Abstract
The emergence of Candida auris as a disheartening fungal pathogen in healthcare settings has prompted urgent re-evaluation of containment and mitigation strategies. This review critically examines the biological persistence, environmental adaptability, and resistance to standard antifungal therapies of the pathogen, particularly regions with limited surveillance infrastructure. Based on regional experiences, such as those in Saudi Arabia and the Middle East in general, the study reveals systemic weaknesses in diagnosis, reporting, and environmental sanitation. Special consideration is paid to the combination of new disinfection technologies, including ultraviolet irradiation systems and hydrogen peroxide vaporisation, with institutional behaviour change strategies. This discussion shows the importance of synchronising technological development with frequent employee contributions and cross-functional planning. It also encourages the international standardisation of diagnostic platforms and the launch of real-time genomic surveillance to reveal evolutionary trends. Finally, the findings justify the shift towards proactive models of infection control that are founded on the resilience of systems and the agility of institutions. This paper is a synthesis of epidemiological patterns, decontamination strategies and behavioural knowledge to contribute to an emerging body of knowledge that can help to fortify healthcare settings against current fungal threats.
1. Introduction
Healthcare-Associated Infections (HAIs) remain a persistent threat to modern healthcare, increasing patient morbidity, straining resources, and affecting institutional reputation [1]. While HAIs were once mostly bacterial, attention has shifted to fungal pathogens, especially Candida auris (C. auris), first identified in 2009 [2]. This high-risk fungus challenges conventional epidemiology due to its ability to colonise patients and clinical surfaces effectively [3]. Its resistance to treatment, difficulty in detection, and environmental persistence complicate infection control. Moreover, inadequate fungal surveillance often delays intervention, allowing C. auris to spread undetected. Its emergence underscores the need for improved diagnostic and containment strategies in healthcare settings.
C. auris distinguishes itself from other Candida species through three defining features: multidrug resistance, outbreak potential, and diagnostic ambiguity. It is often resistant to at least one, and often more than one, category of antifungal agents, resulting in clinicians having few effective treatment options [4]. It is highly transmissible through environmental reservoirs and asymptomatic carriers, which boost its capability to cause prolonged and widespread outbreaks in critical care units. Adding to this multidrug resistance is the fact that it is often misidentified using standard diagnostic systems, thus being misidentified as other Candida species, potentially leading to inappropriate treatment. Such features require immediate consideration by infection control structures that have long been focused on bacterial pathogens [5,6]. The identification of C. auris as a particularly environmental fungal pathogen also shifts the paradigm of hospital-acquired infections, highlighting the need for sustained surface hygiene protocols to prevent persistent contamination, more stringent diagnostic procedures, and a tighter surveillance strategy [7,8].
This review critically examines C. auris as an environmental pathogen in healthcare ecosystems, highlighting its biology, transmission, and systemic response gaps. C. auris embodies microbial adaptation and institutional vulnerability, necessitating revised infection control strategies grounded in environmental and technological insights. The paper integrates global and Middle Eastern data—particularly from Saudi Arabia—to explore diagnostic challenges, infrastructure limitations, and policy gaps. Emphasis is placed on post-COVID-19 epidemiological shifts, limited diagnostic capacity, and the need for scalable, cost-effective infection control applicable even in resource-constrained settings. The pandemic exposed weaknesses in fungal surveillance, with PPE shortages, diagnostic delays, and staff redeployment hindering early containment. Routine screening of high-risk patients with axilla/groin swabs was introduced, leading to updated Ministry of Health (2019) guidelines after the 2018 ICU outbreak. These mandated active surveillance and environmental cleaning protocols. By addressing C. auris’s environmental persistence, this paper supports evidence-based strategies for fungal containment in modern healthcare.
2. Environmental Survival and Transmission
Laboratory studies demonstrate that C. auris can survive on stainless steel and plastics for more than 14 days under nutrient-poor conditions at ≈25 °C and 40–60% humidity, highlighting its environmental resilience [5].
Zhu et al. documented persistent contamination on reusable thermometers despite disinfection efforts [9] while Sansom et al. identified bed rails and infusion pumps as transmission sources during ICU outbreaks [7]. Though its biofilms are less robust than those of C. albicans, they still facilitate strong adherence to diverse surfaces [6], especially under low-moisture conditions. C. auris also colonises axillary, inguinal, and moist skin sites, contaminating surfaces and medical devices. Inadequate infection control allows fomite-mediated spread, with standard decontamination—e.g., quaternary ammonium compounds—proving ineffective. Nosocomial clusters show 30–60% mortality, driven by antifungal resistance [7]. Its tolerance to salinity and heat further enables survival on high-touch surfaces, sustaining prolonged transmission risks [8].
The transmission pathways of C. auris are complex, involving direct contact with colonised individuals and indirect routes via healthcare personnel, contaminated equipment, and inadequately cleaned surfaces. Transmission occurs through: (1) direct skin or mucous membrane contact; (2) indirect spread via hands, gloves, or clothing of healthcare workers; (3) fomite-mediated transfer through shared medical devices like thermometers and ventilators; and (4) environmental persistence on high-touch surfaces such as bed rails and sinks. Reusable items, including temperature probes and blood pressure cuffs, have been implicated in numerous outbreaks, with standard disinfectants like quaternary ammonium and chlorine compounds often proving ineffective due to C. auris’s resistance [9]. Laboratory findings confirm that C. auris survives over 14 days on plastics and steel in nutrient-poor settings, compounded by biofilm formation that impairs disinfection efficacy [5]. Zhu et al. [9] linked an outbreak in a London ICU to a contaminated thermometer, highlighting persistent systemic lapses. Akinbobola et al. [2] and others underscore that controlling C. auris demands proactive environmental surveillance and revised sanitation protocols. The UK, US, and India have shown how minor hygiene lapses can lead to widespread transmission. Without a shift toward prioritising environmental contamination, infection control strategies will remain fragmented and ineffective [10].
3. Experience and Data in Saudi Arabia and the Middle East
The emergence of C. auris across the Middle East (as shown in Figure 1)—especially in Saudi Arabia (KSA)—reveals a pattern of recurring outbreaks, diagnostic delays, and underdeveloped surveillance infrastructure. Tertiary hospitals in Riyadh and Jeddah have reported nosocomial clusters with mortality rates of 30–60%, often linked to antifungal resistance [11]. A 2018 ICU outbreak involving 22 patients high-lighted diagnostic limitations and transmission via healthcare workers and reusable equipment [12]. Containment relied on MALDI-TOF diagnostics, chlorine disinfection, staff retraining, and cohorting. Routine axilla and groin screening was introduced for high-risk patients. These events led to national infection prevention and control (IPC) reforms. Interim IPC guidelines were issued by the Ministry of Health in February 2019, followed by updated directives in August 2020 mandating surveillance, isolation, and reporting protocols [13]. While Saudi Arabia made progress, inconsistent implementation and underreporting remain, and similar outbreaks in the UAE and Oman show elevated resistance patterns but lack centralised, accessible surveillance systems.
