Abstract
Twenty natural products used in traditional medicine to treat dermatophytosis were evaluated for their efficacy against Trichophyton rubrum, the most frequent dermatophyte infecting humans. For this purpose, aqueous and methanolic extracts were prepared from ash, honey, and plant organs as pure (100%) or diluted (75%, 50%, 25%, 12.5%, 6.25%, 3.125%, and 1.56%) preparations. The extracts were then evaluated by incorporating them into a Sabouraud medium and seeding them with T. rubrum as a fungal culture. The results identify fourteen extracts as being able to completely inhibit T. rubrum growth through either fungistatic or fungicidal activity. The five extracts with the highest efficacy to inhibit T. rubrum growth were further analyzed for their potential to alter in vitro reconstructed human epidermis (RHE). An aqueous extract from Allium sativum produced no tissue alteration in RHE, unlike the extracts from Conyza sumatrensis, Rumex abyssinicus, or Pentas longiflora. The data suggest that preparations used in traditional medicine by rural population in South-Kivu (DR-Congo) might represent valid alternatives to fight dermatophytosis. However, they also illustrate that several preparations remain inefficient and that others may be detrimental to the epidermis. This work reveals that traditional preparations, although affordable and easily available, require an evaluation of their efficacy and safety.
1. Introduction
Dermatophytes are pathogen fungi able to invade diverse keratinized structures in humans and animals. The most frequent genera are Trichophyton, Epidermophyton, and Microsporum [1,2,3]. Resulting infections, named dermatophytosis, not only alter the infected skin areas of patients, but they also dramatically reduce self-confidence in affected individuals and often cause financial burdens due to treatment cost and duration. The recurrence of infection, often due to resistance to antifungal treatment, is another increasing concern [4,5,6]. Therefore, dermatophytosis creates numerous public health concerns since it can be easily transmitted and is affecting 20–25% of the global human population, with a still increasing prevalence [7,8]. The most dramatic situation remains in southern countries, where poverty, precarious hygiene conditions, and the inaccessibility of modern efficient antifungal treatments are frequently encountered. In addition, elevated humidity and temperatures favor the growth and spreading conditions of these fungi. Still, correct diagnosis is often lacking in those areas because of insufficient doctor availability or a lack of training, while the cost of treatments is often unaffordable for patients. Consequently, traditional medicine and its recommended treatments, based on easily available and low-cost resources, are very attractive to people in African populations whose cultural values are mainly orally transmitted. Furthermore, traditional medicine is becoming popular since treatments have been recognized as efficient in treating several human diseases [9,10,11]. In addition, several compounds used in modern medicine have been identified from traditional practice, and most likely several others are still to be identified and explored. Nevertheless, traditional medicine has numerous limitations due to its usually oral transmission and the resulting lack of adequate written information, due to some variability and imprecision regarding the prescribed doses, standardization, and preparation of compounds, the surveillance of potential toxic effects, and the uncertainty in diagnosis. Traditional medicine is receiving attention from researchers to list products, identify pros and cons regarding cultural aspects, and screen claimed biological activities of extracts, including antimicrobial effects against bacteria, viruses, or metabolic diseases like diabetes, gut irritation, or infertility. Its use in controlling dermatophytosis caused by Trichophyton rubrum has only been superficially analyzed, especially in several global areas where tinea is highly present. The insertion of traditional medicine into health programs at large is also debated until its efficacy and mechanisms of action are proven [11,12,13,14,15,16]. Our research goal is hereby to scientifically evaluate for efficacy and safety several easily available traditional products and preparations used to fight against dermatophytosis in a specific area of Eastern RDC. Extracts prepared as closely as possible to the practices performed by local inhabitants were first analyzed to determine their eventual efficacy against T. rubrum growth, and whether their efficacy eventually results from cytotoxic (fungicidal) or solely from cytostatic (fungistatic) properties. The concentrations required to produce such effects were determined in vitro and then evaluated for safety by analyzing whether or not they induce anomalies in the usual histology of reconstructed human epidermis in vitro. This study might orient traditional medicine towards antifungal preparations holding a proven efficacy and safety, thereby contributing to better outcomes.
2. Materials and Methods
2.1. Selection of Traditional Preparations Used in South-Kivu to Test Antifungal Properties
Traditional medicine preparations were selected based on testimony collected from traditional practitioners and inhabitants of Kalonge village, near Bukavu, South-Kivu Province, Democratic Republic of Congo, and on personal efficacy reports. Information regarding preparation of treatments and procedures used for their cutaneous administration was collected from the same individuals. Leaves from Ageratum conyzoides, Rumex abyssinicus, Conysa sumatrens, Pentas longiflora (male), Pentas longiflora (female), Commelina diffusa, Jatropha curcas, Rhoicissus spp, garlic cloves (Allium sativum), honey, ashes, oil from Lebrunia bushaie, and palm oil were selected. All materials analyzed in this study were acquired from local sources in South-Kivu. Among selected materials, leaves were harvested, dried in the shade, crushed, and then sifted. Garlic cloves were peeled, then pounded in a mortar. Multifloral dark-brown honey was acquired from a local beekeeper; oils were bought at local market, and ashes were kept from domestic cooking.
