Moringa Reduces Glucose Levels and Alters Wolbachia Abundance in Drosophila melanogaster
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Flies and Diets
2.2. Experimental Design
2.3. Nutritional Indices
2.4. Viable Bacteria Count
2.5. Microbiome Sample Preparation
2.6. 16S rRNA Library Preparation and Sequencing
2.7. Sequence Processing
2.8. Statistical Analysis
3. Results
3.1. Taxonomic Composition of Fly Bacterial Communities
3.2. Impact of Moringa on Fly Bacterial Communities
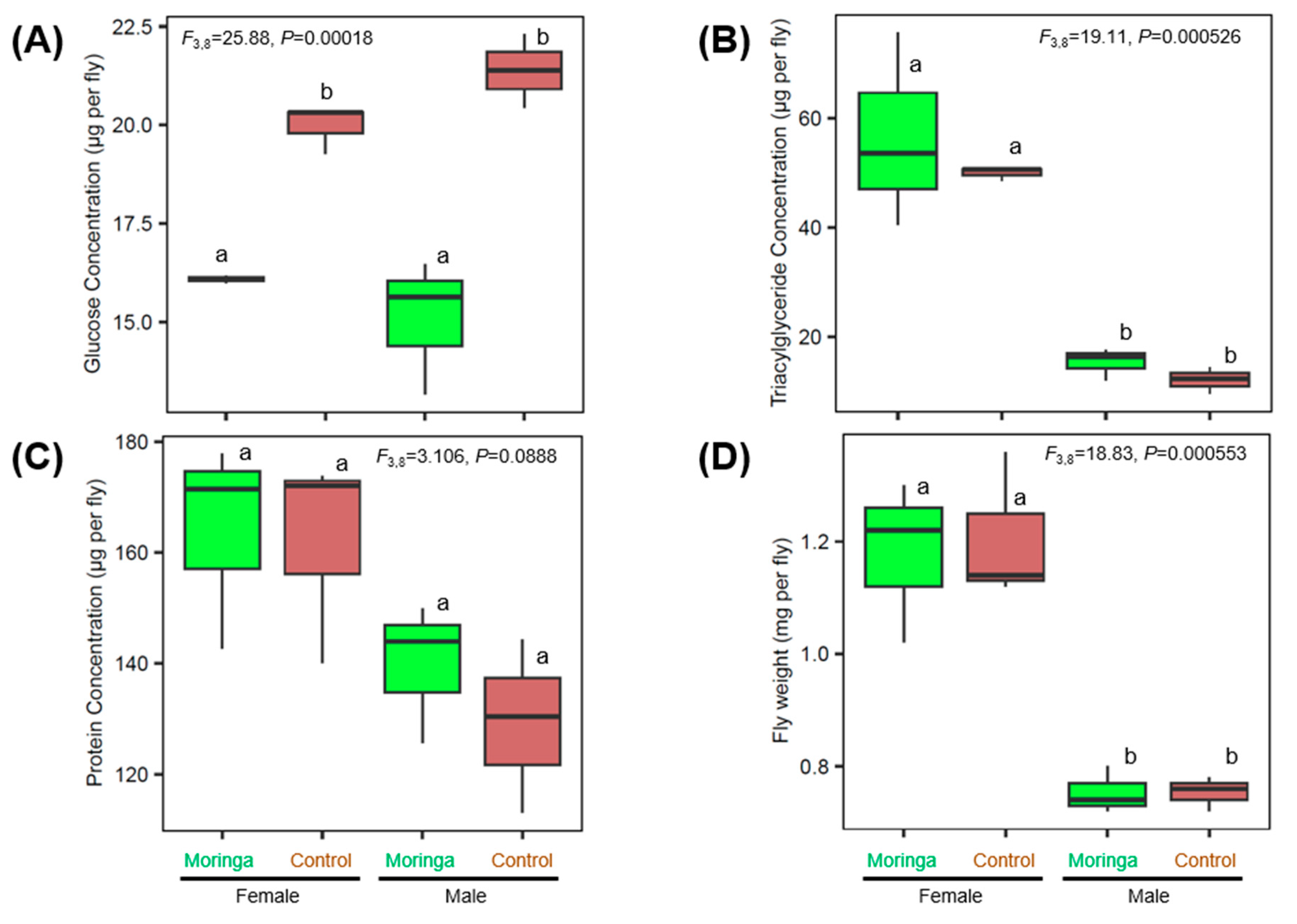
3.3. Impact of Moringa on Fly Glucose, Triacylglyceride, Protein Levels and Fly Weight
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mohamed, S.M.; Shalaby, M.A.; El-Shiekh, R.A.; El-Banna, H.A.; Emam, S.R.; Bakr, A.F. Metabolic Syndrome: Risk Factors, Diagnosis, Pathogenesis, and Management with Natural Approaches. Food Chem. Adv. 2023, 3, 100335. [Google Scholar] [CrossRef]
- Samson, S.L.; Garber, A.J. Metabolic Syndrome. Endocrinol. Metab. Clin. N. Am. 2014, 43, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.S.; Calle, M.; Fernandez, M.L. Healthy Plant-Based Diets Improve Dyslipidemias, Insulin Resistance, and Inflammation in Metabolic Syndrome. A Narrative Review. Adv. Nutr. 2023, 14, 44–54. [Google Scholar] [CrossRef]
- Sonnenburg, E.D.; Sonnenburg, J.L. Starving Our Microbial Self: The Deleterious Consequences of a Diet Deficient in Microbiota-Accessible Carbohydrates. Cell Metab. 2014, 20, 779–786. [Google Scholar] [CrossRef]
- Zmora, N.; Suez, J.; Elinav, E. You Are What You Eat: Diet, Health and the Gut Microbiota. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 35–56. [Google Scholar] [CrossRef]
- Hassoun, A.; Harastani, R.; Jagtap, S.; Trollman, H.; Garcia-Garcia, G.; Awad, N.M.H.; Zannou, O.; Galanakis, C.M.; Goksen, G.; Nayik, G.A.; et al. Truths and Myths about Superfoods in the Era of the COVID-19 Pandemic. Crit. Rev. Food Sci. Nutr. 2024, 64, 585–602. [Google Scholar] [CrossRef]
- Kunyanga, C.; Imungi, J.; Vellingiri, V. Nutritional Evaluation of Indigenous Foods with Potential Food-Based Solution to Alleviate Hunger and Malnutrition in Kenya. J. Appl. Biosci. 2013, 67, 5277. [Google Scholar] [CrossRef]
- Sokhela, H.; Govender, L.; Siwela, M. Complementary Feeding Practices and Childhood Malnutrition in South Africa: The Potential of Moringa Oleifera Leaf Powder as a Fortificant: A Narrative Review. Nutrients 2023, 15, 2011. [Google Scholar] [CrossRef]