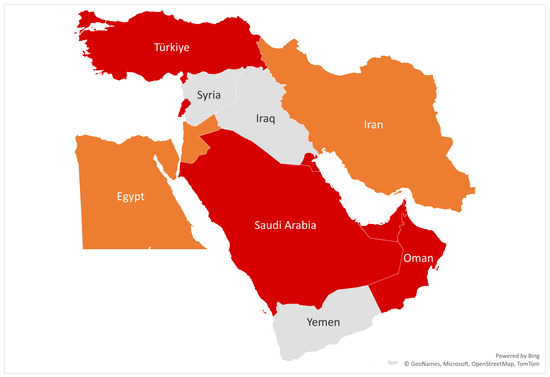
Figure 1.
Reported C. auris outbreaks in selected Middle Eastern countries [13]. Colour code: Red = Confirmed outbreak; Orange = Suspected cases; Grey = No data available.
Surveillance for C. auris in the Middle East remains fragmented, with well-equipped infection control units concentrated in urban tertiary centres, while peripheral facilities lack essential resources. This imbalance impairs early detection, especially where microbiological capacity is limited. Conventional identification tools such as VITEK and API often misidentify C. auris, which requires MALDI-TOF or PCR assays for confirmation [14], yet these are unevenly distributed. The absence of centralised genomic surveillance further restricts the monitoring of clade dynamics, resistance patterns, and interfacility spread. Inconsistent mandatory reporting across countries weakens interagency coordination. Additionally, the lack of standardised environmental culturing protocols [15] prevents recognition of non-clinical reservoirs, resulting in narrowly reactive, case-specific infection control measures.
Saudi Arabia’s Ministry of Health (MOH) has shown initiative in infection prevention, drawing on past experience with MERS-CoV. In response to C. auris, the MOH released interim guidelines prioritising early reporting, environmental decontamination, and patient isolation [16]. However, implementation varies due to gaps in staff training, technology access, and competing institutional priorities. Cultural dynamics—such as hierarchical decision-making and stigma around outbreaks—further complicate compliance. The reliance on expatriate healthcare workers introduces standardisation challenges, including language and educational disparities. To mitigate these, hospitals in Riyadh and Jeddah have launched multilingual IPC training and peer-led workshops. These efforts highlight that containing C. auris requires more than technical solutions; institutional culture must also evolve [12]. Strengthening laboratory networks, improving real-time surveillance, and fostering transparent communication are critical. Without regionally coordinated, culturally informed interventions, C. auris will persist as a multidrug-resistant threat. Sustained investment in both diagnostics and human resources remains essential.
4. Epidemiology and Evolution Post-COVID-19
The COVID-19 pandemic precipitated a marked escalation in C. auris cases globally, revealing critical vulnerabilities in infection control systems, for example, increased antimicrobial use, pressure on ICUs, and changes in protocols. In 2020, nations such as the United States, India, South Africa, and Colombia reported sharp increases in both colonisation and clinical infections, C. auris was first described in 2009, but this pathogen has rapidly become a high-risk, globally persistent pathogen, with mortality rates reported between 30% and 60%, depending on patient comorbidities and access to timely antifungal therapy with the CDC documenting a threefold rise in U.S. healthcare facilities, predominantly driven by silent transmission within overcrowded ICUs [17]. Concurrently, Gulf Cooperation Council (GCC) states, particularly Saudi Arabia and the UAE, experienced emergent outbreaks despite historically limited reporting [18]. Pandemic-induced disruptions—including resource diversion, reduced fungal surveillance, and weakened compliance with routine protocols—created optimal conditions for C. auris proliferation and the undetected spread of resistant strains.
The pathophysiological and systemic characteristics of COVID-19 care environments inadvertently created ideal conditions for the proliferation of C. auris. High-density ICU admissions, reliance on mechanical ventilation, extended hospitalisation, and widespread use of broad-spectrum antibiotics significantly disrupted host microbiota, thereby increasing susceptibility to fungal colonisation [19]. The administration of immunomodulatory agents—such as corticosteroids and tocilizumab—is essential in managing cytokine storm syndromes, further compromising innate immunity, rendering patients vulnerable to opportunistic fungal infections [20]. Simultaneously, operational compromises—including PPE reuse, inadequate environmental cleaning, and deployment of insufficiently trained personnel—undermined fundamental infection control practices. Supply shortages and staff fatigue led to inconsistent decontamination routines, while asymptomatic carriers were seldom screened due to overwhelmed surveillance systems. As a result, colonised individuals acted as unrecognised reservoirs, facilitating silent transmission [21]. These compounded failures, precipitated by emergency conditions, provided fertile ground for sustained nosocomial outbreaks. The rising incidence of C. auris globally and within the Middle East is illustrated in Figure 2, highlighting its opportunistic adaptation to overburdened healthcare infrastructure during the pandemic.
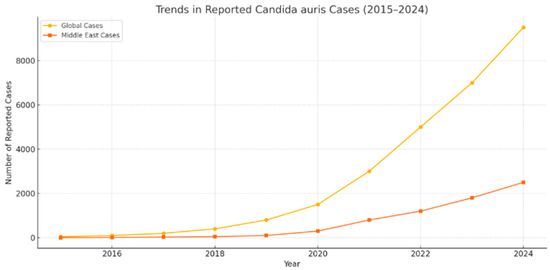
Figure 2.
Trends in reported C. auris cases globally and in the Middle East from 2015 to 2024 [21].
Post-COVID-19 genomic surveillance has revealed concerning patterns of C. auris evolution and global spread. Whole-genome sequencing in the U.S., Europe, and Asia has identified co-circulating clades within healthcare systems, some bearing mutations that confer pan-resistance to all major antifungal classes [22]. This genetic variability complicates diagnosis, limits treatment efficacy, and undermines regional containment. In 2022, the World Health Organisation (WHO) designated C. auris a critical priority pathogen, citing its high mortality and pandemic-fuelled proliferation [23]. The CDC echoed these concerns, urging enhanced surveillance, environmental monitoring, and transparent reporting. Without pre-emptive measures, predictive models forecast increasing outbreaks. Emerging genomic data also reveal that certain strains have adapted to survive in dry, low-nutrient environments, likely due to selective pressure from disinfectant-rich hospital settings [24]. These findings suggest that the post-pandemic landscape represents a new epidemiological era, where C. auris has become a persistent global threat requiring genomically guided, system-level, and policy-driven interventions. These shifts are reflected in Figure 2, which visualises the rising global and Middle Eastern case trends from 2015 to 2024.
5. Diagnostic Challenges
Misidentification remains a critical barrier to controlling C. auris, often mistaken for C. haemulonii, C. famata, or Rhodotorula glutinis due to overlapping sugar assimilation patterns and growth traits [25]. Keighley et al. noted frequent misclassification by VITEK 2, delaying treatment and isolation measures [3]. In a 2018 ICU outbreak in Saudi Arabia, control was only achieved after the introduction of MALDI-TOF MS, highlighting the consequences of diagnostic delay [12]. Conventional assays often miss early colonisation, with species-level identification typically initiated only after infections become invasive or resistant [26]. By then, widespread transmission across patients and healthcare surfaces may have occurred. These errors are clinically significant, leading to inappropriate antifungal use, missed isolation, and prolonged environmental shedding. PCR and LAMP assays now offer improved sensitivity and specificity (>90%) and have shown success in both clinical and environmental detection [2,7]. Validated platforms such as CDC’s LAMP and Bruker Fungiplex enhance early recognition. Yet, diagnostic limitations persist, particularly in overwhelmed or under-resourced settings. These systemic delays obscure C. auris’ true epidemiological footprint, hinder public health response, and enable silent transmission [27]. Given its multidrug resistance and high transmissibility, rapid and accurate identification is essential for effective containment and prevention of institutional outbreaks.
