Abstract
This article explores the diverse array of biologically active compounds derived from microbial symbionts, particularly focusing on the isolation and characterization of diepoxides, highly oxygenated triterpenoids, secosteroids, ergostane-type steroids, and meroterpenoids from various marine and plant-derived fungi. We highlight significant discoveries such as vitamin D variants from fungal species, unique sesterterpenoids from mangrove endophytic fungi, and secosteroids with potential medicinal applications. The study delves into the structural uniqueness and bioactivities of these compounds, including their anti-inflammatory, antibacterial, antifungal, antiviral, and cytotoxic effects. Notable findings include the isolation of compounds with significant activity against cancer cell lines, the inhibition of acetylcholinesterase, and promising antifouling properties. This work underscores the potential of microbial symbionts as a rich source of novel bioactive compounds with diverse therapeutic applications, highlighting the importance of marine and fungal biodiversity in drug discovery and development.
1. Introduction
Microbial symbionts, encompassing a diverse array of microorganisms, establish symbiotic relationships with host organisms, ranging from humans to plants and other microorganisms [1,2,3,4]. These symbiotic interactions are characterized by their nature: mutualistic, where both host and microbe benefit; commensal, where the microbe benefits without impacting the host; or parasitic, where the microbe benefits at the host’s expense [5,6,7]. Microbial symbionts include bacteria, fungi, protozoa, and viruses, each playing a distinct role in their respective ecosystems.
In mutualistic relationships, both parties gain significant advantages. For example, in the human gut, certain bacteria aid in food digestion and vitamin synthesis, while receiving essential nutrients and a habitat from the host [8,9,10]. Conversely, in commensal relationships, the microbe benefits without affecting the host significantly, as seen in the human skin microbiota. Parasitic symbionts, however, can be detrimental, often leading to diseases through pathogenic bacteria, viruses, and fungi [11].
The impact of microbial symbionts is profound, influencing the health and disease states of their hosts. In humans, the gut microbiota plays a vital role in digestion, immune system development, and pathogen defense. Imbalances in these microbial communities, or dysbiosis, can lead to various health issues. In ecological contexts, microbial symbionts are integral to nutrient cycling, soil health, and plant growth, aiding in nutrient acquisition, stress tolerance, and pathogen protection [12,13].
Leveraging microbial symbionts in biotechnology has yielded significant advancements. Their applications span developing biofertilizers, bioremediation agents, and pharmaceuticals. In agriculture, they enhance crop growth and resilience. The field of microbial symbiont research is burgeoning, with a focus on molecular and genetic understandings of these relationships. This knowledge is crucial for developing novel disease treatments, environmental management strategies, and agricultural practices. It also holds the key to future progress in medicine, environmental science, and agriculture [14].
This review primarily discusses microbial symbionts as producers of biologically active compounds, including diepoxy compounds and highly oxygenated triterpenoids. Why are only these two groups included in this review? Both of these groups of natural compounds belong to highly oxidized metabolites that are produced by symbiotic bacteria and fungi, pathogenic fungi or bacteria, and fungi. These organisms are present in both terrestrial and marine organisms or isolated from these organisms.
2. α,β-Diepoxy Metabolites Derived from Bacterial Species and Fungal Endophytes
Diepoxy compounds, characterized by two epoxide groups within their molecular structure, are highly reactive due to this dual epoxide configuration [15,16,17]. An epoxide group is essentially an oxygen atom bonded to two adjacent carbon atoms, forming a three-membered ring structure. The presence of two such groups in diepoxy compounds contributes significantly to their reactivity, making them valuable intermediates in synthesizing polymers, resins, and other materials [18,19,20]. Diepoxies derived from these compounds are notably strong adhesives with excellent chemical resistance [21]. Additionally, some diepoxy compounds exhibit biological activity, with potential applications in drug synthesis in the pharmaceutical industry. However, their inherent reactivity also necessitates caution in handling and usage due to possible interactions with biological molecules [22].
Approximately 300 compounds, classified as α,β-diepoxy-containing compounds, are recognized as a distinct group of natural metabolites [15,18,19]. The presence of an epoxy group is thought to enhance a molecule’s biological activity due to the group’s high reactivity [23,24,25]. It is hypothesized that the inclusion of two epoxy groups in α,β-position could potentially double a molecule’s biological activity, making it a source of highly reactive free oxygen. These molecules, part of the high-energy oxygen-containing non-aromatic heterocycles group, are gaining attention as new pharmacophores with a broad spectrum of biological activity. This group includes endoperoxides, oxetanes, and α,β-epoxides [15,18,19,26,27].
Fungal endophytes, a varied group of fungi that reside within plant tissues without apparent detriment to their hosts, are known for producing a range of secondary metabolites [28]. These compounds can promote plant growth, aid in nutrient acquisition, enhance disease resistance, and improve tolerance to abiotic stresses such as heavy metals or drought. Some of these metabolites possess antimicrobial properties, offering plants protection against pathogens, while others have shown potential in pharmaceutical development, suggesting a promising avenue for novel drug discovery [29,30].
An antibiotic featuring a cyclohexene diepoxide ring system, LL-Z1220 (1; structure, see Figure 1; biological activities, see Table 1), isolated from an unidentified laboratory-cultivated fungus [31,32]. Cepacin B (2), an acetylenic antibiotic derived from Gram-negative bacteria Pseudomonas cepacia SC 11,783, exhibits strong activity against Staphylococci and some Gram-negative bacteria [33]. Produced by Ascomycetous fungi Penicillium rugosum, antibiotic 2188 (3) is effective against Staphylococcus aureus [34]. The soil fungus Trichoderma sp. (family Hypocreaceae) yields the isonitrile antibiotic isonitrin A (4), which demonstrates significant antimicrobial activities against both Gram-positive and negative bacteria, as well as fungi [35]. Trichoviridin (5), originating from the soil fungus Trichoderma sp. TK-1, inhibits S. aureus and E. coli [36].
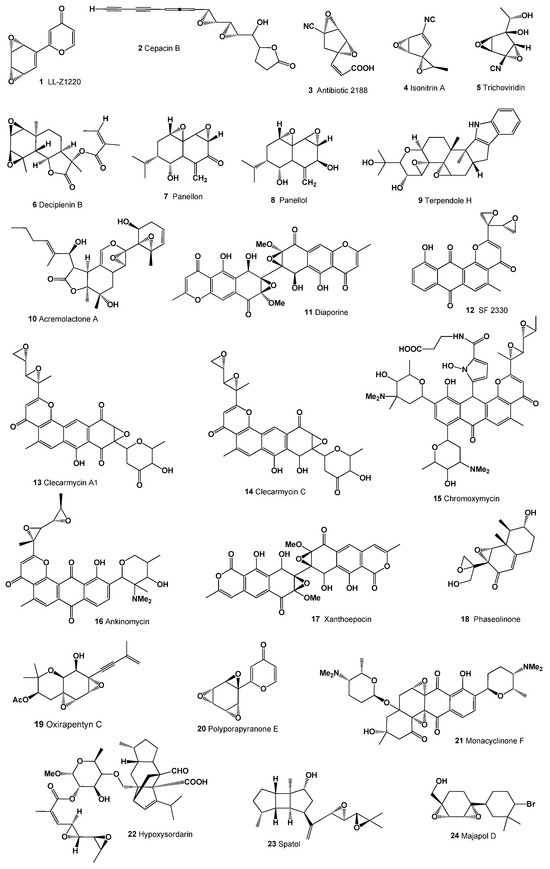
Figure 1.
Bioactive diepoxides derived from bacterial species and fungal endophytes.

Table 1.
Biological activities of diepoxides produced by fungi and fungal endophytes [15,18].
Decipienin B (6), a diepoxide, was isolated from the coprophilous fungus Podopsora decipiens and obtained from a grand landscaping “Hogweed” Melanoselinum decipiens [37,38,39]. Two fungal nor-sesquiterpenes containing a diepoxide fragment, panellon (7) and panellol (8), were isolated from the saprobic fungus Resupinatus leightonii [40]. Terpendole H (9) was identified in the culture broth of Albophoma yamanashiensis [41], while a herbicidal epoxydihydropyranyl γ-lactone, acremolactone A (10, 3D Graph: see Figure 2), was isolated from Acremonium roseum [42].
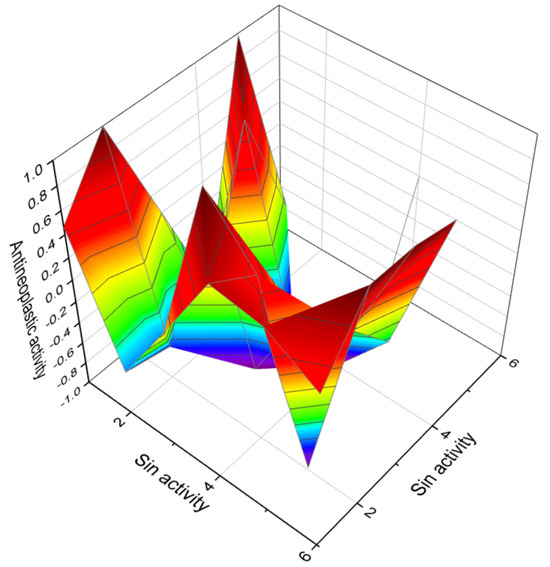
Figure 2.
The 3D graph illustrates the predicted and calculated antineoplastic activity of six diepoxides. Specifically, the graph focuses on compounds numbered as 1, 2, 3, 10, 11, and 12. These compounds have been studied for their potential therapeutic applications. The graph shows that these diepoxides exhibit a high level of confidence, with a maximum prediction accuracy of over 92%. Antineoplastic activity, which refers to the ability of a substance to combat tumor growth or cancer, is characterized through a variety of mechanisms and criteria, both in laboratory settings and in clinical trials. Many antineoplastic agents work by interfering with the cell cycle of cancer cells. This can include blocking DNA replication or mitosis, thereby preventing the cells from dividing and growing. Another common mechanism is the induction of apoptosis, or programmed cell death, in cancer cells. This is a controlled process that leads to the elimination of damaged or unwanted cells. Some antineoplastic agents inhibit angiogenesis, the process by which new blood vessels form. Since tumors need a blood supply to grow, cutting off this supply can inhibit or reduce tumor growth. Certain antineoplastic drugs are directly cytotoxic, meaning they kill cells, particularly those that are rapidly dividing, like cancer cells. Some antineoplastic agents work by enhancing the body’s immune response against cancer cells. The activity of these agents is initially characterized in vitro (in a laboratory setting using cells or biological molecules) and in vivo (in living organisms like animal models). The most definitive characterization comes from clinical trials, where the safety and efficacy of the drug are tested in humans. These trials are conducted in several phases to assess different aspects like safety, efficacy, optimal dosing, and side effects. The effectiveness of antineoplastic agents can also be characterized by monitoring biomarkers—these are biological molecules found in blood, other body fluids, or tissues, which can indicate the presence or progress of cancer. Characterization also involves understanding how and why cancer cells might develop resistance to the drug, which is a significant challenge in cancer treatment. The therapeutic window, which balances efficacy against toxicity, is a crucial aspect of characterizing antineoplastic agents. This includes understanding side effects and how they impact patients’ quality of life.