2.2. Strain of Dermatophytes
T. rubrum TA 13804 P3 strain was available at the University of Namur (Belgium) after previous studies of epidermal infection [3,8]. This species was selected for its incidence on human dermatophytosis. To inoculate T. rubrum, spores were prepared as described [8] and dispersed in PBS.
2.3. Preparation of Extracts and Dilutions
Aqueous or methanol extracts were prepared from most plant materials. Aqueous ones were prepared through a 24–48 h maceration of 1 g of dried material into 9 mL of distilled water. Methanol extracts were prepared with the same procedure in the smallest volume required to completely immerse the dried material. Both types of preparation were cleared by filtration through compress and cotton wool. Extracts in methanol were then heated to 70 °C to evaporate alcohol, then dissolved in a distilled water volume corresponding to the initial powder weight. Aqueous and methanolic extracts were then diluted (i.e., 100%, 75%, 50%, 25%, 12.5%, 6.25%, 3.125%, and 1.5625%) with distilled water.
2.4. Treatments of Cultured T. rubrum with Extracts
Sabouraud agar containing extracts was used to culture T. rubrum. Extracts diluted in water as described above were used to prepare Sabouraud agar (47 g/L), which was then autoclaved for 15 min at 121 °C, then poured in sterile plastic Petri dishes. When cooled, 50 µL of T. rubrum in suspension was pipetted and spread over Sabouraud agar under laminar air flow. Triplicates of each preparation at each precise concentration were compared with a control dish where fungi were cultured in absence of any extract. Dishes were incubated for 6 days at 28 °C before analysis of growth. Lack of growth indicates the inhibitory effect of the preparation at the concentration incorporated in Sabouraud agar.
2.5. Analysis of the Impact of Autoclave Sterilization on Preparation Efficacy
To check whether the sterilization through autoclaving of Sabouraud agar containing the extracts alters the potential efficacy of prepared extracts, those which were found inefficient at their highest concentrations were sterilized with an alternative method. Filtration through 0.2 µm Millex HA was chosen for this purpose. Extracts at various dilutions (100, 75, and 50%) were prepared in Sabouraud broth and mixed with suspended T. rubrum in a tube for a 4-day incubation at 28 °C. A total of 50 µL was then pipetted and spread over Sabouraud agar for a 7-day incubation. An absence of T. rubrum growth allows us to consider the efficacy of the tested preparation and to determine whether the autoclave sterilization process does affect properties of the preparation.
2.6. Determination of Fungicidal or Fungistatic Properties of Extracts Inhibiting T. rubrum Growth
Extracts that exhibited inhibitory properties on T. rubrum growth were then analyzed to assess whether they lethally inhibit fungi (fungicidal) or simply inhibit growth (fungistatic). For this purpose, samples were collected from the surface of Sabouraud agar containing the analyzed extracts and then spread over fresh control Sabouraud agar for a 7-day incubation to determine whether T. rubrum collected from the initial culture may grow again in absence of the extracts. While growth of T. rubrum colonies reveals that the extracts had fungistatic properties during the initial incubation, any absence of growth indicates that the corresponding extract had killed fungi and can thus be considered as fungicidal. Therefore, this analysis allowed us to determine minimal inhibitory concentration (MIC) and minimal lethal concentration (MLC) for each extract analyzed herein.
2.7. Analysis of Morphological Alterations Produced by Extracts in Reconstructed Human Epidermis
Five preparations were selected based on their proven inhibitory effect on T. rubrum growth, and on discomfort reported by users of traditional medicine. They were analyzed through exposure onto human epidermis reconstructed in vitro by cultured keratinocytes exposed at the air–liquid interface [17]. Honey at 25% concentration, Allium sativum aqueous extracts at 25%, Ageratum conyzoides aqueous extracts at 50%, Pentas longiflora (male) methanol extracts at 75%, and Jatropha curcas aqueous extracts at 75% were selected. For this assay, 400 µL of each preparation was laid down over the air-exposed surface of RHE. After 4 h on RHE, 350 µL of the extracts could be aspirated to expose again the apicel surface of RHE to air, and the exposure was repeated for four consecutive days. Tissues were compared with control RHE exposed to PBS instead of extracts. After exposure, tissues were processed for histological analysis [17]. Barrier alteration in RHE is detected by decreased trans-epithelial electrical resistance (TEER) measured as described [18].