- Vergara-Jimenez, M.; Almatrafi, M.; Fernandez, M. Bioactive Components in Moringa Oleifera Leaves Protect against Chronic Disease. Antioxidants 2017, 6, 91. [Google Scholar] [CrossRef]
- Karthikesan, K.; Pari, L.; Menon, V. Combined Treatment of Tetrahydrocurcumin and Chlorogenic Acid Exerts Potential Antihyperglycemic Effect on Streptozotocin-Nicotinamide-Induced Diabetic Rats. Gen. Physiol. Biophys. 2010, 29, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Waterman, C.; Rojas-Silva, P.; Tumer, T.B.; Kuhn, P.; Richard, A.J.; Wicks, S.; Stephens, J.M.; Wang, Z.; Mynatt, R.; Cefalu, W.; et al. Isothiocyanate-rich Moringa oleifera Extract Reduces Weight Gain, Insulin Resistance, and Hepatic Gluconeogenesis in Mice. Mol. Nutr. Food Res. 2015, 59, 1013–1024. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, B.S.; Hussain, M.B.; Omer, R.; Toor, H.A.; Waheed, M.; Shariati, M.A.; Sergey, P.; Heydari, M. Moringa Oleifera in Malnutrition: A Comprehensive Review. Curr. Drug Discov. Technol. 2021, 18, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Newell, P.D.; Douglas, A.E. Interspecies Interactions Determine the Impact of the Gut Microbiota on Nutrient Allocation in Drosophila Melanogaster. Appl. Environ. Microbiol. 2014, 80, 788–796. [Google Scholar] [CrossRef]
- Zhang, F.Q.; McMullen, J.G.; Douglas, A.E.; Ankrah, N.Y.D. Succinate: A Microbial Product That Modulates Drosophila Nutritional Physiology. Insect Sci. 2022, 29, 315–318. [Google Scholar] [CrossRef]
- Walters, W.; Hyde, E.R.; Berg-Lyons, D.; Ackermann, G.; Humphrey, G.; Parada, A.; Gilbert, J.A.; Jansson, J.K.; Caporaso, J.G.; Fuhrman, J.A.; et al. Improved Bacterial 16S rRNA Gene (V4 and V4-5) and Fungal Internal Transcribed Spacer Marker Gene Primers for Microbial Community Surveys. mSystems 2016, 1, e00009-15. [Google Scholar] [CrossRef]
- Weber, N.; Liou, D.; Dommer, J.; MacMenamin, P.; Quiñones, M.; Misner, I.; Oler, A.J.; Wan, J.; Kim, L.; Coakley McCarthy, M.; et al. Nephele: A Cloud Platform for Simplified, Standardized and Reproducible Microbiome Data Analysis. Bioinformatics 2018, 34, 1411–1413. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA Ribosomal RNA Gene Database Project: Improved Data Processing and Web-Based Tools. Nucleic Acids Res. 2012, 41, D590–D596. [Google Scholar] [CrossRef]