The introduction of matrix-assisted laser desorption ionisation-time of flight mass spectrometry (MALDI-TOF MS) and polymerase chain reaction (PCR)-based assays marks a transformative advancement in C. auris diagnostics. These technologies offer rapid, species-level identification and can be integrated into routine workflows with minimal delay. When equipped with updated reference databases, MALDI-TOF MS can reliably differentiate C. auris from closely related species, while real-time PCR and loop-mediated isothermal amplification (LAMP) demonstrate high sensitivity for both clinical and environmental samples [28]. However, widespread adoption is hindered by disparities in diagnostic infrastructure, particularly in low- and middle-income countries (LMICs), where outdated platforms like VITEK 2 and API 20C remain prevalent [29]. These limitations are amplified in LMICs due to the high cost of molecular diagnostics, limited equipment maintenance, irregular reagent supply, and insufficient training for lab personnel. Even in high-income nations, access to molecular diagnostics is often centralised in reference laboratories, delaying confirmation and response. Budgetary limitations further restrict routine screening of asymptomatic carriers, despite growing evidence that colonised individuals constitute persistent reservoirs. This technological divide perpetuates global inequities in outbreak detection and control.
Laboratory biosafety and environmental culturing pose challenges in the diagnosis and control of C. auris. Unlike many fungal pathogens, C. auris survives on surfaces and resists conventional disinfectants, increasing contamination risks during environmental sampling [30]. While laboratory-acquired infections remain theoretical, safety concerns have limited routine culturing. The CDC and WHO deem BSL-2 protocols sufficient, and no occupational infections have been reported globally despite widespread handling [31]. However, in facilities lacking biosafety infrastructure, environmental sampling is rarely conducted, reinforcing a narrow clinical focus that overlooks persistent environmental reservoirs. This omission undermines infection prevention by enabling silent transmission from undetected sources. Cost, training, and limited access to chromogenic or selective media further restrict widespread adoption of environmental culturing protocols. Although occupational risk is low, strict adherence to glove use, laminar flow hoods, and surface decontamination remains essential. Broader implementation of biosafety practices must accompany technological innovation. The absence of coordinated protocols for both clinical and environmental detection means that many health systems remain reactive rather than proactive [32]. Effective containment demands a paradigm shift in diagnostics—one that integrates biosafety, accessibility, and ecological awareness. Environmental surveillance is not an optional adjunct; it is foundational to interrupting transmission cycles and anticipating outbreaks before they escalate.
6. Treatment and Antifungal Resistance
Resistance severely limits therapy. In a South African outbreak, Sansom et al. identified FKS1 mutations in six ICU isolates that caused breakthrough infections despite echinocandin treatment, C. auris exhibits resistance across all major antifungal classes—most notably azoles (e.g., fluconazole), echinocandins (e.g., caspofungin), and polyenes (e.g., amphotericin B)—limiting therapeutic options and increasing reliance on susceptibility testing [7]. Fluconazole resistance exceeds 90% in some regions, while amphotericin B use is limited by toxicity [2]. New antifungals offer promise: Wiederhold et al. reported that ibrexafungerp maintained in vitro activity against echinocandin-resistant isolates, and fosmanogepix has advanced to phase II trials showing broad-spectrum efficacy [32]. However, limited access in low- and middle-income countries highlights the gap between pharmacological innovation and clinical applicability [33]. Echinocandins, including caspofungin, micafungin, and anidulafungin, have shown significant activity [34]. However, recent reports of echinocandin resistance are making therapeutic decision-making more difficult. Amphotericin B has variable activity and is often linked to nephrotoxicity and low applicability. Resistance patterns limit empiric treatment and require early susceptibility data [35]. In the absence of good diagnostics, empirical therapy is likely to be inadequate and toxic. Until recently, no formal EUCAST breakpoints existed. In 2025, EUCAST released updated breakpoints to standardise reporting [36]. These help guide decision-making, especially in multidrug- or pan-resistant isolates. Implementation remains uneven due to infrastructure disparities [22]. Resistance remains a defining challenge. Novel agents provide cautious optimism, but remain inaccessible in many LMICs, highlighting the dual need for pharmacological innovation and equitable stewardship programmes.
Mechanisms underlying antifungal resistance in C. auris are multifaceted and reflect both intrinsic and acquired adaptations. ERG11 and FKS1 genes undergo genetic mutations to confer resistance to azoles and echinocandins, respectively, by either altering the target enzyme of azoles or interfering with the glucan synthase complex targeted by echinocandins [37]. C. auris demonstrates increased expression of ATP-binding cassette (ABC) transporters and major facilitator superfamily (MFS) efflux pumps, actively expelling antifungal drugs from cells and lowering their internal concentrations [38]. The resistance of biofilms is further increased due to physical and biochemical barriers that prevent the penetration of antifungals. These processes tend to be synergistic, leading to the development of multidrug-resistant or pan-resistant strains that are difficult to manage clinically. Improper use of antifungals in healthcare facilities also contributes to resistance, particularly in cases of long-term prophylaxis or empirical treatment lacking species identification [35]. What is created is an evolutionary pressure that favours clones that are highly resistant and can survive intensive treatment procedures, enduring in hospitals. These forces not only make patient care difficult but also complicate the control of infections, as resistant strains are more likely to lead to outbreaks with high morbidity and mortality.
Cross-Regional Resistance Patterns
Table 1 compares the resistance rates of C. auris to three major antifungal drug classes across selected countries, highlighting the geographic variation in susceptibility patterns as of 2020–2024 [35].

Table 1.
Summary of C. auris Antifungal Resistance Rates by Drug Class and Country (2020–2024).
As detailed in Section 6, ibrexafungerp and fosmanogepix demonstrate strong in vitro activity and emerging clinical promise, and may offer therapeutic alternatives in cases of multidrug resistance. Given these complexities, there is growing interest in new and investigational antifungal agents that can overcome current resistance profiles. Ibrexafungerp, a different structural class of glucan synthase inhibitor (not an echinocandin), has demonstrated in vitro activity against C. auris, including echinocandin-resistant strains [13]. Another example of this is Fosmanogepix, which acts on the Gwt1 enzyme in glycosylphosphatidylinositol (GPI) anchor biosynthesis and also exhibits activity in resistant isolates, and is in late-stage clinical trials. They can provide therapeutic alternatives for refractory cases and increase the arsenal of treatments for patients with high risks [39]. In LMICs, access is further constrained by high drug acquisition costs, lack of cold chain logistics, limited diagnostic stewardship capacity, and regulatory delays. Access to advanced antifungals like ibrexafungerp and fosmanogepix remains limited in many Middle Eastern countries due to regulatory delays, cost constraints, and in-consistent pharmaceutical supply chains. Effective stewardship strategies include antifungal de-escalation protocols, early review of empirical therapy within 48–72 h, and formulary restrictions [40,41]. Management protocols increasingly recommend tailoring therapy based on susceptibility profiles.