Diaporine (11), a unique symmetric polyketide from the endophytic fungus 3lp-10, inhibits the conversion from the M2 to the M1 phenotype and regulates the TLR4-MAPK signal pathway and PPARγ activity [43]. Antibiotic SF-2330 (12), effective against Gram-positive bacteria, was isolated from Gram-positive bacteria Streptomyces sp. SF-2330 [44,45]. Antitumor agents clecarmycin A1 (13) and C (14), produced by Streptomyces sp. DO-114, exhibit antibacterial and anti-proliferative activities against human HeLa S3 cells and antitumor effects in mice [46]. The strong antitumor antibiotic chromoxymycin (15) from Streptomyces libani subsp. rubropurpureus 6362 is effective against P388 leukemia, B16 melanoma in mice, and shows weak antibacterial activity [47,48]. Ankinomycin (16), a potent antitumor antibiotic, was identified in the culture broth of Streptomyces sp. SF2587 [49]. An Ascomycetous fungi Penicillium simplicissimum IFO5762 produced the antibiotic xanthoepocin (17) [50], and the phytotoxin phaseolinone (18, 3D Graph: see Figure 3) was identified in plant pathogen fungus Macrophomina phaseolina [51].
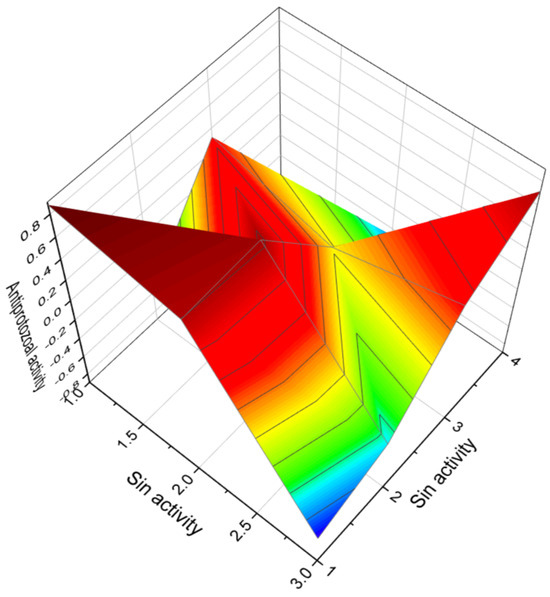
Figure 3.
The 3D graph illustrates the predicted and calculated antiprotozoal activity of three diepoxides. Specifically, the graph focuses on compounds numbered as 8, 17, and 18. The graph shows that these diepoxides exhibit a high level of confidence, with a maximum prediction accuracy of over 88%. Antiprotozoal activity refers to the ability of certain substances to inhibit the growth of or destroy protozoa, which are single-celled organisms that can cause a variety of diseases in humans. These substances are often used in medications to treat protozoal infections. Many protozoa are pathogenic, meaning they can cause diseases. Examples include Plasmodium species causing malaria, Entamoeba histolytica causing amoebiasis, and Giardia lamblia causing giardiasis. Antiprotozoal drugs are crucial for treating these diseases, which can be life-threatening or cause significant morbidity. Some protozoal diseases, like malaria, are major public health concerns in many parts of the world, especially in tropical and subtropical regions. Effective antiprotozoal drugs help control such diseases, preventing them from becoming epidemics. By treating protozoal infections, antiprotozoal drugs can significantly improve the quality of life for affected individuals, allowing them to return to normal activities and reducing the burden on healthcare systems. Understanding antiprotozoal activity is crucial for the development of new drugs, especially as some protozoa develop resistance to existing treatments. Continuous research is necessary to find new, effective compounds. Overall, the study and application of antiprotozoal activity play a vital role in combating infectious diseases caused by protozoa, contributing to better health outcomes globally.
Oxirapentyn C (19), a highly oxygenated chromene derivative, was isolated from the lipophilic extract of the marine-derived fungus Isaria felina KMM 4639 and exhibited weak cytotoxicity against various cell lines [52]. The 2-phenylpyranon-4-one derivative, polyporapyranone E (20), isolated from seagrass-derived fungi Polyporales PSU-ES44 and PSU-ES83, showed cytotoxic activity against human breast cancer (MCF-7) and noncancerous Vero cell lines [53].
Monacyclinone F (21), with two distinct epoxide rings attached to an angucyclinone moiety and an additional aminodeoxysugar linked via an angular oxygen bond, is produced by the Actinomycete Streptomyces sp. strain M715. This strain was derived from the Puerto Rican sponge Scopalina ruetzleri [54]. A sordarin derivative, hypoxysordarin (22), isolated from the facultative marine fungus Hypoxylon croceum, demonstrates potent antifungal activities [55].
Marine endophytic fungi, discovered in every marine algae, seagrass, driftwood, mangrove plant, and marine invertebrates (especially sponges and corals), reside inter- and intra-cellularly without inducing visible symptoms of disease [56]. These endophytic fungi, living in seaweeds and marine plants without causing apparent disease, include strains such as Alternaria sp., Aphanocladium sp., Aspergillus flavus, Aspergillus sp., A. niger, A. terreus, Aureobasidium pullulans, Chaetomium globosum, Cladosporium sp., Colletotrichum sp., Curvularia lunata, Drechslera sp., Fusarium oxysporum, and others [57]. Some of these fungi are noted for producing diepoxides derived from seaweeds, soft corals, or marine sponges [15,18,58,59,60].
Spatol (23), a vicinal diepoxide from the group of spataneous diterpenes, was isolated from the brown alga Stoechospermum marginatum [61] and the brown seaweed Spatoglossum schmittii [62]. However, the activity of these metabolites isolated from algae has not been determined. Majapol D (24), a cytotoxic hydroperoxide, was found in the red alga Laurencia majuscula [63]. The compound 2,10-dibromo-3-chloro- 7,8:9,10-diepoxychamigrane (25; structure, see Figure 4; biological activities, see Table 2) was identified in the red alga Laurencia nidifica [64], along with a chamigrane halogenated sesquiterpene (26) in the same species [65]. Zooxanthellatoxin A (27), a polyhydroxypolyene with significant vasoconstrictive properties, was isolated from the symbiotic marine alga Symbiodinium sp. [66].
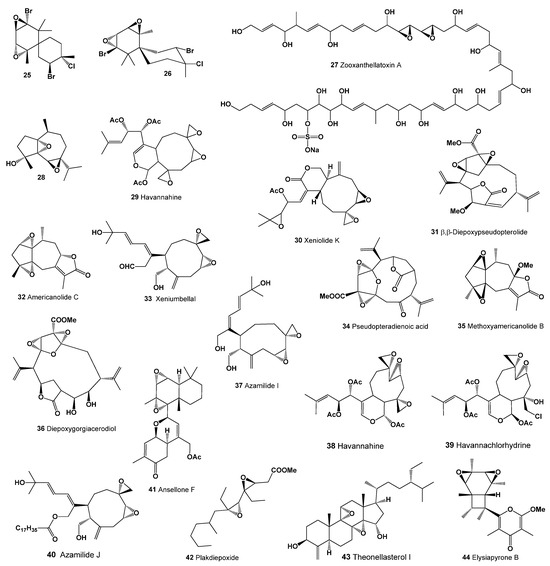
Figure 4.
Bioactive diepoxides derived from marine fungi and fungal endophytes.

Table 2.
Biological activities of diepoxides produced by bacterial and/or fungal endophytes [15,18].
Soft corals and marine sponges are notable for hosting a diverse array of fungal endophytes, known for producing biologically active metabolites [18,67,68,69,70,71]. For instance, a cytotoxic diepoxy sesquiterpene (28) was isolated from the ethyl acetate soluble fraction of the soft coral Sinularia sp. [72]. In soft corals, various other compounds have been discovered: havannahine (29) in Xenia membranacea [73], xeniolide K (30) in the Kenyan Xenia novaebrittanniae [74], and β,β-diepoxy-pseudopterolide (31), americanolide C (32), xeniumbellal (33), and pseudopteradienoic acid (34) in Caribbean plume Pseudopterogorgia acerosa [75,76,77,78]. Additionally, Pseudopterogorgia acerosa yielded guaiane lactone methoxyamericanolide B (35) and diepoxygorgiacerodiol (36) [75,76], while azamilide J (37), a diterpenoid with an opened A-ring, was extracted from Xenia sp. [79]. Xenia membranacea also contained havannahine (38) and havannachlorhydrine (39) [80,81,82], and azamilide J (40) was again found in Kenyan X. novaebrittanniae [74].
Recent advances, particularly in high-throughput sequencing, have significantly expanded our understanding of sponge-associated microbial communities, their composition, host specificity, and spatio-temporal dynamics [83,84,85]. More than 60 bacterial phyla, including newly discovered candidate phyla without any cultured representatives, have been identified in sponges [86,87,88].
From the marine sponge Phorbas sp., a sesterterpenoid named ansellones F with a rare 1,2:3,4-bis-epoxydecalin substructure (41; 3D Graph: see Figure 5) was isolated in Howe Sound, British Columbia [89]. Plakdiepoxide (42), a groundbreaking polyketide with a vicinal diepoxide, extracted from the Chinese sponge Plakortis simplex, acts as a potent modulator of peroxisome proliferator-activated receptor (PPAR)-γ [90]. The apolar fraction of Theonella swinhoei’s methanol extract revealed 10 polyhydroxylated steroids, including theonellasterol I (43) [91].