- Villanueva, R.A.M.; Chen, Z.J. Ggplot2: Elegant Graphics for Data Analysis (2nd ed.). Meas. Interdiscip. Res. Perspect. 2019, 17, 160–167. [Google Scholar] [CrossRef]
- Abdelazim, A.M.; Afifi, M.; Abu-Alghayth, M.H.; Alkadri, D.H. Moringa oleifera: Recent Insights for Its Biochemical and Medicinal Applications. J. Food Biochem. 2024, 2024, 1270903. [Google Scholar] [CrossRef]
- Owens, F.S.; Dada, O.; Cyrus, J.W.; Adedoyin, O.O.; Adunlin, G. The Effects of Moringa Oleifera on Blood Glucose Levels: A Scoping Review of the Literature. Complement. Ther. Med. 2020, 50, 102362. [Google Scholar] [CrossRef] [PubMed]
- Kremer, N.; Voronin, D.; Charif, D.; Mavingui, P.; Mollereau, B.; Vavre, F. Wolbachia Interferes with Ferritin Expression and Iron Metabolism in Insects. PLoS Pathog. 2009, 5, e1000630. [Google Scholar] [CrossRef] [PubMed]
- Gill, A.C.; Darby, A.C.; Makepeace, B.L. Iron Necessity: The Secret of Wolbachia’s Success? PLoS Negl. Trop. Dis. 2014, 8, e3224. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, N.E.; Gerdtzen, Z.P.; Olivera-Nappa, Á.; Salgado, J.C.; Conca, C. A Systems Biology Approach for Studying Wolbachia Metabolism Reveals Points of Interaction with Its Host in the Context of Arboviral Infection. PLoS Negl. Trop. Dis. 2019, 13, e0007678. [Google Scholar] [CrossRef]
- Mandilaras, K.; Pathmanathan, T.; Missirlis, F. Iron Absorption in Drosophila Melanogaster. Nutrients 2013, 5, 1622–1647. [Google Scholar] [CrossRef]
- Brownlie, J.C.; Cass, B.N.; Riegler, M.; Witsenburg, J.J.; Iturbe-Ormaetxe, I.; McGraw, E.A.; O’Neill, S.L. Evidence for Metabolic Provisioning by a Common Invertebrate Endosymbiont, Wolbachia Pipientis, during Periods of Nutritional Stress. PLoS Pathog. 2009, 5, e1000368. [Google Scholar] [CrossRef]
- Newton, I.L.G.; Rice, D.W. The Jekyll and Hyde Symbiont: Could Wolbachia Be a Nutritional Mutualist? J. Bacteriol. 2020, 202, e00589-19. [Google Scholar] [CrossRef]
- Detcharoen, M.; Jiggins, F.M.; Schlick-Steiner, B.C.; Steiner, F.M. Wolbachia Endosymbiotic Bacteria Alter the Gut Microbiome in the Fly Drosophila Nigrosparsa. J. Invertebr. Pathol. 2023, 198, 107915. [Google Scholar] [CrossRef]
- Simhadri, R.K.; Fast, E.M.; Guo, R.; Schultz, M.J.; Vaisman, N.; Ortiz, L.; Bybee, J.; Slatko, B.E.; Frydman, H.M. The Gut Commensal Microbiome of Drosophila Melanogaster Is Modified by the Endosymbiont Wolbachia. mSphere 2017, 2, e00287-17. [Google Scholar] [CrossRef]