7. Infection Control Strategies
The unique environmental persistence of C. auris requires IPC strategies that go beyond bacterial models. The CDC and Public Health England recommend bundled approaches combining patient isolation, enhanced cleaning, and targeted screening of high-risk patients [42]. Empirical studies support these interventions: Zhu et al. [9] confirmed that reusable thermometers acted as outbreak reservoirs, controlled only after device removal. Newer technologies complement these measures—hydrogen peroxide vapour units have been shown to achieve superior bioburden reduction compared with UV-C systems in terminal disinfection trials [43]. Ultimately, IPC effectiveness hinges on embedding these evidence-based measures into routine practice, with sustained investment in both equipment and staff engagement.
7.1. Updated IPC Protocols and Advancements
Traditional IPC protocols, designed primarily for bacterial pathogens, fall short in addressing the bioecological complexities of C. auris. Newer recommendations, including those from the CDC and Public Health England, suggest a bundle-based strategy that incorporates patient isolation, enhanced environmental cleaning, contact precautions, and targeted surveillance [44]. One of the key elements of modern IPC development One of the key elements of modern infection prevention and control (IPC) strategies is the active screening of high-risk patients, particularly upon admission, weekly during prolonged hospital stays, and during outbreaks, using swabs from the axilla, groin, and other colonisation-prone sites, particularly in patients who have been transferred from facilities experiencing active outbreaks. C. auris poses a potential occupational risk that must transcend traditional models focused solely on surface decontamination. Regular screening of both symptomatic and asymptomatic individuals is essential, as undetected colonisation significantly contributes to silent transmission and undermines outbreak control. Especially those who have been transferred to the facility or institution with an outbreak [45]. Although decolonisation strategies are regularly applied to MRSA, they remain debatable in the case of C. auris, as the organism is resistant and there is no evidence-based guideline for clearance.
Key advancements in IPC also include algorithm-based patient movement restrictions, tailored cohorting, and the designation of dedicated nursing staff. These measures aim to prevent cross-contamination by reducing the vectorial movement of colonised patients. The education of staff has also changed, with generic IPC training being replaced by pathogen-specific modules that emphasise the importance of environmental persistence in the epidemiology of C. auris [46]. Sustained adherence to IPC practices relies on ongoing staff training, feedback mechanisms, and active engagement to build a culture of shared accountability and vigilance. In addition, real-time IPC dashboards that combine laboratory data, environmental culture outcomes, and employee compliance indicators have become essential surveillance instruments. These combined platforms enhance decision-making and facilitate the prompt deployment of containment interventions [47]. Nevertheless, the adoption of these innovations is uneven in healthcare systems worldwide, particularly in low- and middle-income countries where resources are scarce and laboratory capacity is limited.
7.2. Disinfection Materials
The selection and application of effective disinfectants are paramount to interrupting environmental transmission cycles. C. auris has demonstrated notable resistance to quaternary ammonium compounds (QACs), a mainstay in routine hospital cleaning protocols. As such, the reliance on more potent agents has become an IPC imperative.
Chlorine-Based Agents: Chlorine-based agents, particularly sodium hypochlorite at concentrations between 1000 and 10,000 ppm, have demonstrated potent fungicidal efficacy against C. auris, especially on nonporous surfaces commonly found in healthcare environments [48]. The oxidising nature of the compound enables inactivation of microbes by disrupting the integrity of cellular membranes and denaturing essential intracellular proteins, making the organism nonviable. However, its regular use has significant drawbacks, such as corrosive properties on medical equipment and facility infrastructure, a pungent smell that can negatively affect tolerability, and mucosal irritation that can inhibit long-term staff adherence [49]. Nevertheless, sodium hypochlorite remains a staple disinfectant in lower-resource healthcare facilities because of its low cost and accessibility.
Hydrogen Peroxide Vapour (HPV): Hydrogen peroxide vapour (HPV) systems represent a highly efficacious method for automated, room-wide decontamination, delivering broad-spectrum sporicidal and fungicidal activity through controlled vapour dispersal [50]. Their proven efficacy against C. auris is especially remarkable when combined with stringent pre-cleaning measures. HPV systems are essential in terminal disinfection procedures and outbreak control in high-risk environments, such as intensive care units [51]. Their use is, however, limited by high operational downtimes, which require complete room evacuation and environmental isolation, making them unsuitable for use in continuously occupied patient zones [45]. Moreover, the need for specialised staff and real-time monitoring of the environment creates logistical and training overheads, particularly in high-throughput clinical settings.
Ultraviolet-C (UV-C) Systems: Ultraviolet-C (UV-C) disinfection systems, which emit light within the germicidal spectrum of 200–280 nm, function by inducing photodimerisation of DNA and RNA, thereby disrupting the genomic integrity of microorganisms and halting their replication [52]. These systems have demonstrated considerable efficacy in reducing C. auris surface contamination, particularly on high-touch, nonporous surfaces such as bed rails, infusion pumps, and procedural carts [53]. Their no-touch, chemical-free application offers operational advantages, including minimal surface degradation and reduced occupational exposure risks. However, UV-C efficacy is inherently dependent on direct exposure, with performance markedly diminished in shadowed or obstructed areas, as well as on porous materials. As such, UV-C units must be deployed in conjunction with thorough manual cleaning and cannot substitute for conventional chemical disinfection protocols in high-risk clinical environments.
Peracetic Acid-Based Disinfectants: Peracetic acid (PAA), particularly when combined with hydrogen peroxide, is a highly potent oxidising agent that exhibits broad-spectrum antimicrobial efficacy, including fungicidal activity against resilient pathogens such as C. auris [54]. Its mechanism of action involves oxidative damage to microbial cell walls, proteins, and nucleic acids, rendering it effective even against biofilm-associated organisms. Notably, PAA decomposes into environmentally benign byproducts—acetic acid, water, and oxygen—making it a preferred choice for sustainable disinfection strategies [55]. Its utility is especially pronounced in fogging systems and high-level disinfection of complex reusable medical instruments, including those used in surgical theatres and endoscopy units. However, widespread adoption remains constrained by several operational challenges, including elevated procurement and maintenance costs, rigorous safety handling requirements, and the potential for mucosal and dermal irritation among healthcare workers.
7.3. Equipment and Technologies
The emergence of advanced decontamination technologies has significantly enhanced the infection prevention and control (IPC) landscape by facilitating consistent, reproducible, and high-efficacy environmental cleaning practices that extend beyond the limitations of manual disinfection [56]. Technologies such as automated vapour systems, ultraviolet-C irradiation units, and electrostatic sprayers bring precision and standardisation to routine cleaning processes, reducing the variability associated with human-based protocols. Their use in high-acuity environments guarantees a thorough reduction in bioburden on complex surfaces and equipment, especially in microbial-prone areas [47]. Incorporating these innovations into IPC frameworks, healthcare institutions will be able to enhance their ability to combat resilient pathogens, such as C. auris, thereby promoting patient safety and institutional resilience.
UV-C Disinfection Robots: Ultraviolet-C (UV-C) disinfection robots represent a sophisticated advancement in no-touch environmental decontamination, utilising autonomous navigation systems to deliver calibrated doses of germicidal light across patient rooms and high-risk zones [57].