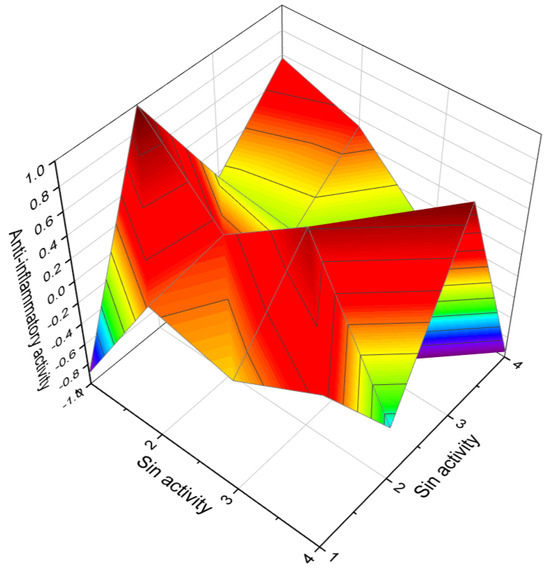
Figure 5.
The 3D graph illustrates the predicted and calculated anti-inflammatory activity of four diepoxides. Specifically, the graph focuses on compounds numbered as 28, 32, 40, and 41. The graph shows that these diepoxides exhibit a high level of confidence, with a maximum prediction accuracy of over 86%. Anti-inflammatory activity is characterized through various mechanisms and approaches, both in laboratory settings and clinically. Anti-inflammatory agents often work by inhibiting the production or action of inflammatory mediators like cytokines (e.g., interleukins, tumor necrosis factor), prostaglandins, and leukotrienes. Nonsteroidal anti-inflammatory drugs, for example, inhibit the enzyme cyclooxygenase, which is involved in the production of prostaglandins. Some anti-inflammatory drugs reduce the activation or migration of immune cells (like macrophages and neutrophils) to the site of inflammation. This can be assessed by examining tissue samples for the presence of these cells. Techniques such as Western blotting, ELISA, and RT-PCR are used to quantify the expression of inflammatory markers at the molecular level in both animal models and in vitro cell culture studies. In the clinical setting, the effectiveness of anti-inflammatory drugs is evaluated through their ability to reduce symptoms of inflammation in patients, such as pain, swelling, redness, and loss of function. This is often assessed using various scales or indices, depending on the specific condition being treated. Blood tests can be used to measure levels of C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), and other markers of inflammation, which can indicate the effectiveness of anti-inflammatory treatment. Research into the genetic basis of inflammation has helped in understanding how different individuals respond to anti-inflammatory treatment, leading to more personalized approaches in managing inflammatory conditions. These methods collectively help in characterizing the anti-inflammatory activity of various substances and are crucial in the development and assessment of new anti-inflammatory drugs and therapies.
Elysiapyrone B (44), a polypropionate-derived metabolite featuring a unique bicyclo [4.2.0]octane core, was isolated from the sea slug Elysia diomedea [92]. This compound showcases an unprecedented carbon skeleton.
Based on the information in Table 1 and Table 2, diepoxides numbered 1–44, which are derived from bacteria, fungi, and fungal endophytes across diverse ecological environments, demonstrate significant biological activity. Over 90% of these secondary metabolites display antineoplastic properties and related activities. Furthermore, a substantial number of these compounds exhibit robust antineoplastic effects with a confidence level exceeding 92%. In addition to their cancer-fighting potential, certain diepoxides are also recognized for their antiprotozoal and anti-inflammatory properties, which are predominant in some instances.
3. Highly Oxygenated Triterpenoids Derived from Terrestrial Microbial Symbionts
Another group of bioactive natural metabolites belongs to oxygenated triterpenoids and steroids derived from terrestrial microbial symbionts. Highly oxygenated secosteroids belong to a unique subclass of steroid compounds characterized by a distinct structural feature: a broken carbon ring in their standard four-ring structure [93,94,95]. The term ‘secosteroid’, derived from the Latin ‘seco’, meaning ‘cut’, refers to this ‘broken’ ring aspect, differentiating them from conventional steroids [95,96,97].
Among the most renowned secosteroids is vitamin D and its derivatives [97]. Vitamin D3 (45, cholecalciferol), for example, is synthesized in the skin from 7-dehydrocholesterol under ultraviolet radiation, forming a secosteroid. Vitamin D, extensively studied for its role in calcium homeostasis and metabolism, is vital for bone formation and maintenance, and also modulates immune system function [97,98,99]. Vitamin D undergoes hydroxylation in the liver to form 25-hydroxyvitamin D, and a subsequent hydroxylation in the kidney produces the active 1,25-dihydroxyvitamin D. Secosteroid imbalances, particularly in vitamin D, are linked to various health conditions, including bone disorders like rickets and osteomalacia, and potentially a broader spectrum of diseases such as certain cancers, autoimmune disorders, and cardiovascular diseases. Vitamin D analogs, due to their critical roles in bone health and immune function, are employed in treating conditions like psoriasis, renal osteodystrophy, and secondary hyperparathyroidism [67,69,95,97,100,101].
Secosteroids and/or highly oxygenated triterpenoids from bacterial and fungal endophytes represent a fascinating class of natural lipids [18,93,94,95]. The Ganoderma species, for instance, produce biologically active secosteroids with anti-cancer and antibacterial properties, among others [102,103]. Fornicatin B (46; structure, see Figure 6; biological activities, see Table 3), D (47), F (48), and H (49) from Ganoderma cochlear have shown potential hepatoprotective activities in HepG2 cells treated with H2O2 [104]. Cochlearic acids A (49) and B (50), cochlate C (54), and ganocochlearic acid A (56) from the same species exhibited moderate cytotoxicity against various human tumor cell lines (HL-60, SMMC-7721, A-549, MCF-7, and SW480), with IC50 values ranging from 8 to 30 μM [103]. Additionally, Ganoderma cochlear produced secosteroids cochlate A (52) and B (53) [104], and Ganoderma sinense yielded methyl ganosinensate A (55) [70,105,106].
Figure 6.
Bioactive oxygenated triterpenoids derived from fungi and fungal endophytes.

Table 3.
Biological activities of secosteroids produced by fungi and fungal endophytes [67,70,71].
Lanostane-type australic acid (57) from Ganoderma austral has been shown to inhibit cell growth, partly by inducing apoptosis [107]. Elfvingic acid H methyl ester (59) displayed significant cytotoxicity against Kato III and Ehlrich cells (IC50 1 mg/mL), found in the fruiting body of Elfvingia applanata [108]. Additionally, colossolactone V (58) and colossolactone VII (62) are two other lanostane-type triterpenes isolated from Ganoderma colossum [109].
Two ergostane-type steroids, phomopsterones B (60) and dankasterone A (61), were isolated from the plant-derived fungus Phomopsis sp. TJ507A. Phomopsterone B (60) displayed notable anti-inflammatory activity [110]. From the solid cultures of Gloeophyllum abietinum, two unique ergosteroids, gloeophyllin A (63) and J (64), were extracted. Gloeophyllin A (63) features a rare C-nor-D-homosteroid skeleton, while gloeophyllin J (64) is the first-known ergosteroid with a C8–C14 bond cleavage. Both compounds exhibited cytotoxicity against human cancer cell lines K562 and HCT116 [111].
The 8,14-Seco-ergosterol derivative, childinin (65), obtained from the fruiting bodies of Daldinia childiae, showed antibacterial activity [112]. Ganoboninketal B (66) and C (67), nortriterpenes with rearranged 3,4-seco-27-norlanostane skeletons and complex polycyclic systems, were isolated from the medicinal mushroom Ganoderma boninense. These compounds demonstrated anti-plasmodial activity against Plasmodium falciparum with IC50 values of 7.9 and 1.7 μM, respectively. Additionally, ganoboninketal B (66) showed weak cytotoxicity towards the HeLa cell line (IC50 = 65.5 μM), and ganoboninketal C (67) exhibited weak cytotoxicity against the A549 cell line (IC50 = 35.8 μM). Both also presented NO inhibitory activity in LPS-induced macrophages with IC50 values of 24.3 and 60.9 μM, respectively [113].
Aspterpenacid A (68), with a unique 5/3/7/6/5 ring system, was isolated from the mangrove endophytic fungus Aspergillus terreus H010 [114]. The biotransformation of hyodeoxycholic acid by various Gram-positive bacteria Rhodococcus spp. yielded two compounds: 6α-hydroxy-3-oxo-23,24-dinor-5β-cholan-22-oic acid (69; structure, see Figure 7; biological activities, see Table 4) and 6α-hydroxy-3-oxo-23, 24-dinorchol-1,4-dien-22-oic acid (70), with specific strains (Rhodococcus zopfii, Rh. ruber, and Rh. aetherivorans) facilitating the degradation [115].
Figure 7.
Bioactive oxygenated triterpenoids derived from fungal endophytes and fungi.

Table 4.
Biological activities of secosteroids produced by bacteria and fungal endophytes [67,70].
Albocisterols A (71) and C (72) degraded steroids from the fungus Antrodiella albocinnamomea showed significant inhibitory activities against protein tyrosine phosphatase 1B [116]. Two secoergosterols, tylopiol A (73) and B (74), were isolated from the fresh fruit bodies of Tylopilus plumbeoviolaceus (for a sample of the fungi, see Figure 8). These compounds are scientifically named 3β-hydroxy-8α,9α-oxido-8,9- secoergosta-7,9(11),22-triene (73) and 3β-hydroxy-8α,9α-oxido-8,9-secoergosta-7,22- dien-12-one (74), respectively [117].

Figure 8.
Samples of some fungi produce secondary bioactive metabolites. (a) Daldinia childiae, commonly known as Carbon balls, is a beneficial fungus found on decaying conifer and hardwood logs. This fungus plays a crucial role in the ecosystem by deriving its nutrients from deadwood and aiding in its decomposition. The distinctive feature of Carbon balls is its ball-shaped cap, which has a crusty surface. This surface is colored brownish-black, resembling a ball of carbon, which is the inspiration behind its common name “carbon ball”. D. childiae, a wood-inhabiting ascomycete fungus, belongs to the Hypoxylaceae family and the Xylariales order. Notably, strain JS-1345 of this fungus, which was isolated from the stem tissue of Abies koreana (Korean fir), has demonstrated significant anti-inflammatory activity. (b) White rot fungus or Ganoderma boninense, a major pathogen that attacks the oil palm and eventually kills it. Extracts of this fungus demonstrate antiplasmodial, antimicrobial, and antibacterial activity, and have an antioxidant effect. (c) Tylopilus plumbeoviolaceus, commonly known as the violet-grey bolete, is a fungus of the bolete family. First described in 1936, the mushroom has a disjunct distribution, and is distributed in eastern North America and Korea. (d) Antrodiella sp. or tinder fungus is a polypore living on deciduous trees. Tinder fungus is a decaying fungus that lives on dead trees laying on the ground and living trees. It forms white rot and decays wood quite quickly. It grows most commonly on birch (Betula sp.) and alders (Alnus sp.), but also on other deciduous trees.