3. Results
3.1. Evaluation of Traditional Preparations for Their Efficiency to Inhibit Growth of T. rubrum on Sabouraud Agar
Twenty traditional preparations were selected for an evaluation of their potential to inhibit growth of T. rubrum dermatophytes. The extracts, from a 100% concentration to diverse dilutions down to 1.5625%, were incorporated into a Sabouraud agar before seeding T. rubrum in each dish. The results are fully illustrated in the Supplementary Materials, Supplementary Figure S1a–t. This Figure S1 includes the data obtained with dishes containing unseeded Sabouraud agar to collect pictures of dishes holding the culture medium only, as well as control dishes containing no extracts to assess the potential growth of seeded T. rubrum in the assay. Developing white mycelium over the Sabouraud medium indicates T. rubrum growth. Among the 20 different extracts evaluated using this approach, 14 of them were found to exert differential inhibition of T. rubrum growth. Honey, diluted down to 25% of the initial concentration, ash (50%), aqueous and methanolic extracts of Allium sativum (50%), Pentas longiflora female (50%), roots of Rumex abyssicus (12.5%) and Conyza sumatrensis (75%), aqueous extracts of Ageratum conyzoides (50%), Pentas longiflora male (50%), Pentas longiflora w/Lebruna bushaie oil (25%), and indigenous Colocasi esculentea (50%) were all found to be able to inhibit T. rubrum growth (Table 1).

Table 1.
Inhibitory effects of the different extracts tested on T. rubrum growth.
Interestingly, the inhibition brought on by efficient preparations decreases with their dilution in the Sabouraud agar. Overall, the aqueous extracts were found to be the most successful in inhibiting T. rubrum growth. Upon dilution, the aqueous extracts prepared from Rumex abyssinicus roots appear the most powerful, as their dilution down to 12.5–6.25% remains efficient for impeding T. rubrum growth. Conversely, several preparations, including those prepared from Commelina diffusa leaves or from Rumex abyssinicus leaves, were totally unable to inhibit the growth of T. rubrum.
3.2. Evaluation of Fungicidal and Fungistatic Properties of Extracts
Cultures that exhibited an inhibition of T. rubrum growth were further analyzed over a Sabouraud medium containing no extract to determine whether fungi were still able to grow after the initial culture condition (fungistatic property) or not (fungicidal property). The results are extensively displayed in the Supplementary Materials, Supplementary Figure S2a–n, and all observations are compiled in Table 2, presenting all tests in a single panel. Although each selected extract demonstrated fungicidal properties when incorporated into the Sabouraud agar without dilution (100%), one could observe a re-growth of T. rubrum after incubation with 75% ashes, with 75% methanolic extract of Pentas longiflora female, with 25% aqueous extract of Pentas longiflora, with Lebruna bushaie oil, with 6.25% aqueous extract of Rumex abyssicus roots, and with 50% aqueous extract of Conyza sumatrensis, thereby indicating that these concentrations hold fungistatic properties while being too low to demonstrate fungicidal activity. Other extracts demonstrated their expected efficiency at inhibiting T. rubrum growth when included in Sabouraud at elevated concentrations. However, the concentrations with fungistatic properties were not identified in this assay.

Table 2.
Fungicidal and fungistatic effects of the different extracts tested.
Using this approach, the minimal fungicidal concentration for each preparation is 25% for honey, 100% for ash, 25% for aqueous and methanolic extracts of Allium sativum, 25% for aqueous extract of Ageratum conyzoides, 25% and 100%, respectively, for aqueous and methanolic extracts of Pentas longiflora female, 50% for aqueous extracts of Pentas longiflora male, 50% for aqueous extracts of Pentas longiflora w/Lebruna bushaie oil, 12.5% for either aqueous or methanolic extracts of Rumex abyssicus roots, 75% for aqueous or methanolic extracts of Conyza sumatrensis, and 50% for aqueous extract of indigenous Colocasi esculentea.
3.3. Potential Influence of the Sterilization Procedure on the Efficacy of Extracts to Inhibit T. rubrum Growth
Since several extracts have been found to be unable to inhibit T. rubrum growth, the sterilization procedure in an autoclave was compared to a sterilization obtained by filtration through a 0.2 µm porous membrane, which requires no heating of the samples. The data demonstrate that heat-sterilized extracts (either aqueous or methanolic) were not less active than extracts sterilized through filtration. The results are extensively displayed in Supplementary Figure S3a–j.