Empirical studies have demonstrated that they have the potential to reduce C. auris surface Colony-Forming Units (CFUs) by up to 99.9%, particularly when applied following intensive manual cleaning. These units enhance the precision of decontamination by accessing complex geometries in clinical spaces, thereby minimising operator variability [58]. However, their performance is controlled by environmental factors such as room size, surface reflectivity, and the duration of exposure to light to achieve microbicidal levels. Additionally, their high cost of capital and maintenance remain significant barriers to their large-scale implementation, particularly in under-resourced public healthcare systems.
HPV Decontamination Units: Hydrogen peroxide vapour (HPV) decontamination units are a pivotal component of terminal disinfection protocols, particularly following patient discharge or in the context of outbreak containment involving resilient pathogens such as C. auris [59]. The system works by vaporising concentrated hydrogen peroxide in a hermetically sealed space, allowing the agent to achieve even diffusion and access otherwise inaccessible surfaces, including equipment crevices and architectural recesses. HPV units have been demonstrated to be more effective in reducing log counts of fungal contaminants than ultraviolet-based disinfection systems, and therefore offer more effective bioburden control [60]. Nevertheless, they must be used in operations with strict preparatory measures, including complete evacuation of rooms, physical sealing, and long exposure times. These needs can cause significant disruptions to clinical operations, particularly in busy or high-volume settings; therefore, scheduling and resource planning are crucial.
Electrostatic Sprayers: Electrostatic sprayers represent a technologically advanced approach to enhancing surface disinfection by imparting an electrostatic charge to disinfectant droplets, enabling them to adhere uniformly to a broad range of surfaces, including vertical, curved, and otherwise inaccessible areas [61]. This process significantly enhances contact time and surface saturation, particularly in clinical settings with high-touch and complex geometric infrastructure. Their adoption in day-to-day environmental hygiene procedures has been on the rise due to ease of operation, reduced chemical waste, and improved efficiency [46]. Electrostatic sprayers significantly enhance decontamination results when applied in conjunction with C. auris-effective agents, including sodium hypochlorite or peracetic acid-based formulations, and reduce the risk of pathogenic reservoirs’ persisting in healthcare facilities.
Continuous Environmental Decontamination Systems: Continuous environmental decontamination systems, such as those integrating high-efficiency particulate air (HEPA) filtration with ultraviolet germicidal irradiation (UVGI), provide a proactive approach to mitigating microbial contamination in high-risk clinical areas [62]. C. auris is not considered an airborne pathogen; however, its high shedding rates on colonised skin and subsequent deposition on nearby surfaces suggest that ambient air currents could contribute to secondary environmental seeding. The systems are designed to minimise both airborne particulates and surface bioburden simultaneously, which may interfere with indirect transmission routes [53]. Although initial implementations in intensive care units demonstrate that the systems are operationally viable, the effectiveness of such systems in controlling C. auris needs to be empirically validated, and cost-effectiveness analyses should be conducted with fungal-specific transmission risks in mind. Due to its environmental persistence, C. auris poses a potential occupational risk in laboratory settings, particularly where biosafety protocols are suboptimal. Nonetheless, documented cases of laboratory-acquired infections remain rare.
8. Environmental Monitoring, Surface Culturing, and Staff Compliance Mechanisms
Environmental detection of C. auris relies on a combination of culture-based and molecular surveillance techniques that are critical for verifying cleaning efficacy and identifying environmental reservoirs during both routine monitoring and outbreak containment. Surface culturing methods typically involve the use of salt-enriched broths for selective enrichment and CHROMagar Candida Plus as a differential chromogenic medium capable of distinguishing C. auris from other Candida species through colony colour and morphology [63]. Swabbing techniques include the use of flocked swabs, sponge-stamping, and contact plates, with environmental samples collected from high-touch surfaces such as bed rails, infusion pumps, ventilator controls, sink drains, floor junctions, and mobile medical equipment. These surfaces are known to support persistent colonisation and have been frequently implicated in transmission events. Complementary detection methods, such as quantitative PCR (qPCR), offer high sensitivity and faster turnaround times, especially when evaluating low-level contamination in critical care units [36]. Additionally, ATP bioluminescence assays are increasingly employed to assess residual organic matter as a proxy for cleaning thoroughness, although they do not confirm fungal presence directly. These methods, when implemented in alignment with CDC and WHO guidelines, contribute significantly to outbreak prevention and response by enabling targeted decontamination, auditing of cleaning protocols, and verification of IPC compliance. Continuous environmental monitoring, particularly in high-risk units such as ICUs, is critical for detecting persistent fungal reservoirs and informing timely decontamination efforts. Ultimately, standardising environmental sampling techniques and thresholds across institutions enhances comparability and strengthens systemic resilience against fungal threats.
Staff compliance remains the linchpin of IPC efficacy. Behavioural audits, direct observation tools, and electronic compliance tracking systems are increasingly employed to monitor adherence to hand hygiene, PPE use, and disinfection protocols [53]. The inclusion of compliance measures in electronic health record dashboards enables real-time feedback and management control. However, surveillance is not enough to maintain compliance; it needs a cultural change. It is essential to embed IPC as a non-negotiable standard of care supported by education, incentives, and leadership modelling [63]. Moreover, the ability to report protocol violations without fear of punishment will empower frontline personnel to develop a safety-focused culture that is critical to fungal containment.
9. Conclusions and Future Directions
Effective infection control strategies targeting C. auris must transcend the traditional model of superficial decontamination and embrace a dynamic, systems-level framework that reflects the pathogen’s unique biological, ecological, and institutional challenges. The exceptional resilience of C. auris on both porous and nonporous surfaces, coupled with its capacity for multidrug resistance and asymptomatic colonisation, renders conventional ad hoc protocols not only insufficient but potentially dangerous when deployed in isolation. The complexity of its transmission dynamics—spanning from biofilm-mediated environmental persistence to cryptic spread via healthcare personnel and contaminated equipment—necessitates a recalibration of infection prevention and control (IPC) models toward sustained, multicomponent interventions.
While technological innovations, such as ultraviolet-C (UV-C) disinfection robots and hydrogen peroxide vapour (HPV) decontamination systems, represent substantial advances in environmental hygiene, their standalone use offers limited benefits if not embedded within an overarching IPC ecosystem. These tools are only as effective as the protocols that govern their deployment—protocols that must integrate real-time environmental monitoring, precision diagnostics, and a culture of procedural adherence among healthcare personnel. The establishment of standardised protocols for environmental culturing and surface sampling is required to move beyond reactive outbreak containment toward pre-emptive risk identification. Development and distribution of fungal-specific diagnostic platforms, including MALDI-TOF MS and isothermal amplification assays, must be prioritised. However, high cost and limited availability continue to hinder adoption.
Furthermore, the global health community must adopt coordinated genomic surveillance strategies to track emerging C. auris clades, resistance mutations, and transmission corridors. Such surveillance will inform local epidemiological profiles and guide treatment guidelines and containment protocols. Equally essential is the exploration of anti-biofilm surface coatings and long-acting protectants for environmental protection. These approaches remain in early stages and require rigorous clinical trials. These agents warrant trials, particularly in critical care settings. Implementation science and organisational psychology must institutionalise a culture of safety, reinforced through education, leadership modelling, and feedback. Staff engagement must transition from passive compliance to proactive IPC ownership.