Vitamin D2, known chemically as 9,10-seco(5Z,7E)-5,7,10(19),22- ergostatetraene-3β-ol (75), along with vitamin D4 (76), have been isolated from ten different fungal species [118]. Additionally, two distinct sesterterpenoids, asperterpenol A (77) and B (78), were extracted from a mangrove endophytic fungus, Aspergillus sp. 085242. These compounds feature unusual tetracyclic ring skeletons, with asperterpenol A (77) displaying a 5/7/6/5 structure and asperterpenol B (78) having a 5/8/6/6 structure. Both sesterterpenoids demonstrated the ability to inhibit acetylcholinesterase, with IC50 values of 2.3 μM and 3.0 μM, respectively [119].
4. Highly Oxygenated Triterpenoids Derived from Marine Bacterial Species and Fungal Endophytes
Marine bacterial species and fungal endophytes are both significant sources of secondary metabolites, each with unique characteristics and potentials [1,2,3,9,10,11,12]. Marine bacteria are found in various marine environments, including deep-sea vents, coral reefs, and ocean sediments [3,10,56]. These unique habitats contribute to the diversity of secondary metabolites they produce. They are known to produce a wide range of secondary metabolites, including antibiotics, antitumor agents, anti-inflammatory compounds, and others that have potential pharmaceutical applications [2,3,4,8,9,10,11]. The secondary metabolites from marine bacteria often have unique chemical structures not found in terrestrial organisms, which is attributed to the different environmental pressures and ecological niches in the ocean. There is a growing interest in bioprospecting marine bacteria for novel compounds, especially for drug discovery and development [1,2,3,4,5,6,7,8,10,11,12].
Fungal endophytes live inside marine invertebrates or seaweeds tissues without causing apparent harm to the host. This symbiotic relationship is crucial for the production of certain secondary metabolites. These fungi produce a variety of secondary metabolites, including alkaloids, terpenoids, phenolics, and flavonoids. These compounds can have antimicrobial, anticancer, and other bioactive properties [28,29,30,31].
Like marine bacteria, fungal endophytes are a rich source of novel compounds for drug discovery. They have been the source of several clinically important drugs. In both cases, the exploration of marine bacteria, and/or fungal endophytes for secondary metabolites is an active area of research, driven by the need for new and effective bioactive compounds in medicine, agriculture, and other fields. The unique environments and symbiotic relationships these organisms engage in contribute to their ability to produce diverse and novel compounds [2,3,4,5,6,7,8,11,12,26,27,28,29,30].
Austalide N (79; structure, see Figure 9; biological activities, see Table 5), produced by Aspergillus sp., exhibited antibacterial activity against various marine bacteria including Halomonas aquamarina, Polaribacter irgensii, Pseudoalteromonas elyakovii, and others, with MIC values ranging from 0.01 to 0.1 μg/mL [120,121]. The antiviral potential of arisugacin A (80), isolated from marine fungus Aspergillus terreus SCSGAF0162, was demonstrated against HSV-1 with an IC50 value of 12.76 μM. This fungus was extracted from gorgonian corals Echinogorgia aurantiaca in the South China Sea [122].
Figure 9.
Bioactive oxygenated triterpenoids derived from marine fungi and fungal endophytes.

Table 5.
Biological activities of secosteroids produced by marine bacteria.
The secosteroid dankasterone A (81) was found in the culture filtrate extract of marine sponge-associated fungus Neosartorya fennelliae KUFA 0811 [123]. A rare 7-norsteroid, 7-Nor-ergosterolide (82), with a unique pentalactone B-ring system, was characterized from Aspergillus ochraceus EN-31, an endophytic fungus isolated from the marine brown alga Sargassum kjellmanianum. It displayed cytotoxicity against several human cancer cell lines [124].
Chermesin A (83), a spiromeroterpenoid with a drimane-type sesquiterpene skeleton, was isolated from Penicillium chermesinum EN-480 [125], an endophytic fungus from the marine red alga Pterocladiella tenuis. It exhibited antibacterial activity against Micrococcus luteus [126]. The phenylspirodrimane derivative, stachybotrysin (84), from marine-derived fungus Stachybotrys sp. KCB13F013, inhibited osteoclast differentiation in bone marrow macrophage cells [127]. Brevione I (85, 3D Graph: see Figure 10), a spiroditerpene isolated from deep sea sediment-derived Penicillium sp., exhibited cytotoxicity against MCF-7 cells, comparable to cisplatin [128]. From marine red alga Chondrus ocellatus, Penicillium echinulatum pt-4 yielded meroterpene arisugacin K (86), displaying inhibitory activity against E. coli [129].
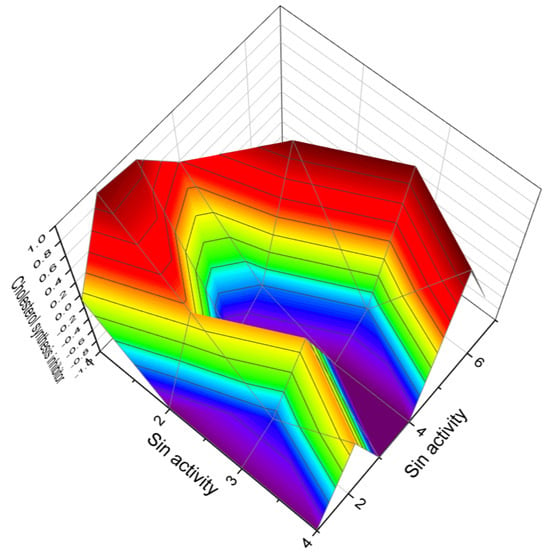
Figure 10.
The 3D graph illustrates the predicted and calculated anti-hypercholesterolemic activity and/or as cholesterol synthesis inhibitors of highly oxygenated triterpenoids. Specifically, the graph focuses on compounds numbered as 64, 81, 85, and 89. The graph shows that these highly oxygenated triterpenoids exhibit a high level of confidence, with a maximum prediction accuracy of over 91%. “Anti-hypercholesterolemic activity” and “cholesterol synthesis inhibitors” refer to related but distinct concepts in the management of cholesterol levels. Anti-hypercholesterolemic activity—this term encompasses any activity or mechanism that leads to the reduction in high cholesterol levels in the body. It is a general term that includes various approaches and mechanisms. Anti-hypercholesterolemic actions can be achieved through different mechanisms, such as reducing cholesterol absorption in the gut, enhancing the elimination of cholesterol or its conversion to bile acids, inhibiting cholesterol synthesis in the liver, or increasing the uptake of LDL cholesterol from the bloodstream. Statins (which are also cholesterol synthesis inhibitors), bile acid sequestrants, cholesterol absorption inhibitors (like ezetimibe), and even lifestyle interventions such as diet and exercise can all be considered antihypercholesterolemic. Cholesterol synthesis inhibitors—these specifically refer to drugs or substances that inhibit the synthesis of cholesterol within the liver. They target a particular enzyme in the cholesterol synthesis pathway. The most common target of these inhibitors is the HMG-CoA reductase enzyme. This enzyme plays a crucial role in the mevalonate pathway, which is responsible for cholesterol biosynthesis. Cholesterol synthesis inhibitors are a subset of anti-hypercholesterolemic agents. They are among several ways to achieve anti-hypercholesterolemic activity. Anti-hypercholesterolemic activity is a broader term and includes various methods and drugs, while cholesterol synthesis inhibitors are specifically focused on inhibiting the biosynthesis of cholesterol in the liver. While cholesterol synthesis inhibitors have a targeted biochemical action, other antihypercholesterolemic strategies might work differently, like increasing cholesterol excretion or reducing absorption. In summary, while all cholesterol synthesis inhibitors are anti-hypercholesterolemic (as they lower cholesterol levels), not all anti-hypercholesterolemic methods involve inhibiting cholesterol synthesis. Each approach has its place in managing high cholesterol, depending on individual patient needs and specific health conditions.
Terretonin G (87), a terretonin-type meroterpene from sponge-derived fungus Aspergillus sp. OPMF00272, showed potent antibacterial activity against Gram-positive bacteria [130]. Insuetolide C (88), from sponge-derived fungus Aspergillus insuetus isolated from Mediterranean sponge Psammocinia sp., exhibited moderate cytotoxicity against MOLT-4 cancer cell lines [131].
Chrodrimanin K (89), a meroterpenoid from Ascomycetous fungus Penicillium sp. SCS-KFD09 isolated from marine worm Sipunculus nudus, showed anti-H1N1 activity [132]. Sartorypyrone C (90) and chevalone B (91), two meroditerpenes, were derived from marine sponge-associated fungus Neosartorya paulistensis [133].
Aspergillus terreus SCSGAF0162, isolated from gorgonian corals Echinogorgia aurantiaca, produced a territrem derivative (92, 3D Graph: see Figure 11) with strong acetylcholinesterase inhibitory and antifouling activities [122].
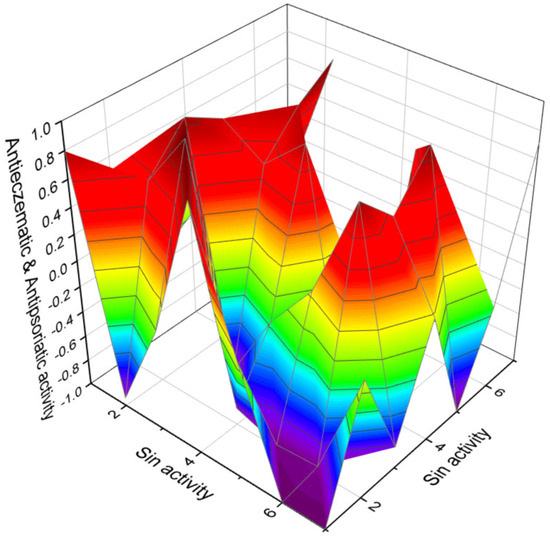
Figure 11.