3.4. Analysis of Morphological Alterations Produced by Extracts on Reconstructed Human Epidermis
Among extracts that were able to inhibit T. rubrum growth, five of them were randomly chosen to evaluate the potential consequences of their exposure over reconstructed human epidermis (RHE) for four consecutive days. The highest concentrations of extracts were analyzed. Thus, aqueous extracts of Allium sativum, Ageratum conyzoïdes, and Pentas longiflora (male and female), and methanolic extracts of Rumex abyssinicus were analyzed. The results are presented in Figure 1 and illustrate that Allium sativum extracts were the only ones to produce no morphological alteration in the histology of RHE. Therefore, the data suggest that a prolonged exposure of RHE to certain extracts might be responsible for some alterations linked to a potential yet undefined toxicity. These observations argue for the safety evaluation of products used in traditional medicine to fight against dermatophytes.
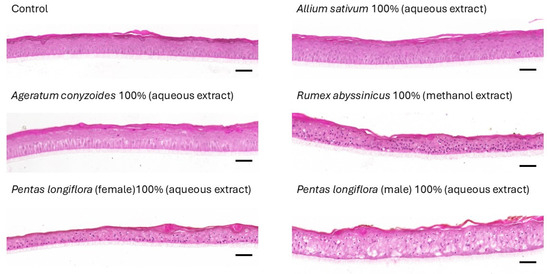
Figure 1.
Morphological alterations of RHE by 100% extracts. RHE were either exposed to PBS (control) or exposed to aqueous extract of Allium sativum, Ageratum conyzoides, or Pentas longiflora (female and male), or to methanol extracts of Rumex abyssinicus before fixation and histological processing and observation. Bars: 50 µm.
4. Discussion
Dermatophytes of the Trichophyton genus are widely spread in the global population and harmful for human health. An increasing incidence and resistance to current treatments render these cutaneous fungal infections a major threat worldwide and a sanitary challenge of concern [19,20]. In the fight against infecting fungi, traditional medicine provides opportunities for natural, easily available, and affordable products that bring valuable help to treat dermatophytosis [21]. To assess the efficacy of traditional recipes commonly used in Kalonge near Bukavu, South-Kivu province, DR-Congo, for the treatment of tinea tonsurans, this study analyzed aqueous and methanolic extracts of Allium sativum, Pentas longiflora (male or female, with and without Lebrunia bushaie oil), Rumex abyssinicus, Ageratum conyzoides, and indigenous Colocasia esculentum, as well as aqueous solutions of ash and honey. The data reveal the effectiveness of 14 out of the 20 tested extracts, confirming the vast potential of many plants to inhibit fungal growth, including during dermatophytosis. However, in addition, the data showcase that certain preparations (6 out of 20) remain inefficient in inhibiting T. rubrum growth, suggesting that scientific advice could improve the benefits brought on by traditional medicine.
Interestingly, the efficacy of one specific extract might depend on several factors, including the species of plants considered, the composition and concentration of active principles, the extraction procedure, and the environmental factors or strategies developed by fungi to resist treatments. Such factors likely explain the differences observed in the efficacy of the various tested extracts [22,23]. For several extracts, the lowest concentration able to inhibit T. rubrum growth was identified as fungicidal. For others like honey, the lowest inhibiting concentration did not kill all dermatophytes, since re-culturing them in a medium devoid of inhibiting extracts demonstrated a survival to the treatment. Those extracts were thus reported as holding fungistatic properties.
Numerous extracts from plants have been tested already against dermatophytes, precisely against species of the Trichophyton genus [14,24,25,26,27,28,29,30,31,32,33,34], demonstrating their inhibiting potential and often identifying the chemical nature of active principles. Notably, these principles were found to be polyphenols, alkaloids, tannins, terpenes, geraniol, citronellol, saponins, essential oils, and anthraquinones, some of them being reported more efficient than synthesized preparations. Reports analyzed, e.g., Allium sativum, Curcuma longa, Senna occidentalis, Pergularia tomentosa, Vernonia amygdalina, Rhapis excelsa, Syzygium myrtifolium, Punica granatum, Lawsonia inermis, Nandina domestica, Eucalyptus Camaldulensis, Cymbopogon Citratus, and Senna Alata, this list being not exhaustive.
Although the nature of active compounds in the various extracts analyzed herein has not been determined, it is very likely that the observed efficacy was due to similar active principles. It is currently admitted that natural antifungal compounds extracted from plants exert their fungicidal or fungistatic effects by acting on the structure and synthesis of cell wall components, on membrane permeability, or by direct growth inhibition [35,36,37]. We hereby confirm the observations performed by Aala et al. [38], reporting that Allium sativum extracts are efficient against T. rubrum, with aqueous extracts (2–4 mg/mL) causing a partial degradation of T. rubrum hyphae, while pure allicine (6.25–12.5 µg/mL extracted from garlic) destroyed the membrane and cell wall of T. rubrum.