- Ye, Y.H.; Seleznev, A.; Flores, H.A.; Woolfit, M.; McGraw, E.A. Gut Microbiota in Drosophila Melanogaster Interacts with Wolbachia but Does Not Contribute to Wolbachia -Mediated Antiviral Protection. J. Invertebr. Pathol. 2017, 143, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Fahey, J.W. Moringa oleifera: A Review of the Medicinal Potential. Acta Hortic. 2017, 1158, 209–224. [Google Scholar] [CrossRef]
- Romeo, L.; Iori, R.; Rollin, P.; Bramanti, P.; Mazzon, E. Isothiocyanates: An Overview of Their Antimicrobial Activity against Human Infections. Molecules 2018, 23, 624. [Google Scholar] [CrossRef] [PubMed]
- Maggi, A.; Della Torre, S. Sex, Metabolism and Health. Mol. Metab. 2018, 15, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Maric, I.; Krieger, J.-P.; Van Der Velden, P.; Börchers, S.; Asker, M.; Vujicic, M.; Wernstedt Asterholm, I.; Skibicka, K.P. Sex and Species Differences in the Development of Diet-Induced Obesity and Metabolic Disturbances in Rodents. Front. Nutr. 2022, 9, 828522. [Google Scholar] [CrossRef]
- Pontifex, M.G.; Vauzour, D.; Muller, M. Sexual Dimorphism in the Context of Nutrition and Health. Proc. Nutr. Soc. 2024, 83, 109–119. [Google Scholar] [CrossRef]
- Ankrah, N.Y.D.; Barker, B.E.; Song, J.; Wu, C.; McMullen, J.G.; Douglas, A.E. Predicted Metabolic Function of the Gut Microbiota of Drosophila Melanogaster. mSystems 2021, 6, e01369-20. [Google Scholar] [CrossRef]
- Ankrah, N.Y.D.; Douglas, A.E. Nutrient Factories: Metabolic Function of Beneficial Microorganisms Associated with Insects. Environ. Microbiol. 2018, 20, 2002–2011. [Google Scholar] [CrossRef]
- Carneiro Dutra, H.L.; Deehan, M.A.; Frydman, H. Wolbachia and Sirtuin-4 Interaction Is Associated with Alterations in Host Glucose Metabolism and Bacterial Titer. PLoS Pathog. 2020, 16, e1008996. [Google Scholar] [CrossRef]
- Lesperance, D.N.; Broderick, N.A. Microbiomes as Modulators of Drosophila Melanogaster Homeostasis and Disease. Curr. Opin. Insect Sci. 2020, 39, 84–90. [Google Scholar] [CrossRef]
- Shin, S.C.; Kim, S.-H.; You, H.; Kim, B.; Kim, A.C.; Lee, K.-A.; Yoon, J.-H.; Ryu, J.-H.; Lee, W.-J. Drosophila Microbiome Modulates Host Developmental and Metabolic Homeostasis via Insulin Signaling. Science 2011, 334, 670–674. [Google Scholar] [CrossRef] [PubMed]
- Storelli, G.; Defaye, A.; Erkosar, B.; Hols, P.; Royet, J.; Leulier, F. Lactobacillus Plantarum Promotes Drosophila Systemic Growth by Modulating Hormonal Signals through TOR-Dependent Nutrient Sensing. Cell Metab. 2011, 14, 403–414. [Google Scholar] [CrossRef] [PubMed]