The 3D graph illustrates the predicted and calculated anti-eczematic and antipsoriatic activities of highly oxygenated triterpenoids. Specifically, the graph focuses on compounds numbered as 46, 47, 48, 49, 50, 90, and 91. The graph shows that these highly oxygenated triterpenoids exhibit a high level of confidence, with a maximum prediction accuracy of over 92%. Eczema and psoriatic diseases, such as psoriasis, are both chronic skin conditions that can cause discomfort and distress, but they have different causes, symptoms, and treatments. Eczema—also known as atopic dermatitis, it is often linked to allergies and is more common in people with a family history of allergies, asthma, or atopic dermatitis. It is thought to be related to an overactive immune response to environmental triggers and a defect in skin barrier function. Psoriatic diseases (like psoriasis)—these are autoimmune diseases. In psoriasis, the immune system mistakenly attacks healthy skin cells, accelerating the skin cell life cycle. This rapid turnover leads to the build-up of cells on the skin’s surface. The differences between anti-eczematic and antipsoriatic drugs are based on the underlying causes and characteristics of eczema and psoriasis, as these conditions have distinct pathologies and thus respond to different types of treatment. Eczema, particularly atopic dermatitis, is often treated with a combination of skin care and medication to reduce inflammation and alleviate itching. Topical corticosteroids are the mainstay for reducing inflammation and itchiness in eczema. They come in various strengths and are used according to the severity of the eczema. Psoriasis treatment aims to slow down the rapid growth of skin cells and reduce inflammation. Psoriasis is similar to eczema, but often stronger. Vitamin D analogues, such as calcipotriene, help to regulate skin cell growth.
Based on the information from Table 4 and Table 5 regarding highly oxygenated triterpenoids, it is evident that their predominant activity is antineoplastic. However, they also exhibit a range of other biological activities. Specifically, triterpenoids numbered 46, 47, 48, 49, 50, 90, and 91 demonstrate notable anti-eczematic and antipsoriatic properties. Additionally, oxygenated triterpenoids 64, 81, 85, and 89 are identified as having anti-hypercholesterolemic capabilities or function as cholesterol synthesis inhibitors. Consequently, these highly oxygenated triterpenoids emerge as potential candidates for the development of pharmaceuticals aimed at preventing or treating cancer, various skin disorders, and managing blood cholesterol levels. The overarching conclusions of the study highlight that both natural diepoxy compounds and highly oxygenated triterpenoids exhibit significant antitumor activity, making them subjects of considerable interest for biologists, pharmacologists, chemists, and pharmaceutical companies, particularly in the context of preclinical trials. This discovery marks a significant achievement in the realm of academic science.
5. Conclusions
This comprehensive survey of bioactive compounds derived from microbial symbionts, primarily from marine and plant-associated fungi, has revealed a remarkable diversity of structurally unique and biologically potent molecules. The study underscores the vast potential of these symbionts in bioprospecting for novel pharmacological agents. The isolation of highly oxygenated triterpenoids, secosteroids, ergostane-type steroids, and meroterpenoids not only enriches our understanding of natural compound chemistry but also presents a multitude of therapeutic possibilities. From anti-inflammatory and antibacterial activities to antifungal, antiviral, and cytotoxic effects against various cancer cell lines, these compounds showcase a wide spectrum of biological activities.
Furthermore, the findings of this research highlight the intricate interplay between microbial symbionts and their host organisms, contributing significantly to the field of natural product chemistry and marine biotechnology. The study also brings to light the importance of preserving marine and fungal biodiversity, as it is a critical reservoir for discovering new substances with potential pharmaceutical applications.
In conclusion, the diverse array of bioactive compounds identified in this study not only represents a promising avenue for future drug discovery but also emphasizes the need for continued exploration of microbial symbionts. As we advance our understanding of these complex biological systems, we pave the way for innovative treatments and therapies, harnessing the power of nature in addressing some of the most challenging health issues.
Funding
This work did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The author declares that he has no known competing financial interest or personal relationships that could affect the work described in this article.
References
- Koide, R.T. On holobionts, holospecies, and holoniches: The role of microbial symbioses in ecology and evolution. Microb. Ecol. 2023, 85, 1143–1149. [Google Scholar] [CrossRef] [PubMed]
- Bright, M.; Bulgheresi, S. A complex journey: Transmission of microbial symbionts. Nat. Rev. Microbiol. 2010, 8, 218–230. [Google Scholar] [CrossRef] [PubMed]
- Haygood, M.G.; Schmidt, E.W.; Davidson, S.K.; Faulkner, D.J. Microbial symbionts of marine invertebrates: Opportunities for microbial biotechnology. J. Mol. Microbiol. Biotechnol. 1999, 1, 33–43. [Google Scholar] [PubMed]
- Hays, G.; Patrick, W.G.; Ziesack, M.; Oxman, N.; Silver, P.A. Better together: Engineering and application of microbial symbioses. Curr. Opin. Biotechnol. 2015, 36, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Parmentier, E.; Michel, L. Boundary lines in symbiosis forms. Symbiosis 2013, 60, 1–5. [Google Scholar] [CrossRef]
- Álvarez, C.; Jiménez-Ríos, L.; Iniesta-Pallarés, M.; Jurado-Flores, A.; Molina-Heredia, F.P.; Ng, C.K.Y.; Mariscal, V. Symbiosis between cyanobacteria and plants: From molecular studies to agronomic applications. J. Exp. Bot. 2023, 74, 6145–6157. [Google Scholar] [CrossRef] [PubMed]
- Neves, A.; Godina, R.; Azevedo, S.G.; Matias, J.C.O. A comprehensive review of industrial symbiosis. J. Clean. Prod. 2020, 247, 119113. [Google Scholar] [CrossRef]
- Hooper, L. Do symbiotic bacteria subvert host immunity? Nat. Rev. Microbiol. 2009, 7, 367–374. [Google Scholar] [CrossRef]
- Piel, J. Metabolites from symbiotic bacteria. Nat. Prod. Rep. 2009, 26, 338–362. [Google Scholar] [CrossRef]
- Grube, M.; Berg, G. Microbial consortia of bacteria and fungi with focus on the lichen symbiosis. Fungal Biol. Rev. 2009, 23, 72–85. [Google Scholar] [CrossRef]
- Vorburger, C.; Perlman, S.J. The role of defensive symbionts in host–parasite coevolution. Biol. Rev. 2018, 93, 1747–1764. [Google Scholar] [CrossRef] [PubMed]
- Myers, S.P.; Hawrelak, J. The causes of intestinal dysbiosis: A Review. Altern. Med. Rev. 2004, 9, 180–197. [Google Scholar]
- Ducatelle, R.; Eeckhaut, V.; Haesebrouck, F.; Van Immerseel, F. A review on prebiotics and probiotics for the control of dysbiosis: Present status and future perspectives. Animal 2015, 9, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Carballo-Sánchez, M.P.; Alarcón, A.; Pérez-Moreno, J.; Ferrera-Cerrato, R. Agricultural and forestry importance of microorganism-plant symbioses: A microbial source for biotechnological innovations. Rev. Agric. Sci. 2022, 10, 344–355. [Google Scholar] [CrossRef] [PubMed]
- Vil, V.; Gloriozova, T.A.; Poroikov, V.V.; Dembitsky, V.M. Naturally occurring of α,β-diepoxy-containing compounds: Origin, structures, and biological activities. Appl. Microbiol. Biotechnol. 2019, 103, 3249–3264. [Google Scholar] [CrossRef] [PubMed]
- Fretland, A.J.; Omiecinski, C.J. Epoxide hydrolases: Biochemistry and molecular biology. Chem. Biol. Interact. 2000, 129, 41–59. [Google Scholar] [CrossRef] [PubMed]
- Newman, J.W.; Morisseau, C.; Hammock, B.D. Epoxide hydrolases: Their roles and interactions with lipid metabolism. Prog. Lipid Res. 2005, 44, 1–51. [Google Scholar] [CrossRef]
- Dembitsky, V.M. Bioactive steroids bearing oxirane ring. Biomedicines 2023, 11, 2237. [Google Scholar] [CrossRef]
- Kuklev, D.V.; Dembitsky, V.M. Epoxy acetylenic lipids: Their analogues and derivatives. Prog. Lipid Res. 2014, 56, 67–91. [Google Scholar] [CrossRef]
- Arbuzov, Y.A. The diels–alder reaction with molecular oxygen as dienophile. Russ. Chem. Rev. 1965, 34, 558. [Google Scholar] [CrossRef]