When analyzing the activity of Australian honey on the growth of dermatophytes, Guttentag et al. [39] demonstrated an inhibition against T. rubrum. This consequence on T. rubrum has been attributed to the osmotic effect and to its content of hydrogen peroxide and of volatile phenolic compounds.
Regarding the use of Pentas longiflora extracts in traditional medicine, they were reported as being efficient against pityriasis versicolor [40], indirectly suggesting their efficacy against T. rubrum. Indeed, we hereby demonstrate that this plant extract is among those with an inhibiting potential against dermatophytes.
Ethanolic and methanolic extracts from plants of the Rumex genus, precisely Rumex acetosella have been analyzed [41,42] for their activity against Microsporum canis, Microsporum gypseum, and Trichophyton mentagrophytes. The published data demonstrate the better antifungal activity of those extracts against dermatophytes, in good accordance with our observations. Indeed, although we analyzed a different species of this genus, Rumex abyssinicus, the strongest activity among our extracts was found when using aqueous extracts from tubercules, which were still efficient when diluted up to 6.25% to inhibit the growth of T. rubrum.
Other studies [43,44] have proven the antifungal activity in extracts from several plants, including Ageratum comyzoides. This activity was tested against fungi of the Aspergillus, Alternaria, Candida, Fusarium, Phytophthora, and Pythium genus. Our current observations extend this activity against T. rubrum, even at a dilution of 25% of aqueous extracts.
Besides their efficacy against fungal growth on the Sabouraud medium, antifungal preparations should be evaluated for their safe use on human skin. For this purpose, we exposed a few extracts over an in vitro reconstructed epidermis produced by cell culture techniques described by our team [17]. Histological alterations to RHE are observed with undiluted extracts of Ageratum conyzoides and Pentas longiflora, as well as methanolic extracts from Rumex abyssinicus tubercules. Conversely, no alteration can be observed after the exposure of RHE to Allium sativum extracts. These results recall that remedies based on natural products are not always devoid of safety concerns. Our results concur with studies [45,46,47], previously reporting that species of the Rumex genus and Ageratum conyzoides contain secondary metabolites holding some toxicity, even if beneficial effects to treat dermatoses can be obtained with such extracts.
Finally, we herein observed that the heat sterilization of our extracts did not alter their efficacy against T. rubrum, in good accordance with Albrecht et al. [25], who studied heat-sterilized medicinal plant extracts against dermatophytes.
5. Conclusions
Among recipes used in traditional medicine near Bukavu, DR-Congo, the efficacy of several aqueous and methanolic plant extracts, honey, and ash against T. rubrum has been assessed. Most of them can inhibit the growth of dermatophytes in a culture on Sabouraud medium, but several were found to alter epidermal histology when undiluted preparations were applied in vitro over RHE. Variable efficacies are reported, and fungistatic versus fungicidal activity was further determined. The phytochemical screening of all extracts and evaluations on RHE of standardized recipes should now complete the study to eventually identify the active principles and improve safety when using such easy and cheap recipes to fight dermatophytosis in poor populations.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/microbiolres16100223/s1. Figure S1: Analysis of the various preparations of extracts on the growth of T. rubrum on Sabouraud agar; Figure S2: Analysis of the fungistatic or fungicidal effect for various extracts on the growth of T. rubrum on Sabouraud agar; Figure S3: Analysis of the influence of sterilization procedures on the efficacity of extracts.