Ultimately, the management of C. auris must reflect a paradigm shift toward proactive, data-driven, and ecologically informed IPC strategies. Institutions must foster system resilience by investing in interdisciplinary collaboration, adaptive policy frameworks, and flexible response mechanisms that can absorb evolving mycological threats. The enduring containment of C. auris will not be achieved through singular interventions but through a convergence of technological, procedural, and behavioural innovations that collectively reinforce the integrity of healthcare ecosystems.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
No new data were created or analysed in this study.
Conflicts of Interest
The author declares no conflicts of interest.
References
- Voidazan, S.; Albu, S.; Toth, R.; Grigorescu, B.; Rachita, A.; Moldovan, I. Healthcare associated infections—A new pathology in medical practice? Int. J. Environ. Res. Public Health 2020, 17, 760. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, A.; Sood, P. On the emergence, spread and resistance of Candida auris: Host, pathogen and environmental tipping points. J. Med. Microbiol. 2021, 70, 001318. [Google Scholar] [CrossRef] [PubMed]
- Keighley, C.; Garnham, K.; Harch, S.A.; Robertson, M.; Chaw, K.; Teng, J.C.; Chen, S.A. Candida auris: Diagnostic challenges and emerging opportunities for the clinical microbiology laboratory. Curr. Fungal Infect. Rep. 2021, 15, 116–126. [Google Scholar] [CrossRef]
- Hau, P.T.; Shiu, A.; Tam, E.W.; Chau, E.C.; Murillo, M.; Humer, E.; Po, W.W.; Yu, R.C.; Fung, J.; Seto, S.W.; et al. Diversity and Antifungal Susceptibilities of Yeasts from Mangroves in Hong Kong, China—A One Health Aspect. J. Fungi 2024, 10, 728. [Google Scholar] [CrossRef]
- Akinbobola, A.B.; Kean, R.; Hanifi, S.M.; Quilliam, R.S. Environmental reservoirs of the drug-resistant pathogenic yeast Candida auris. PLoS Pathog. 2023, 19, e1011268. [Google Scholar] [CrossRef]
- Tharp, B.; Zheng, R.; Bryak, G.; Litvintseva, A.P.; Hayden, M.K.; Chowdhary, A.; Thangamani, S. Role of microbiota in the skin colonisation of Candida auris. Msphere 2023, 8, e00623-22. [Google Scholar] [CrossRef]
- Sansom, S.E.; Gussin, G.M.; Schoeny, M.; Singh, R.D.; Adil, H.; Bell, P.; Benson, E.C.; Bittencourt, C.E.; Black, S.; Del Mar Villanueva Guzman, M.; et al. Rapid environmental contamination with Candida auris and multidrug-resistant bacterial pathogens near colonised patients. Clin. Infect. Dis. 2024, 78, 1276–1284. [Google Scholar] [CrossRef]
- Motallebirad, T.; Mohammadi, M.R.; Jadidi, A.; Safarabadi, M.; Kerami, A.; Azadi, D.; Hussein, E.S. Tracheal tube infections in critical care: A narrative review of influencing factors, microbial agents, and mitigation strategies in intensive care unit settings. SAGE Open Med. 2024, 12, 20503121241306951. [Google Scholar] [CrossRef]
- Zhu, Y.; O’Brien, B.; Leach, L.; Clarke, A.; Bates, M.; Adams, E.; Ostrowsky, B.; Quinn, M.; Dufort, E.; Southwick, K.; et al. Laboratory analysis of an outbreak of Candida auris in New York from 2016 to 2018: Impact and lessons learned. J. Clin. Microbiol. 2020, 58, 10–128. [Google Scholar] [CrossRef]
- Noshili, A.I.; Almutairi, F.A.; Shahbal, S.; Alotaibi, F.A.; Alotaibi, B.; Refaei, R.A.A.; Almutairi, M.M.; Hmed Al Balawi, S.A.; Alshammari, R.T.; Almotairi, A.K.; et al. Systematic Review Are We Ready for New Emerging Infection Candida auris; Review of Preparedness Measure and Strategies for Infection Prevention in the Saudi Arabian Health System. Migr. Lett. 2023, 20, 678–697. [Google Scholar]
- Hata, D.J.; Humphries, R.; Lockhart, S.R.; College of American Pathologists Microbiology Committee. Candida auris: An emerging yeast pathogen posing distinct challenges for laboratory diagnostics, treatment, and infection prevention. Arch. Pathol. Lab. Med. 2020, 144, 107–114. [Google Scholar] [CrossRef]
- Chowdhary, A.; Tarai, B.; Singh, A.; Sharma, A. Multidrug-resistant Candida auris infections in critically ill coronavirus disease patients, India, April–July 2020. Emerg. Infect. Dis. 2020, 26, 2694. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.R.; Neill, C.; Borman, A.M.; Budd, E.L.; Cummins, M.; Fry, C.; Guy, R.L.; Jeffery, K.; Johnson, E.M.; Manuel, R.; et al. The laboratory investigation, management, and infection prevention and control of Candida auris: A narrative review to inform the 2024 national guidance update in England. J. Med. Microbiol. 2024, 73, 001820. [Google Scholar] [CrossRef]
- Thomsen, J.; Abdulrazzaq, N.M.; AlRand, H.; UAE AMR Surveillance Consortium; Elhag Ahmed, A.; Yousef, A.F.; AlBlooshi, A.; Latoom, D.A.; Abdulkareem Al Hammadi, D.A.; Enshasy, D.A.; et al. Surveillance of antimicrobial resistance in the United Arab Emirates: The early implementation phase. Front. Public Health 2023, 11, 1247627. [Google Scholar] [CrossRef]
- Najeeb, H.; Siddiqui, S.A.; Anas, Z.; Ali, S.H.; Usmani, S.U.; Jawed, F.; Jatoi, H.N. The menace of Candida auris epidemic amidst the COVID-19 pandemic: A systematic review. Diseases 2022, 10, 58. [Google Scholar] [CrossRef]
- Siopi, M.; Georgiou, P.C.; Paranos, P.; Beredaki, M.I.; Tarpatzi, A.; Kalogeropoulou, E.; Damianidou, S.; Vasilakopoulou, A.; Karakosta, P.; Pournaras, S.; et al. Increase in candidemia cases and emergence of fluconazole-resistant Candida parapsilosis and C. auris isolates in a tertiary care academic hospital during the COVID-19 pandemic, Greece, 2020 to 2023. Eurosurveillance 2024, 29, 2300661. [Google Scholar] [CrossRef]