- Jacobsen, E.N. Asymmetric catalysis of epoxide ring-opening reactions. Acc. Chem. Res. 2000, 33, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Bučko, M.; Kaniaková, K.; Hronská, H.; Gemeiner, P.; Rosenberg, M. Epoxide Hydrolases: Multipotential Biocatalysts. Int. J. Mol. Sci. 2023, 24, 7334. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Xia, J.; Zhou, W.; Zhou, L.; Hussain, G.; Li, Q.; Ostrikov, K. Adhesion and cohesion of epoxy-based industrial composite coatings. Compos. Part B Eng. 2020, 193, 108035. [Google Scholar] [CrossRef]
- Scotti, M.T.; Fernandes, M.B.; Ferreira, M.J.P.; Emerenciano, V.P. Quantitative structure–activity relationship of sesquiterpene lactones with cytotoxic activity. Bioorg. Med. Chem. 2007, 15, 2927–2934. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.R.; Varela, C.L.; Tavares-da-Silva, E.J.; Roleira, F.M.F. Epoxide containing molecules: A good or a bad drug design approach. Eur. J. Med. Chem. 2020, 201, 112327. [Google Scholar] [CrossRef] [PubMed]
- Vil, V.; Terent’ev, A.O.; Al Quntar, A.A.A.; Gloriozova, T.A.; Savidov, N.; Dembitsky, V.M. Oxetane-containing metabolites: Origin, structures, and biological activities. Appl. Microbiol. Biotechnol. 2019, 103, 2449–2467. [Google Scholar] [CrossRef] [PubMed]
- Dembitsky, V.M.; Gloriozova, T.A.; Poroikov, V.V. Naturally occurring marine α, β-epoxy steroids: Origin and biological activities. Vietnam J. Chem. 2018, 56, 409–433. [Google Scholar] [CrossRef]
- Lugtenberg, B.J.; Caradus, J.R.; Johnson, L.J. Fungal endophytes for sustainable crop production. FEMS Microbiol. Ecol. 2016, 92, fiw194. [Google Scholar] [CrossRef]
- Xu, K.; Li, X.-Q.; Zhao, D.-L.; Zhang, P. Antifungal secondary metabolites produced by the fungal endophytes: Chemical diversity and potential use in the development of biopesticides. Front. Microbiol. 2021, 12, 689527. [Google Scholar] [CrossRef]
- Schulz, B.; Boyle, C.; Draeger, S.; Römmert, A.K.; Krohn, K. Endophytic fungi: A source of novel biologically active secondary metabolites. Mycol. Res. 2002, 106, 996–1004. [Google Scholar] [CrossRef]
- Borders, D.B.; Barbatschi, F.; Shay, A.J.; Shu, P. Fermentation, isolation, and characterization of LL-Z1220, a new antibiotic. Antimicrob. Agents Chemother. (Bethesda) 1969, 9, 233–235. [Google Scholar] [PubMed]
- Borders, D.B.; Shu, P.; Lancaster, J.E. Structure of LL-Z1220. New antibiotic containing a cyclohexene diepoxide ring system. J. Am. Chem. Soc. 1972, 94, 2540–2541. [Google Scholar] [CrossRef] [PubMed]
- Parker, W.L.; Rathnum, M.L.; Seiner, V.; Trejo, W.H.; Principe, P.A.; Sykes, R.B. Cepacin A and cepacin B, two new antibiotics produced by Pseudomonas cepacia. J. Antibiot. 1984, 37, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Sivakumari, V.; Dhinakaran, J.; Rajendran, A. Screening and productivity of penicillin antibiotic from Penicillium sp. J. Environ. Sci. Eng. 2009, 51, 247–248. [Google Scholar] [PubMed]
- Fujiwara, A.; Okuda, T.; Masuda, S.; Shiomi, Y.; Miyamoto, C.; Sekine, Y.; Tazoe, M.; Fujiwara, M. Fermentation, isolation and characterization of isonitrile antibiotics. Agric. Biol. Chem. 1982, 46, 1803–1809. [Google Scholar] [CrossRef]
- Tamura, A.; Kotani, H.; Naruto, S. Trichoviridin and dermadin from Trichoderma sp. TK-1. J. Antibiot. 1975, 28, 161–162. [Google Scholar] [CrossRef]
- Che, Y.; Gloer, J.B.; Koster, B.; Malloch, D. Decipienin A and decipienolides A-B: New bioactive metabolites from the coprophilous fungus Podopsora decipiens. J. Nat. Prod. 2002, 65, 916–919. [Google Scholar] [CrossRef]
- Holub, M.; Buděšínský, M.; Smítalová, Z.; Šaman, D.; Rychłewska, U. Structure of isosilerolide, relative and absolute configuration of silerolide and lasolide—Sesquiterpenic lactones of new stereoisomeric type of eudesmanolides. Collect. Czechoslov. Chem. Commun. 1985, 51, 903–929. [Google Scholar] [CrossRef]
- Massanet, G.M.; Guerra, F.M.; Jorge, Z.D.; Astorga, C. Sesquiterpenolides from Melanoselinum decipiens. Phytochemistry 1997, 45, 1645–1651. [Google Scholar] [CrossRef]
- Sundin, A.; Anke, H.; Bergquist, K.E.; Mayer, A.; Sheldrick, W.S.; Stadler, M.; Sterner, O. The structure determination of panellon and panellol, two 14-noreudesmanes isolated from Resupinatus leightonii. Tetrahedron 1993, 49, 7519–7524. [Google Scholar] [CrossRef]
- Tomoda, H.; Tabata, N.; Yang, D.J.; Takayanagi, H.; Omura, S. Terpendoles, novel ACAT inhibitors produced by Albophoma yamanashiensis. III. Production, isolation and structure elucidation of new components. J. Antibiot. 1995, 48, 793–804. [Google Scholar] [CrossRef] [PubMed]
- Sassa, T.; Kinoshita, H.; Nukina, M. Acremolactone A, a novel herbicidal epoxy-hydropyranyl γ-lactone from Acremonium roseum I4267. J. Antibiot. 1998, 51, 967–969. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wu, H.C.; Ge, H.M.; Zang, L.Y.; Bei, Y.C.; Niu, Z.Y.; Wei, W.; Feng, X.J.; Ding, S.; Ng, S.W.; Shen, P.P.; et al. Diaporine, a novel endophyte-derived regulator of macrophage differentiation. Org. Biomol. Chem. 2014, 12, 6545–6548. [Google Scholar] [CrossRef] [PubMed]
- Itoh, J.; Shomura, T.; Tsuyuki, T.; Yoshida, J.; Ito, M.; Sezaki, M.; Kojima, M. Studies on a new antibiotic SF-2330. I. Taxonomy, isolation and characterization. J. Antibiot. 1986, 39, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Itoh, J.; Tsuyuki, T.; Fujita, K.; Sezaki, M. Studies on a new antibiotic SF-2330. II. The structural elucidation. J. Antibiot. 1986, 39, 780–783. [Google Scholar] [CrossRef] [PubMed]
- Fujii, N.; Katsuyama, T.; Kobayashi, E.; Hara, M.; Nakano, H. The clecarmycins, new antitumor antibiotics produced by Streptomyces: Fermentation, isolation and biological properties. J. Antibiot. 1995, 48, 768–772. [Google Scholar] [CrossRef] [PubMed]
- Iwami, M.; Kawai, Y.; Kiyoto, S.; Terano, H.; Kohsaka, M.; Aoki, H.; Imanaka, H. A new antitumor antibiotic, chromoxymycin. I. Taxonomic studies on the producing strain: A new subspecies of the genus Streptomyces. J. Antibiot. 1986, 39, 6–11. [Google Scholar] [CrossRef]
- Hori, Y.; Hino, M.; Kawai, Y.; Kiyoto, S.; Terano, H.; Kohsaka, M.; Aoki, H.; Hashimoto, M.; Imanaka, H. A new antitumor antibiotic, chromoxymycin. II. Production, isolation, characterization and antitumor activity. J. Antibiot. 1986, 39, 12–16. [Google Scholar] [CrossRef]
- Sato, Y.; Watabe, H.; Nakazawa, T.; Shomura, T.; Yamamoto, H.; Sezaki, M.; Kondo, S. Ankinomycin, a potent antitumor antibiotic. J. Antibiot. 1989, 42, 149–152. [Google Scholar] [CrossRef][Green Version]
- Igarashi, Y.; Kuwamori, Y.; Takagi, K.; Ando, T.; Fudou, R.; Furumai, T.; Oki, T. Xanthoepocin, a new antibiotic from Penicillium simplicissimum IFO5762. J. Antibiot. 2000, 53, 928–933. [Google Scholar] [CrossRef]
- Dhar, T.K.; Siddiqui, K.A.I.; Ali, E. Structure of phaseolinone, a novel phytotoxin from Macrophomina phaseolina. Tetrahedron Lett. 1982, 23, 5459–5462. [Google Scholar]
- Smetanina, O.F.; Yurchenko, A.N.; Afiyatullov, S.S.; Kalinovsky, A.I.; Pushilin, M.A.; Khudyakova, Y.A.; Slinkina, N.N.; Ermakova, S.P.; Yurchenko, E.A. Oxirapentyns B–D produced by a marine sediment-derived fungus Isaria felina (DC.) Fr. Phytochem. Lett. 2012, 5, 165–169. [Google Scholar] [CrossRef]
- Rukachaisirikul, V.; Kannai, S.; Klaiklay, S.; Phongpaichit, S.; Sakayaroj, J. Rare 2-phenylpyran-4-ones from the seagrass-derived fungi Polyporales PSU-ES44 and PSU-ES83. Tetrahedron 2013, 69, 6981–6986. [Google Scholar] [CrossRef]
- Vicente, J.; Stewart, A.K.; van Wagoner, R.M.; Elliott, E.; Bourdelais, A.J.; Wright, J.L.C. Monacyclinones, new angucyclinone metabolites isolated from Streptomyces sp. M7_15 associated with the Puerto Rican sponge Scopalina ruetzleri. Mar. Drugs 2015, 13, 4682–4700. [Google Scholar] [CrossRef] [PubMed]
- Daferner, M.; Mensch, S.; Anke, T.; Sterner, O. Hypoxysordarin, a new sordarin derivative from Hypoxylon croceum. Z. Naturforsch. C 1999, 54, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Debbab, A.; Aly, A.H.; Proksch, P. Endophytes and associated marine derived fungi—Ecological and chemical perspectives. Fungal Divers. 2012, 57, 45–83. [Google Scholar] [CrossRef]
- Govinda Rajulu, M.B.; Thirunavukkarasu, N.; Suryanarayanan, T.S.; Venkatachalam, A. Endophytic fungi of marine algae and seagrasses: A novel source of chitin modifying enzymes. Mycosphere 2015, 6, 345–355. [Google Scholar]
- Elkhateeb, W.; Daba, G.M. Marine endophytes a natural novel source for a treasure of bioactive compounds. J. Adv. Microbiol. 2022, 5, 018. [Google Scholar]
- Gareth, J.E.B.; Stanley, S.J.; Pinruan, U. Marine endophyte sources of new chemical natural products: A review. Bot. Mar. 2008, 51, 163–170. [Google Scholar]