Author Contributions
Conceptualization, A.C.M. and Y.P.; methodology, A.C.M. and V.D.G.; investigation, A.C.M., V.D.G., and E.D.; writing—original draft preparation, A.C.M.; writing—review and editing, E.D. and Y.P.; funding acquisition, Y.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by FONDS ADRIEN BAUCHAU in support of A.C.M. fellowship. E.D. is supported by REGION WALLONNE, grant TINEADIAG number 2410077.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author. The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
Help and advice from Emilie Faway and Pierre Devos (University of Namur) before and during this research were deeply appreciated.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviation
The following abbreviation is used in this manuscript:
| RHE | Reconstructed Human Epidermis |
References
- Kromer, C.; Celis, D.; Hipler, U.; Zampeli, V.A.; Mößner, R.; Lippert, U. Dermatophyte infections in children compared to adults in Germany: A retrospective multicenter study in Germany. J. Deutsche Derma. Gesell. 2021, 19, 993–1001. [Google Scholar] [CrossRef]
- Meena, S.; Bhadauria, S. Clinical Manifestations of Dermatophytoses and its Treatment: A Review. Asian J. Biol. 2024, 20, 78–89. [Google Scholar] [CrossRef]
- Faway, É.; Cambier, L.; Mignon, B.; Poumay, Y.; Lambert De Rouvroit, C. Modeling dermatophytosis in reconstructed human epidermis: A new tool to study infection mechanisms and to test antifungal agents. Med. Myco. 2016, 55, 485–494. [Google Scholar] [CrossRef]
- Chowdhary, A.; Singh, A.; Kaur, A.; Khurana, A. The emergence and worldwide spread of the species Trichophyton indotineae causing difficult-to-treat dermatophytosis: A new challenge in the management of dermatophytosis. PLoS Pathog. 2022, 18, e1010795. [Google Scholar] [CrossRef]
- Ishrat, S.; Ahmad, Z.; Ahmer, S.; Fatima, N. A comprehensive review on Qooba (Dermatophytosis). Int. J. Unani. Integ. Med. 2022, 6, 27–32. [Google Scholar] [CrossRef]
- Abdul Jamil, R.; Saxena, K.; Koti, V.R.; Mohanty, S. Dermatophytosis- its impact on quality of life and financial burden: Research Article. Int. J. Med. Biomed. Stud. 2023, 7, 7–13. [Google Scholar] [CrossRef]
- AL-Khikani, F.O. Dermatophytosis a worldwide contiguous fungal infection: Growing challenge and few solutions. Biomed. Biotechnol. Res. J. 2020, 4, 117. [Google Scholar] [CrossRef]
- Faway, E.; Staerck, C.; Danzelle, C.; Vroomen, S.; Courtain, C.; Mignon, B.; Poumay, Y. Towards a Standardized Procedure for the Production of Infective Spores to Study the Pathogenesis of Dermatophytosis. J. Fungi 2021, 7, 1029. [Google Scholar] [CrossRef]
- Lucariello, G.; Cicia, D.; Capasso, R. Pharmacological Studies on Traditional Plant-Based Remedies. Biomedicines 2021, 9, 315. [Google Scholar] [CrossRef]
- Che, C.-T.; George, V.; Ijinu, T.P.; Pushpangadan, P.; Andrae-Marobela, K. Traditional medicine. In Pharmacognosy, 2nd ed.; Badal McCreath, S., Clement, Y.N., Eds.; Academic Press: Cambridge, MA, USA, 2024; pp. 11–28. [Google Scholar]
- Singh, S.; Rai, V.; Kanojia, A.; Yadav, A. Natural Products and Traditional Medicine: Investigating the Role of Natural Products and Traditional Medicine in Modern Pharmacology. J. Res. Appl. Sci. Biotechnol. 2024, 3, 40–53. [Google Scholar] [CrossRef]
- Maina Mwaura, F. Integrating Traditional Medicine with Modern Healthcare: Addressing Maternal and Mental Health in Uganda. IDOSR J. Appl. Sci. 2024, 9, 76–82. [Google Scholar] [CrossRef]
- Jakir Hossain, M.; Al Mamun, A.; Shamsuddin Sultan Khan, M. Traditional Ethnobotanical Practice in Indigenous Communities of Australia, Asia, and South Asia, and Modern Medicinal Prospects. Aust. Herb Insight 2020, 3, 1–11. [Google Scholar] [CrossRef]
- Singh, P.; Vidyasagar, G. Antifungal screening of 61 folkloric medicinal plant extracts against dermatophytic fungi Trichophyton rubrum. J. App. Pharm. Sci. 2015, 5, 38–44. [Google Scholar] [CrossRef]