- Chaston, J.M.; Newell, P.D.; Douglas, A.E. Metagenome-Wide Association of Microbial Determinants of Host Phenotype in Drosophila Melanogaster. mBio 2014, 5, e01631-14. [Google Scholar] [CrossRef]
- Consuegra, J.; Grenier, T.; Akherraz, H.; Rahioui, I.; Gervais, H.; Da Silva, P.; Leulier, F. Metabolic Cooperation among Commensal Bacteria Supports Drosophila Juvenile Growth under Nutritional Stress. iScience 2020, 23, 101232. [Google Scholar] [CrossRef] [PubMed]
- Karpova, E.K.; Bobrovskikh, M.A.; Deryuzhenko, M.A.; Shishkina, O.D.; Gruntenko, N.E. Wolbachia Effect on Drosophila Melanogaster Lipid and Carbohydrate Metabolism. Insects 2023, 14, 357. [Google Scholar] [CrossRef]
- Zhang, H.-B.; Cao, Z.; Qiao, J.-X.; Zhong, Z.-Q.; Pan, C.-C.; Liu, C.; Zhang, L.-M.; Wang, Y.-F. Metabolomics Provide New Insights into Mechanisms of Wolbachia-Induced Paternal Defects in Drosophila Melanogaster. PLoS Pathog. 2021, 17, e1009859. [Google Scholar] [CrossRef]
- Simcox, J.A.; McClain, D.A. Iron and Diabetes Risk. Cell Metab. 2013, 17, 329–341. [Google Scholar] [CrossRef]
- Kaneto, H.; Katakami, N.; Matsuhisa, M.; Matsuoka, T. Role of Reactive Oxygen Species in the Progression of Type 2 Diabetes and Atherosclerosis. Mediators Inflamm. 2010, 2010, 453892. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schaffer, M.; Grant, D.; Berge, K.; Ankrah, N.Y.D. Moringa Reduces Glucose Levels and Alters Wolbachia Abundance in Drosophila melanogaster. Microbiol. Res. 2024, 15, 1870-1879. https://doi.org/10.3390/microbiolres15030125
Schaffer M, Grant D, Berge K, Ankrah NYD. Moringa Reduces Glucose Levels and Alters Wolbachia Abundance in Drosophila melanogaster. Microbiology Research. 2024; 15(3):1870-1879. https://doi.org/10.3390/microbiolres15030125
Chicago/Turabian StyleSchaffer, Michaela, D’Andre Grant, Katherine Berge, and Nana Yaw Darko Ankrah. 2024. "Moringa Reduces Glucose Levels and Alters Wolbachia Abundance in Drosophila melanogaster" Microbiology Research 15, no. 3: 1870-1879. https://doi.org/10.3390/microbiolres15030125
APA StyleSchaffer, M., Grant, D., Berge, K., & Ankrah, N. Y. D. (2024). Moringa Reduces Glucose Levels and Alters Wolbachia Abundance in Drosophila melanogaster. Microbiology Research, 15(3), 1870-1879. https://doi.org/10.3390/microbiolres15030125