- Liu, Y.; Palaniveloo, K.; Alias, S.A.; Sathiya Seelan, J.S. Species diversity and secondary metabolites of Sarcophyton-associated marine fungi. Molecules 2021, 26, 3227. [Google Scholar] [CrossRef]
- Fernandes, L.; Kamat, S.Y.; Paknikar, S.K. New diterpenoids of the brown seaweed Stoechospermum marginatum: Structure of stoechospermol. Tetrahedron Lett. 1980, 21, 2249–2252. [Google Scholar]
- Gerwick, W.H.; Fenical, W.; Ven Engen, D.; Clardy, J. Isolation and structure of spatol, a potent inhibitor of cell reolication from the brown seaweed Spatoglossum schmittii. J. Am. Chem. Soc. 1980, 102, 7991–7993. [Google Scholar] [CrossRef]
- Erickson, K.L.; Beutler, J.A.; Gray, G.N.; Cardellina, J.H., II; Boyd, M.R. Majapolene A, a cytotoxic peroxide, and related sesquiterpenes from the red alga Laurencia majuscula. J. Nat. Prod. 1995, 58, 1848–1860. [Google Scholar] [CrossRef]
- Kimura, J.; Kamada, N.; Tsujimoto, Y. Fourteen chamigrane derivatives from a red alga, Laurencia nidifica. Bull. Chem. Soc. Jpn. 1999, 72, 289–292. [Google Scholar] [CrossRef]
- Cikoš, A.M.; Jurin, M.; Čož-Rakovac, R.; Gašo-Sokač, D.; Jokić, S.; Jerković, I. Update on sesquiterpenes from red macroalgae of the Laurencia genus and their biological activities (2015–2020). Algal Res. 2021, 56, 102330. [Google Scholar] [CrossRef]
- Nakamura, H.; Asari, T.; Murai, A.; Kan, Y.; Kondo, T.; Yoshida, K.; Ohizumi, Y. Zooxanthellatoxin-A, a potent vasoconstrictive 62-membered lactone from a symbiotic Dinoflagellate. J. Am. Chem. Soc. 1995, 117, 550–551. [Google Scholar] [CrossRef]
- Ermolenko, E.V.; Imbs, A.B.; Gloriozova, T.A.; Poroikov, V.V.; Sikorskaya, T.V.; Dembitsky, V.M. Chemical diversity of soft coral steroids and their pharmacological activities. Mar. Drugs 2020, 18, 613. [Google Scholar] [CrossRef]
- Damare, S.; Singh, P.; Raghukumar, S. Biotechnology of Marine Fungi. In Biology of Marine Fungi. Progress in Molecular and Subcellular Biology; Raghukumar, C., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; Volume 53. [Google Scholar] [CrossRef]
- Imbs, A.B.; Dembitsky, V.M. Coral Lipids. Mar. Drugs 2023, 21, 539. [Google Scholar] [CrossRef]
- Dembitsky, V.M. Fascinating furanosteroids and their pharmacological profile. Molecules 2023, 28, 5669. [Google Scholar] [CrossRef]
- Dembitsky, V.M. Biological activity and structural diversity of steroids containing aromatic rings, phosphate groups, or halogen atoms. Molecules 2023, 28, 5549. [Google Scholar] [CrossRef]
- Zhang, G.W.; Ma, X.Q.; Su, J.Y.; Zhang, K.; Kurihara, H.; Yao, X.S. Two new bioactive sesquiterpenes from the soft coral Sinularia sp. Nat. Prod. Res. 2006, 20, 659–664. [Google Scholar] [CrossRef] [PubMed]
- Lelong, H.; Ahond, A.; Chiaroni, A.; Poupat, C.; Riche, C.; Potier, P.; Pusset, J.; Pusset, M.; Laboute, P.; Menou, J.L. Invertébrés marins du lagon Néo-Calédonien, VIII. métabolites terpéniques de Xenia membranacea. J. Nat. Prod. 1987, 50, 203–210. [Google Scholar] [CrossRef]
- Bishara, A.; Rudi, A.; Goldberg, I.; Benayahu, Y.; Kashman, Y. Novaxenicins A–D and xeniolides I–K, seven new diterpenes from the soft coral Xenia novaebrittanniae. Tetrahedron 2006, 62, 12092–12097. [Google Scholar] [CrossRef]
- Rodríguez, A.D.; Boulanger, A.; Martínez, J.R.; Huang, S.D. Sesquiterpene lactones from the Caribbean Sea plume Pseudopterogorgia americana. J. Nat. Prod. 1998, 61, 451–455. [Google Scholar] [CrossRef]
- Rodríguez, A.D.; Soto, J.J. Pseudopterane and norcembrane diterpenoids from the Caribbean Sea plume Pseudopterogorgia acerosa. J. Nat. Prod. 1998, 61, 401–404. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, A.D.; Boulanger, A. Americanolides A−C, new guaianolide sesquiterpenes from the Caribbean Sea plume Pseudopterogorgia americana. J. Nat. Prod. 1996, 59, 653–657. [Google Scholar] [CrossRef]
- Berrueab, F.; Kerr, R.G. Diterpenes from gorgonian corals. Nat. Prod. Rep. 2009, 26, 681–710. [Google Scholar] [CrossRef] [PubMed]
- Iwagawa, T.; Amano, Y.; Nakatani, M.; Hase, T. New xenia diterpenoids from a soft coral, Xenia species containing fatty acyl side chains. Bull. Chem. Soc. Jpn. 1996, 69, 1309–1312. [Google Scholar] [CrossRef]
- König, G.M.; Coll, J.C.; Bowden, B.F.; Gulbis, J.M.; MacKay, M.F.; Labarre, S.C.; Laurent, D. The structure determination of a xenicane diterpene from Xenia garciae. J. Nat. Prod. 1989, 52, 294–299. [Google Scholar] [CrossRef]
- Ng, S.Y.; Phan, C.S.; Ishii, T.; Kamada, T.; Hamada, T.; Santhanaraju Vairappan, C. Terpenoids from marine soft coral of the genus Xenia in 1977 to 2019. Molecules 2020, 25, 5386. [Google Scholar] [CrossRef]
- Almourabit, A.; Ahond, A.; Chiaroni, A.; Poupat, C.; Riche, C.; Potier, P.; Laboute, P.; Menou, J.L. Invertébrés marins du lagon Néo-Calédonien, IX. Havannachlorhydrines, nouveaux métabolites de Xenia membranacea: Étude structurale et configuration absolue. J. Nat. Prod. 1988, 51, 282–292. [Google Scholar] [CrossRef]
- Schmitt, S.; Tsai, P.; Bell, J.; Fromont, J.; Ilan, M.; Lindquist, N. Assessing the complex sponge microbiota: Core, variable and species-specific bacterial communities in marine sponges. ISME J. 2012, 6, 564–576. [Google Scholar] [CrossRef] [PubMed]
- Dat, T.T.H.; Steinert, G.; Cuc, N.T.K.; Smidt, H.; Sipkema, D. Archaeal and bacterial diversity and community composition from 18 phylogenetically divergent sponge species in Vietnam. Peer J. 2018, 6, e4970. [Google Scholar] [CrossRef]
- Steinert, G.; Rohde, S.; Janussen, D.; Blaurock, C.; Schupp, P.J. Host-specific assembly of sponge-associated prokaryotes at high taxonomic ranks. Sci. Rep. 2017, 7, 2542. [Google Scholar] [CrossRef]
- Taylor, J.A.; Palladino, G.; Wemheuer, B.; Steinert, G.; Sipkema, D.; Williams, T.J. Phylogeny resolved, metabolism revealed: Functional radiation within a widespread and divergent clade of sponge symbionts. ISME J. 2021, 15, 503–519. [Google Scholar] [CrossRef] [PubMed]
- Moitinho-Silva, L.; Nielsen, S.; Amir, A.; Gonzalez, A.; Ackermann, G.L.; Cerrano, C. The sponge microbiome project. Giga Science 2017, 6, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Thomas, T.; Moitinho-Silva, L.; Lurgi, M.; Bjork, J.R.; Easson, C.; Astudillo-Garcia, C. Diversity, structure and convergent evolution of the global sponge microbiome. Nat. Commun. 2016, 7, 11870. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Tietjen, I.; Chen, M.; Williams, D.E.; Daoust, J.; Brockman, M.A.; Andersen, R.J. Sesterterpenoids isolated from the sponge Phorbas sp. activate latent HIV-1 provirus expression. J. Org. Chem. 2016, 81, 11324–11334. [Google Scholar] [CrossRef]
- Chianese, G.; Yu, H.B.; Yang, F.; Sirignano, C.; Luciano, P.; Han, B.N.; Khan, S.; Lin, H.W.; Taglialatela-Scafati, O. PPAR Modulating polyketides from a Chinese Plakortis simplex and clues on the origin of their chemodiversity. J. Org. Chem. 2016, 81, 5135–5143. [Google Scholar] [CrossRef]
- De Marino, S.; Ummarino, R.; D’Auria, M.V.; Chini, M.G.; Bifulco, G.; Renga, B.; D’Amore, C.; Fiorucci, S.; Debitus, C.; Zampella, A. Theonellasterols and conicasterols from Theonella swinhoei. Novel marine natural ligands for human nuclear receptors. J. Med. Chem. 2011, 54, 3065–3075. [Google Scholar] [CrossRef]
- Cueto, M.; D’Croz, L.; Mate, J.L.; San-Martın, A.; Darias, J. Elysiapyrones from Elysia diomedea. Do such metabolites evidence an enzymatically assisted electrocyclization cascade for the biosynthesis of their bicyclo [4.2.0]octane core? Org. Lett. 2005, 7, 415–418. [Google Scholar] [CrossRef] [PubMed]
- Olatunji, T.L.; Odebunmi, C.A.; Adetunji, A.E. Biological activities of limonoids in the Genus Khaya (Meliaceae): A review. Future J. Pharm. Sci. 2021, 7, 74. [Google Scholar] [CrossRef]
- Joshi, R.K. Bioactive usual and unusual triterpenoids derived from natural sources used in traditional medicine. Chem. Biodiver. 2023, 20, e202200853. [Google Scholar] [CrossRef] [PubMed]
- Dembitsky, V.M.; Dzhemileva, L.; Gloriozova, T.; D’yakonov, V.D. Natural and synthetic drugs used for the treatment of the dementia. Biochem. Biophys. Res. Commun. 2020, 524, 772–783. [Google Scholar] [CrossRef] [PubMed]
- Dembitsky, V.M. In Silico Prediction of steroids and triterpenoids as potential regulators of lipid metabolism. Mar. Drugs 2021, 19, 650. [Google Scholar] [CrossRef] [PubMed]
- Gezen-Ak, D.; Dursun, E. Vitamin D, a secosteroid hormone and its multifunctional receptor, vitamin D receptor, in Alzheimer’s type neurodegeneration. J. Alzheimer’s Disease 2023, 95, 1273–1299. [Google Scholar] [CrossRef] [PubMed]