- Mohammed, M.A. Fighting cytokine storm and immunomodulatory deficiency: By using natural products therapy up to now. Front. Pharmacol. 2023, 14, 1111329. [Google Scholar] [CrossRef]
- Tavares, L.P.; Galvão, I.; Ferrero, M.R. Novel immunomodulatory therapies for respiratory pathologies. Compr. Pharmacol. 2022, 2022, 554–594. [Google Scholar]
- Christie, M.J.; Irving, A.T.; Forster, S.C.; Marsland, B.J.; Hansbro, P.M.; Hertzog, P.J.; Nold-Petry, C.A.; Nold, M.F. Of bats and men: Immunomodulatory treatment options for COVID-19 guided by the immunopathology of SARS-CoV-2 infection. Sci. Immunol. 2021, 6, eabd0205. [Google Scholar] [CrossRef]
- Huang, C. Pathogenesis of coronaviruses through human monocytes and tissue macrophages. Viral Immunol. 2021, 34, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Novak Babič, M.; Gunde-Cimerman, N. Potable water as a source of intermediate and borderline-resistant Aspergillus and Candida strains. J. Water Health 2025, 23, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Arendrup, M.C.; Guinea, J.; Arikan-Akdagli, S.; Meijer, E.F.; Meis, J.F.; Buil, J.B.; Dannaoui, E.; Giske, C.G.; Lyskova, P.; Meletiadis, J.; et al. How to interpret MICs of amphotericin B, echinocandins and flucytosine against Candida auris (Candidozyma auris) according to the newly established EUCAST breakpoints. Clin. Microbiol. Infect. 2025; in press. [Google Scholar]
- Garcia-Bustos, V.; Cabanero-Navalon, M.D.; Ruiz-Saurí, A.; Ruiz-Gaitán, A.C.; Salavert, M.; Tormo, M.Á.; Pemán, J. What do we know about Candida auris? State of the art, knowledge gaps, and future directions. Microorganisms 2021, 9, 2177. [Google Scholar] [CrossRef]
- Gómez-Gaviria, M.; Martínez-Álvarez, J.A.; Chávez-Santiago, J.O.; Mora-Montes, H.M. Candida haemulonii complex and Candida auris: Biology, virulence factors, immune response, and multidrug resistance. Infect. Drug Resist. 2023, 16, 1455–1470. [Google Scholar] [CrossRef]
- Foo, P.C.; Nurul Najian, A.B.; Muhamad, N.A.; Ahamad, M.; Mohamed, M.; Yean Yean, C.; Lim, B.H. Loop-mediated isothermal amplification (LAMP) reaction as viable PCR substitute for diagnostic applications: A comparative analysis study of LAMP, conventional PCR, nested PCR (nPCR) and real-time PCR (qPCR) based on Entamoeba histolytica DNA derived from faecal sample. BMC Biotechnol. 2020, 20, 34. [Google Scholar]
- Hu, X.; Deng, Q.; Li, J.; Chen, J.; Wang, Z.; Zhang, X.; Fang, Z.; Li, H.; Zhao, Y.; Yu, P.; et al. Development and clinical application of a rapid and sensitive loop-mediated isothermal amplification test for SARS-CoV-2 infection. MSphere 2020, 5, 10–128. [Google Scholar] [CrossRef] [PubMed]
- Dire, O.; Ahmad, A.; Duze, S.; Patel, M. Survival of Candida auris on environmental surface materials and low-level resistance to disinfectant. J. Hosp. Infect. 2023, 137, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Kramer, A.; Lexow, F.; Bludau, A.; Köster, A.M.; Misailovski, M.; Seifert, U.; Eggers, M.; Rutala, W.; Dancer, S.J.; Scheithauer, S. How long do bacteria, fungi, protozoa, and viruses retain their replication capacity on inanimate surfaces? A systematic review examining environmental resilience versus healthcare-associated infection risk by “fomite-borne risk assessment”. Clin. Microbiol. Rev. 2024, 37, e00186-23. [Google Scholar] [CrossRef]
- Bäumler, W.; Eckl, D.; Holzmann, T.; Schneider-Brachert, W. Antimicrobial coatings for environmental surfaces in hospitals: A potential new pillar for prevention strategies in hygiene. Crit. Rev. Microbiol. 2022, 48, 531–564. [Google Scholar] [CrossRef]
- Hoenigl, M.; Arastehfar, A.; Arendrup, M.C.; Brüggemann, R.; Carvalho, A.; Chiller, T.; Chen, S.; Egger, M.; Feys, S.; Gangneux, J.P.; et al. Novel antifungals and treatment approaches to tackle resistance and improve outcomes of invasive fungal disease. Clin. Microbiol. Rev. 2024, 37, e00074-23. [Google Scholar] [CrossRef]
- Cavassin, F.B.; Baú-Carneiro, J.L.; Vilas-Boas, R.R.; Queiroz-Telles, F. Sixty years of amphotericin B: An overview of the main antifungal agent used to treat invasive fungal infections. Infect. Dis. Ther. 2021, 10, 115–147. [Google Scholar] [CrossRef]
- Wiederhold, N.P. Emerging fungal infections: New species, new names, and antifungal resistance. Clin. Chem. 2022, 68, 83–90. [Google Scholar] [CrossRef]
- Nivoix, Y.; Ledoux, M.P.; Herbrecht, R. Antifungal therapy: New and evolving therapies. InSeminars Respir. Crit. Care Med. 2020, 41, 158–174. [Google Scholar] [CrossRef]
- Mroczyńska, M.; Brillowska-Dąbrowska, A. Review on current status of echinocandins use. Antibiotics 2020, 9, 227. [Google Scholar] [CrossRef]
- Wiederhold, N.P.; Najvar, L.K.; Olivo, M.; Morris, K.N.; Patterson, H.P.; Catano, G.; Patterson, T.F. Ibrexafungerp demonstrates in vitro activity against fluconazole-resistant Candida auris and in vivo efficacy with delayed initiation of therapy in an experimental model of invasive candidiasis. Antimicrob. Agents Chemother. 2021, 65, 10–128. [Google Scholar] [CrossRef]
- EUCAST. Breakpoint Tables for Interpretation of MICs and Zone Diameters, version 12.0. European Committee on Antimicrobial Susceptibility Testing. 2022. Available online: https://www.eucast.org/clinical_breakpoints (accessed on 2 December 2024).