- Telles, S.; Pathak, S.; Singh, N.; Balkrishna, A. Research on Traditional Medicine: What Has Been Done, the Difficulties, and Possible Solutions. Evid.-Based Complement. Altern. Med. 2014, 2014, 495635. [Google Scholar] [CrossRef]
- Nguyen, T.L.A.; Bhattacharya, D. Antimicrobial Activity of Quercetin: An Approach to Its Mechanistic Principle. Molecules 2022, 27, 2494. [Google Scholar] [CrossRef]
- De Vuyst, E.; Charlier, C.; Giltaire, S.; De Glas, V.; De Rouvroit, C.L.; Poumay, Y. Reconstruction of Normal and Pathological Human Epidermis on Polycarbonate Filter. Methods Mol. Biol. 2014, 1195, 191–201. [Google Scholar] [CrossRef]
- Faway, E.; Cambier, L.; De Vuyst, E.; Evrard, C.; Thiry, M.; Lambert De Rouvroit, C.; Mignon, B.; Poumay, Y. Responses of Reconstructed Human Epidermis to Trichophyton rubrum Infection and Impairment of Infection by the Inhibitor PD169316. J. Investig. Dermatol. 2019, 139, 2080–2089.e6. [Google Scholar] [CrossRef]
- Legaspi, C.-L.B.; Maramba-Lazarte, C. Medicinal plants for Dermatophytosis: Senna Alata (Linn.) Roxb., Allium sativum (Linn.) and Cymbopogon citratus (DC.) Stapf. Pediatr. Infect. Dis. Soc. Philipp. J. 2020, 21, 59–70. [Google Scholar] [CrossRef]
- Urban, K.; Chu, S.; Scheufele, C.; Giesey, R.L.; Mehrmal, S.; Uppal, P.; Delost, G.R. The global, regional, and national burden of fungal skin diseases in 195 countries and territories: A cross-sectional analysis from the Global Burden of Disease Study 2017. JAAD Int. 2021, 2, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Hafidh, R.R.; Abdulamir, A.S.; Vern, L.S.; Bakar, F.A. The Inhibition of Human Pathogens: Trichophyton rubrum and Trichoderma harzianum by a Natural Product. Am. J. Biochem. Biotechnol. 2010, 6, 40–46. [Google Scholar] [CrossRef]
- Ismael, H.R.; Ramadan, N.A. The anti-dermatophyte activities of some plant extracts against Trichophyton mentagrophytes and Microsporum canis. J. Univ. Zakho 2013, 1, 463–468. [Google Scholar]
- Ennaghra, N.; Bourzama, G. Effect of aqueous extract of Eucalyptus sp., Nerium oleander and Allium sativum on the growth of dermatophytes. Fungal Territ. 2019, 2, 6–11. [Google Scholar] [CrossRef]
- Al-Juraifani, A.A. Comparative study between effect of Lawsonia inermis, Punica granatum and miconazole nitrate on Trichophyton rubrum. J. Am. Sci. 2013, 9, 51–53. [Google Scholar]
- Albrecht, J.; Köberle, M.; Preis, S.; Biedermann, T.; Seidl, H.P.; Lindner, M.; Florig, E.; Zink, A. Herbal Agents Against Dermatophytes: Effect of 43 Herbal Agents on Dermatophyte Growth. Dermatol. Ther. 2024, 2024, 6727040. [Google Scholar] [CrossRef]
- Robles-Martínez, M.; González, J.F.C.; Pérez-Vázquez, F.J.; Montejano-Carrizales, J.M.; Pérez, E.; Patiño-Herrera, R. Antimycotic Activity Potentiation of Allium sativum Extract and Silver Nanoparticles against Trichophyton rubrum. Chem. Biodivers. 2019, 16, e1800525. [Google Scholar] [CrossRef] [PubMed]
- Hammadi, H.A.; Hanawi, M.J.; Al-Inizi, A.H.H.; Kadhim, K.J. in Vitro Antimycotic Activity of Eucalyptus Camaldulensis Extract Against Trichophyton Rubrum. South East Eur. J. Public Health 2024, 24, 414–422. [Google Scholar] [CrossRef]
- Pereira, F.D.O.; Mendes, J.M.; Lima, I.O.; Mota, K.S.D.L.; Oliveira, W.A.D.; Lima, E.D.O. Antifungal activity of geraniol and citronellol, two monoterpenes alcohols, against Trichophyton rubrum involves inhibition of ergosterol biosynthesis. Pharm. Biol. 2015, 53, 228–234. [Google Scholar] [CrossRef]
- Zhou, R.; Dzomba, P.; Gwatidzo, L. Phytochemicals as potential active principal components for formulation of alternative antifungal remedies against Trichophyton spp.: A systematic review. Pure Appl. Chem. 2023, 96, 1455–1498. [Google Scholar] [CrossRef]
- Jha, A. Plant terpenes. In Phytoconstituents and Antifungals; Developments in Applied Microbiology and Biotechnology; Academic Press: Cambridge, MA, USA, 2022; pp. 77–87. [Google Scholar]
- Bhavsar, M.A.N. Herbal Anti-fungal Cream: A Natural Approach to Onychomycosis Treatment. Int. J. Res. Appl. Sci. Eng. Technol. 2024, 12, 143–153. [Google Scholar] [CrossRef]