- Karampela, I.; Sakelliou, A.; Vallianou, N. Vitamin D and obesity: Current evidence and controversies. Curr. Obes. Rep. 2021, 10, 162–180. [Google Scholar] [CrossRef]
- Amrein, K.; Scherkl, M.; Hoffmann, M. Vitamin D deficiency 2.0: An update on the current status worldwide. Eur. J. Clin. Nutr. 2020, 74, 1498–1513. [Google Scholar] [CrossRef]
- Duecker, F.L.; Reuß, F.; Heretsch, F. Rearranged ergostane-type natural products: Chemistry, biology, and medicinal aspects. Org. Biomol. Chem. 2019, 17, 1624–1633. [Google Scholar] [CrossRef]
- Wu, J.; Zhao, J.; Zhang, T.; Gu, Y.; Khan, I.A.; Zou, Z.; Xu, Q. Naturally occurring physalins from the genus Physalis: A review. Phytochemistry 2021, 191, 112925. [Google Scholar] [CrossRef]
- Grishko, V.V.; Galaiko, N.V. Structural diversity, natural sources and pharmacological potential of naturally occurring A-seco-triterpenoids studies. Nat. Prod. Chem. 2016, 51, 51–149. [Google Scholar]
- Peng, X.R.; Wang, X.; Zhou, L.; Hou, B.; Zuo, Z.L.; Qiu, M.H. Ganocochlearic acid A, a rearranged hexanorlanostane triterpenoid, and cytotoxic triterpenoids from the fruiting bodies of Ganoderma cochlear. RSC Adv. 2015, 5, 95212–95222. [Google Scholar] [CrossRef]
- Peng, X.R.; Liu, J.Q.; Wang, C.F.; Li, X.Y.; Shu, Y.; Zhou, L.; Qiu, M.H. Hepatoprotective effects of triterpenoids from Ganoderma cochlear. J. Nat. Prod. 2014, 77, 737–743. [Google Scholar] [CrossRef] [PubMed]
- Galappaththi, M.C.A.; Patabendige, N.M.; Premarathne, B.M.; Hapuarachchi, K.K.; Tibpromma, S.; Dai, D.-Q.; Suwannarach, N.; Rapior, S.; Karunarathna, S.C. A review of Ganoderma triterpenoids and their bioactivities. Biomolecules 2023, 13, 24. [Google Scholar] [CrossRef] [PubMed]
- Hill, R.A.; Connolly, V.M. Triterpenoids. Nat. Prod. Rep. 2020, 37, 962–998. [Google Scholar] [CrossRef]
- Leon, F.; Valencia, M.; Rivera, A.; Nieto, I.; Quintana, J.; Estevez, F.; Bermejo, J. Novel cytostatic lanostanoid triterpenes from Ganoderma australe. Helv. Chim. Acta 2003, 86, 3088–3095. [Google Scholar] [CrossRef]
- Yoshikawa, K.; Nishimura, N.; Bando, S.; Arihara, S.; Matsumura, E.; Katayama, S. New lanostanoids, elfvingic acids A−H, from the fruit body of Elfvingia applanata. J. Nat. Prod. 2002, 65, 548–552. [Google Scholar] [CrossRef]
- Kleinwächter, P.; Anh, N.; Kiet, T.T.; Schlegel, B.; Dahse, H.M.; Härtl, A.; Gräfe, U. Colossolactones, new triterpenoid metabolites from a Vietnamese mushroom Ganoderma colossum. J. Nat. Prod. 2001, 64, 236–239. [Google Scholar] [CrossRef]
- Hu, Z.; Wu, Y.; Xie, S.; Sun, W.; Guo, Y.; Li, X.N.; Liu, J.; Li, H.; Wang, J.; Luo, Z.; et al. Phomopsterones A and B, two functionalized ergostane-type steroids from the endophytic fungus Phomopsis sp. TJ507A. Org. Lett. 2017, 19, 258–261. [Google Scholar] [CrossRef]
- Han, J.J.; Bao, L.; Tao, Q.Q.; Yao, Y.J.; Liu, X.Z.; Yin, W.B.; Liu, H.W. Gloeophyllins A–J, cytotoxic ergosteroids with various skeletons from a Chinese Tibet fungus Gloeophyllum abietinum. Org. Lett. 2015, 17, 2538–2541. [Google Scholar] [CrossRef]
- Zhao, Z.Z.; Chen, H.P.; Huang, Y.; Zhang, S.B.; Li, Z.H.; Feng, T.; Liu, J.K. Bioactive polyketides and 8,14-seco-ergosterol from fruiting bodies of the ascomycete Daldinia childiae. Phytochemistry 2017, 142, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Ren, J.; Han, J.; Bao, L.; Li, L.; Yao, Y.; Sun, C.; Zhou, B.; Liu, H. Ganoboninketals A–C, antiplasmodial 3,4-seco-27-norlanostane triterpenes from Ganoderma boninense Pat. J. Nat. Prod. 2014, 77, 1847–1852. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Chen, Y.; Chen, S.; Liu, Y.; Lu, Y.; Chen, D.; Lin, Y.; Huang, X. Aspterpenacids A and B, two sesterterpenoids from a Mangrove endophytic fungus Aspergillus terreus H010. Org. Lett. 2016, 18, 1406–1409. [Google Scholar] [CrossRef] [PubMed]
- Costa, S.; Giovannini, P.P.; Fantin, G.; Medici, A.; Pedrini, P. New 9,10-secosteroids from biotransformations of hyodeoxycholic acid with Rhodococcus spp. Helv. Chim. Acta 2013, 96, 1062–1071. [Google Scholar] [CrossRef]
- Chen, Z.M.; Yang, X.Y.; Fan, Q.Y.; Lia, Z.H.; Weia, K.; Chena, H.P.; Fenga, T.; Liu, J.K. Three novel degraded steroids from cultures of the Basidiomycete Antrodiella albocinnamomea. Steroids 2014, 87, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.H.; Luo, X.D.; Ma, Y.B.; Liu, J.K.; Wu, D.G.; Zhao, B.; Lu, Y.; Zheng, Q.T. Two novel secoergosterols from the fungus Tylopilus plumbeoviolaceus. J. Nat. Prod. 2000, 63, 534–536. [Google Scholar] [CrossRef] [PubMed]
- Phillips, K.M.; Horst, R.L.; Koszewski, N.J.; Simon, R.R. Vitamin D4 in mushrooms. PLoS ONE 2012, 7, e40702. [Google Scholar] [CrossRef]
- Xiao, Z.; Huang, H.; Shao, C.; Xia, X.; Ma, L.; Huang, X.; Lu, Y.; Lin, Y.; Long, Y.; She, Z. Asperterpenols A and B, new sesterterpenoids isolated from a mangrove endophytic fungus Aspergillus sp. 085242. Org. Lett. 2013, 15, 2522–2525. [Google Scholar] [CrossRef]
- Zhou, Y.M.; Mandi, A.; Debbab, A.; Wray, V.; Schulz, B.; Muller, W.E.G.; Lin, W.H.; Proksch, P.; Kurtan, T.; Aly, A.H. New austalides from the sponge-associated fungus Aspergillus sp. Eur. J. Org. Chem. 2011, 30, 6009–6019. [Google Scholar] [CrossRef]
- Zhou, Y.M.; Debbab, A.; Wray, V.; Lin, W.H.; Schulz, B.; Trepos, R.; Pile, C.; Hellio, C.; Proksch, P.; Aly, A.H. Marine bacterial inhibitors from the sponge-derived fungus Aspergillus sp. Tetrahedron Lett. 2014, 55, 2789–2792. [Google Scholar] [CrossRef]
- Nong, X.H.; Wang, Y.F.; Zhang, X.Y.; Zhou, M.P.; Xu, X.Y.; Qi, S.H. Territrem and butyrolactone derivatives from a marine-derived fungus Aspergillus terreus. Mar. Drugs 2014, 12, 6113–6124. [Google Scholar] [CrossRef] [PubMed]
- Kumla, D.; Aung, T.S.; Buttachon, S.; Dethoup, T.; Gales, L.; Pereira, J.A.; Inácio, Â.; Costa, P.M. A new dihydrochromone dimer and other secondary metabolites from cultures of the marine sponge-associated fungi Neosartorya fennelliae KUFA 0811 and Neosartorya tsunodae KUFC 9213. Mar. Drugs 2017, 15, 375. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.M.; Li, X.M.; Meng, L.; Li, C.S.; Huang, C.G.; Wang, B.G. 7-Nor-ergosterolide, a pentalactone-containing norsteroid and related steroids from the marine-derived endophytic Aspergillus ochraceus EN-31. J. Nat. Prod. 2010, 73, 1780–1784. [Google Scholar] [CrossRef] [PubMed]
- Onodera, H.; Ichimura, M.; Baba, K.; Agatsuma, T.; Sasho, S.; Suzuki, M.; Iwamoto, S.; Kakita, S. Nerve Trunk Cell Propagation Accelerator. International Patent Application No. PCT/JP2009/051417; WO 2009096445A1, 6 August 2009. [Google Scholar]
- Liu, H.; Li, X.M.; Liu, Y.; Zhang, P.; Wang, J.N.; Wang, B.G. Chermesins A–D: Meroterpenoids with a drimane-type spirosesquiterpene skeleton from the marine algal-derived endophytic fungus Penicillium chermesinum EN-480. J. Nat. Prod. 2016, 79, 806–811. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Ko, S.K.; Kim, H.M.; Kim, G.H.; Son, S.; Kim, G.S.; Hwang, G.J. Stachybotrysin, an osteoclast differentiation inhibitor from the marine-derived fungus Stachybotrys sp. KCB13F013. J. Nat. Prod. 2016, 79, 2703–2708. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ye, D.; Shao, Z.; Cui, C.; Che, Y. A sterol and spiroditerpenoids from a Penicillium sp. isolated from a deep sea sediment sample. Mar. Drugs 2012, 10, 497–508. [Google Scholar] [CrossRef]
- Li, X.; Miao, F.; Liang, X.; Ji, N. Meroterpenes from an algicolous strain of Penicillium echinulatum. Magn. Reson. Chem. 2014, 52, 247–250. [Google Scholar] [CrossRef]
- Fukuda, T.; Tomoda, H.; Kurihara, Y.; Kanamoto, A. Terretonin G, a new sesterterpenoid antibiotic from marine-derived Aspergillus sp. OPMF00272. J. Antibiot. 2014, 67, 593–595. [Google Scholar] [CrossRef]
- Trisuwan, K.; Rukachaisirikul, V.; Kaewpet, M.; Phongpaichit, S.; Hutadilok-Towatana, N.; Preedanon, S.; Sakayaroj, J. Sesquiterpene and xanthone derivatives from the sea fan-derived fungus Aspergillus sydowii PSU-F154. J. Nat. Prod. 2012, 74, 1663–1667. [Google Scholar] [CrossRef]
- Kong, F.D.; Ma, Q.Y.; Huang, S.Z.; Wang, P.; Wang, J.F.; Zhou, L.M.; Yuan, J.Z.; Dai, H.F.; Zhao, Y.X. Chrodrimanins K–N and related meroterpenoids from the fungus Penicillium sp. SCS-KFD09 isolated from a marine worm, Sipunculus nudus. J. Nat. Prod. 2017, 80, 1039–1047. [Google Scholar]
- Gomes, N.M.; Bessa, L.J.; Buttachon, S.; Costa, P.M.; Buaruang, J.; Dethoup, T.; Silva, A.M.; Kijjoa, A. Antibacterial and antibiofilm activities of tryptoquivalines and meroditerpenes isolated from the marine-derived fungi Neosartorya paulistensis, N. laciniosa, N. tsunodae, and the soil fungi N. fischeri and N. siamensis. Mar. Drugs 2014, 12, 822–839. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).