- Lockhart, S.R.; Chowdhary, A.; Gold, J.A. The rapid emergence of antifungal-resistant human-pathogenic fungi. Nat. Rev. Microbiol. 2023, 21, 818–832. [Google Scholar] [CrossRef]
- Arendrup, M.C.; Friberg, N.; Mares, M.; Kahlmeter, G.; Meletiadis, J.; Guinea, J.; Andersen, C.T.; Arikan-Akdagli, S.; Barchiesi, F.; Chryssanthou, E.; et al. How to interpret MICs of antifungal compounds according to the revised clinical breakpoints v. 10.0 European committee on antimicrobial susceptibility testing (EUCAST). Clin. Microbiol. Infect. 2020, 26, 1464–1472. [Google Scholar] [CrossRef] [PubMed]
- Silva, I.; Miranda, I.M.; Costa-de-Oliveira, S. Potential environmental reservoirs of Candida auris: A systematic review. J. Fungi 2024, 10, 336. [Google Scholar] [CrossRef] [PubMed]
- Thoma, R.; Seneghini, M.; Seiffert, S.N.; Vuichard Gysin, D.; Scanferla, G.; Haller, S.; Flury, D.; Boggian, K.; Kleger, G.R.; Filipovic, M.; et al. The challenge of preventing and containing outbreaks of multidrug-resistant organisms and Candida auris during the coronavirus disease 2019 pandemic: Report of a carbapenem-resistant Acinetobacter baumannii outbreak and a systematic review of the literature. Antimicrob. Resist. Infect. Control. 2022, 11, 12. [Google Scholar]
- Yahaya, H.; Sule, H. Candida auris: An emergent virulent and multidrug-resistant yeast associated with serious health implications. Acad. Biol. 2023, 1, 1–12. [Google Scholar] [CrossRef]
- De Melo, C.C.; de Sousa, B.R.; da Costa, G.L.; Oliveira, M.M.; de Lima-Neto, R.G. Colonised patients by Candida auris: Third and largest outbreak in Brazil and impact of biofilm formation. Front. Cell. Infect. Microbiol. 2023, 13, 1033707. [Google Scholar] [CrossRef]
- Addetia, A.; Crawford, K.H.; Dingens, A.; Zhu, H.; Roychoudhury, P.; Huang, M.L.; Jerome, K.R.; Bloom, J.D.; Greninger, A.L. Neutralizing antibodies correlate with protection from SARS-CoV-2 in humans during a fishery vessel outbreak with a high attack rate. J. Clin. Microbiol. 2020, 58, e02107-20. [Google Scholar] [CrossRef]
- Muñoz, J.E.; Ramirez, L.M.; Dias, L.D.; Rivas, L.A.; Ramos, L.S.; Santos, A.L.; Taborda, C.P.; Parra-Giraldo, C.M. Pathogenicity levels of Colombian strains of Candida auris and Brazilian strains of Candida haemulonii species complex in both murine and Galleria mellonella experimental models. J. Fungi 2020, 6, 104. [Google Scholar] [CrossRef] [PubMed]
- Bandara, H.M.; Samaranayake, L.P. Emerging strategies for environmental decontamination of the nosocomial fungal pathogen Candida auris. J. Med. Microbiol. 2022, 71, 001548. [Google Scholar] [CrossRef]
- Douglas, A.P.; Stewart, A.G.; Halliday, C.L.; Chen, S.C. Outbreaks of fungal infections in hospitals: Epidemiology, detection, and management. J. Fungi 2023, 9, 1059. [Google Scholar] [CrossRef]
- Totaro, M.; Casini, B.; Profeti, S.; Tuvo, B.; Privitera, G.; Baggiani, A. Role of hydrogen peroxide vapor (HPV) for the disinfection of hospital surfaces contaminated by multiresistant bacteria. Pathogens 2020, 9, 408. [Google Scholar] [CrossRef]
- Courti, I.; Allix, S. Qualitative comparison of hydrogen peroxide decontamination systems: Vapor vs. aerosol. Laboratories 2024, 1, 124–134. [Google Scholar] [CrossRef]
- Xie, X.; Gao, N.; Zhu, L.; Hunter, M.; Chen, S.; Zang, L. PEDOT: PSS/PEDOT film chemiresistive sensors for hydrogen peroxide vapor detection under ambient conditions. Chemosensors 2023, 11, 124. [Google Scholar] [CrossRef]
- Pereira, A.R.; Braga, D.F.; Vassal, M.; Gomes, I.B.; Simões, M. Ultraviolet C irradiation: A promising approach for the disinfection of public spaces? Sci. Total Environ. 2023, 879, 163007. [Google Scholar] [CrossRef] [PubMed]
- Shah, D.; Gandhi, H.; Shah, M.; Nagda, M.; Rajpurohit, Y. Ultraviolet light (UV-C): An effective tool to mitigate pandemic spread—A review. Int. J. Eng. Res. Technol. 2021, 10, 26–33. [Google Scholar]
- Zhu, Y.; Kilburn, S.; Kapoor, M.; Chaturvedi, S.; Shaw, K.J.; Chaturvedi, V. In vitro activity of manogepix against multidrug-resistant and panresistant Candida auris from the New York outbreak. Antimicrob. Agents Chemother. 2020, 64, 10–128. [Google Scholar] [CrossRef]
- Thakur, H.; Rao, R. Emphasis of infection prevention and control: A review. J. Popul. Therap. Clin. Pharmacol. 2024, 31, 2238–2249. [Google Scholar]
- Jimenez, Y.A.; Lewis, S.J. Infection prevention and control in the medical imaging environment: A scoping review. Insights Into Imaging 2023, 14, 121. [Google Scholar] [CrossRef] [PubMed]
- Godbole, A.A.; Paras; Mehra, M.; Banerjee, S.; Roy, P.; Deb, N.; Jagtap, S. Enhancing Infection Control in ICUS Through AI: A Literature Review. Health Sci. Rep. 2025, 8, e70288. [Google Scholar] [CrossRef] [PubMed]
- Sartelli, M.; Pagani, L.; Iannazzo, S.; Moro, M.L.; Viale, P.; Pan, A.; Ansaloni, L.; Coccolini, F.; D’Errico, M.M.; Agreiter, I.; et al. A proposal for a comprehensive approach to infections across the surgical pathway. World J. Emerg. Surg. 2020, 15, 1–26. [Google Scholar] [CrossRef]
- McGinn, C.; Scott, R.; Donnelly, N.; Roberts, K.L.; Bogue, M.; Kiernan, C.; Beckett, M. Exploring the Applicability of Robot-Assisted UV Disinfection in Radiology. Front. Robot. AI 2021, 7, 590306. [Google Scholar] [CrossRef]
- Wood, J.P.; Magnuson, M.; Touati, A.; Gilberry, J.; Sawyer, J.; Chamberlain, T.; McDonald, S.; Hook, D. Evaluation of electrostatic sprayers and foggers for the application of disinfectants in the era of SARS-CoV-2. PLoS ONE 2021, 16, e0257434. [Google Scholar] [CrossRef]
- Weber, D.J.; Rutala, W.A.; Anderson, D.J.; Chen, L.F.; Sickbert-Bennett, E.E.; Boyce, J.M. Effectiveness of ultraviolet devices and hydrogen peroxide systems for terminal room decontamination: Focus on clinical trials. Am. J. Infect. Control. 2016, 44, e77–e84. [Google Scholar] [CrossRef]
- Kompatscher, K.; van der Vossen, J.M.; van Heumen, S.P.; Traversari, A.A. Scoping review on the efficacy of filter and germicidal technologies for capture and inactivation of micro-organisms and viruses. J. Hosp. Infect. 2023, 142, 39–48. [Google Scholar] [CrossRef]
- Izadyar, N.; Miller, W. Ventilation strategies and design impacts on indoor airborne transmission: A review. Build. Environ. 2022, 218, 109158. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Roy, S.; Shaikh, N.I.; Malave, P.; Mishra, A.; Alam, M.A.; Ghorpade, Y.; Hasan, M.R.; Nizam, A. Recent advances in multiplex aptasensor detection techniques for food-borne pathogens: A comprehensive review of novel approaches. Biosens. Bioelectron. X 2024, 16, 100417. [Google Scholar] [CrossRef]
- Albers, B.; Verweij, L.; Blum, K.; Oesch, S.; Schultes, M.T.; Clack, L.; Naef, R. Firm, yet flexible: A fidelity debate paper with two case examples. Implement. Sci. 2024, 19, 79. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).