microbiolres16100222
Ullah Q, Ahmad S, Khan S, Ahmad I, Khan S, Khan R, Khan FA. Hydroxamic Acid Isolated from Maize Roots Exhibits Potent Antimicrobial Activity Against Pathogenic Escherichia coli in Broiler Chickens. Microbiology Research. 2025; 16(10):222. https://doi.org/10.3390/microbiolres16100222
Chicago/Turabian StyleUllah, Qudrat, Shakoor Ahmad, Sarzamin Khan, Ijaz Ahmad, Samiullah Khan, Rajwali Khan, and Farhan Anwar Khan. 2025. "Hydroxamic Acid Isolated from Maize Roots Exhibits Potent Antimicrobial Activity Against Pathogenic Escherichia coli in Broiler Chickens" Microbiology Research 16, no. 10: 222. https://doi.org/10.3390/microbiolres16100222
APA StyleUllah, Q., Ahmad, S., Khan, S., Ahmad, I., Khan, S., Khan, R., & Khan, F. A. (2025). Hydroxamic Acid Isolated from Maize Roots Exhibits Potent Antimicrobial Activity Against Pathogenic Escherichia coli in Broiler Chickens. Microbiology Research, 16(10), 222. https://doi.org/10.3390/microbiolres16100222