- Husseini, H.A.; Olonitola, O.S.; Aliyu, M.S. Phytoconstituents and antidermatophytic activity of crude extracts of Senna occidentalis. UMYU J. Microbiol. Res. 2023, 8, 152–160. [Google Scholar] [CrossRef]
- Sit, N.W.; Chan, Y.S.; Lai, S.C.; Lim, L.N.; Looi, G.T.; Tay, P.L.; Tee, Y.T.; Woon, Y.Y.; Khoo, K.S.; Ong, H.C. In vitro antidermatophytic activity and cytotoxicity of extracts derived from medicinal plants and marine algae. J. Mycol. Médicale 2018, 28, 561–567. [Google Scholar] [CrossRef]
- Xiao, D.; Sun, H.; Li, X.; Meng, F.; Sun, T.; Shao, X.; Ding, Y.; Li, Y. Rumex japonicus Houtt. leaves: The chemical composition and anti-fungal activity. J. Med. Mycol. 2024, 34, 101513. [Google Scholar] [CrossRef]
- Long, N.; Li, F. Antifungal Mechanism of Natural Products Derived from Plants: A Review. Nat. Prod. Commun. 2024, 19, 1934578X241271747. [Google Scholar] [CrossRef]
- Zuzarte, M.; Lopes, G.; Pinto, E.; Salgueiro, L. Are natural products an alternative therapy for dermatophytosis? In Dermatophytes and Dermatophytoses; Springer: Cham, Switzerland, 2021; pp. 473–519. [Google Scholar]
- Jain, N.; Sharma, M. Inhibitory Effect of Some Selected Essential Oil Terpenes on Fungi Causing Superficial Infection in Human Beings. J. Essent. Oil Bear. Plants 2020, 23, 862–869. [Google Scholar] [CrossRef]
- Aala, F.; Yusuf, U.K.; Nulit, R.; Rezaie, S. Inhibitory effect of allicin and garlic extracts on growth of cultured hyphae. Iran J. Basic Med. Sci. 2014, 17, 150. [Google Scholar]
- Guttentag, A.; Krishnakumar, K.; Cokcetin, N.; Hainsworth, S.; Harry, E.; Carter, D. Inhibition of Dermatophyte Fungi by Australian Jarrah Honey. Pathogens 2021, 10, 194. [Google Scholar] [CrossRef]
- Kagisha, V.; Djang’eing’a, R.M.; Muganga, R.; Bonnet, O.; Tchinda, A.T.; Jansen, O.; Tomani, J.C.; Njakarinala, R.; Ledoux, A.; Nyirimigabo, A.; et al. Pentas longiflora Oliv. (Rubiaceae), a plant used in the treatment of Pityriasis Versicolor in Rwanda: Chemical composition and standardization of leaves and roots. Fitoterapia 2021, 153, 104974. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-J.; Li, Y.-X.; Li, N.; Zhu, H.-T.; Wang, D.; Zhang, Y.-J. The genus Rumex (Polygonaceae): An ethnobotanical, phytochemical and pharmacological review. Nat. Prod. Bioprospect. 2022, 12, 21. [Google Scholar] [CrossRef] [PubMed]
- Husein, A.; Al-Nuri, M.A.; Zatar, N.; Jondi, W.; Shtayeh, M.S.A. Isolation and Antifungal Evaluation of Rumex cyprius Murb Extracts. J. Chem. Eng. Data 2012, 6, 547–550. [Google Scholar]
- Chahal, R.; Nanda, A.; Akkol, E.K.; Sobarzo-Sánchez, E.; Arya, A.; Kaushik, D.; Dutt, R.; Bhardwaj, R.; Rahman, M.H.; Mittal, V. Ageratum conyzoides L. and Its Secondary Metabolites in the Management of Different Fungal Pathogens. Molecules 2021, 26, 2933. [Google Scholar] [CrossRef]
- Fogang Dongmo, A.-J.; Chimi Yetendje, L.; Njateng, G.S.S.; Gatsing, D.; Kuiate, J.R. Antidermatophytic activity and adverse side effects of the methanolic extract from leaves of Ageratum conyzoides (Asteraceae). Investig. Med. Chem. Pharmacol. 2018, 1, 19. [Google Scholar] [CrossRef]
- Belete, Y.; Wondu, K.; Wiedenfeld, H.; Debella, A. Natural Toxins of Ageratum conyzoides from Hepatic Vein Occlusive Disease Affected Community in Ethiopia. Nat. Prod. Chem. Res. 2020, 8, 370. [Google Scholar] [CrossRef]
- Bosi, C.F.; Rosa, D.W.; Grougnet, R.; Lemonakis, N.; Halabalaki, M.; Skaltsounis, A.L.; Biavatti, M.W. Pyrrolizidine alkaloids in medicinal tea of Ageratum conyzoides. Rev. Bras. Farmacogn. 2013, 23, 425–432. [Google Scholar] [CrossRef]
- Shokan, A.K.; Yergozova, D.M.; Kobylina, T.N.; Kudrina, N.O.; Litvinenko, Y.A.; Seitimova, G.A.; Kulmanov, T.E.; Terletskaya, N.V.; Zharkova, I.M. Effect of the complex extract from Rumex plants on quantitative parameters of blood cells and bone marrow in vivo. Int. J. Biol. Chem. 2024, 17, 31–39. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).