Comparative Analysis of Cyanotoxins in Fishponds in Nigeria and South Africa
Abstract
1. Introduction
2. Methodology
2.1. Study Area and Sampling Sites
2.2. Water Sampling
2.3. Cyanotoxin Extraction in Water Samples
2.4. LCMS Analyses
2.5. Data Analyses
3. Results and Discussion
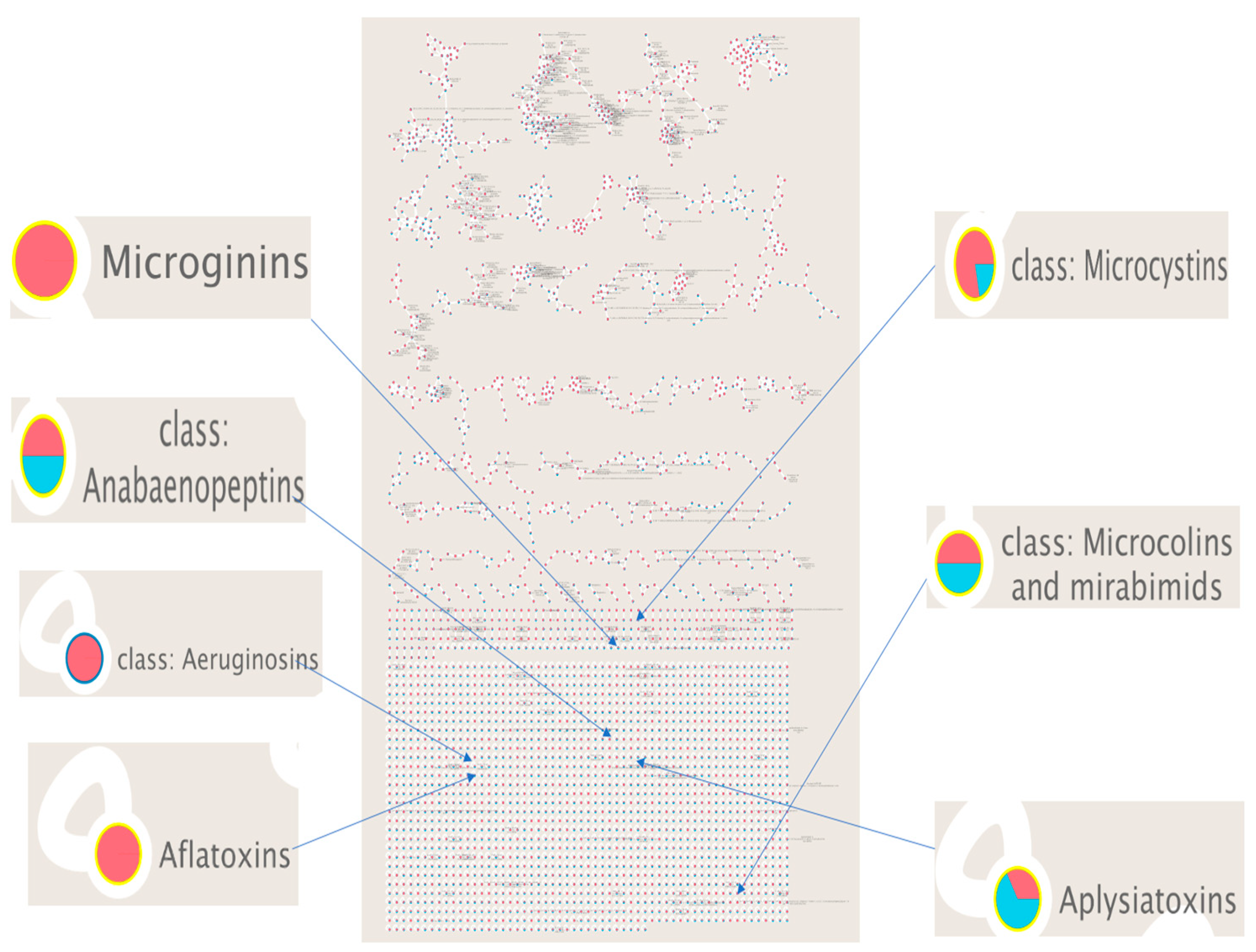
3.1. Molecular Network
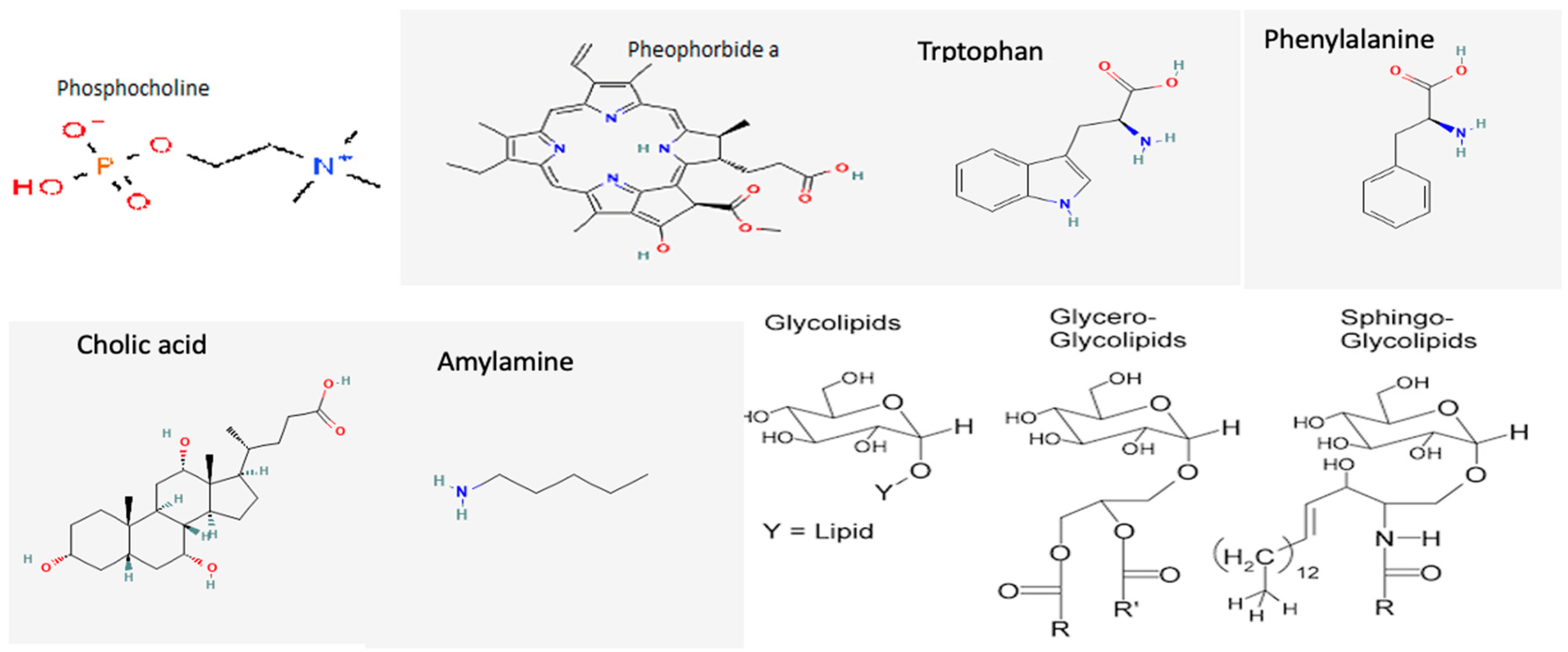
3.2. Cyanobacterial Toxins and Other Bioactive Metabolites
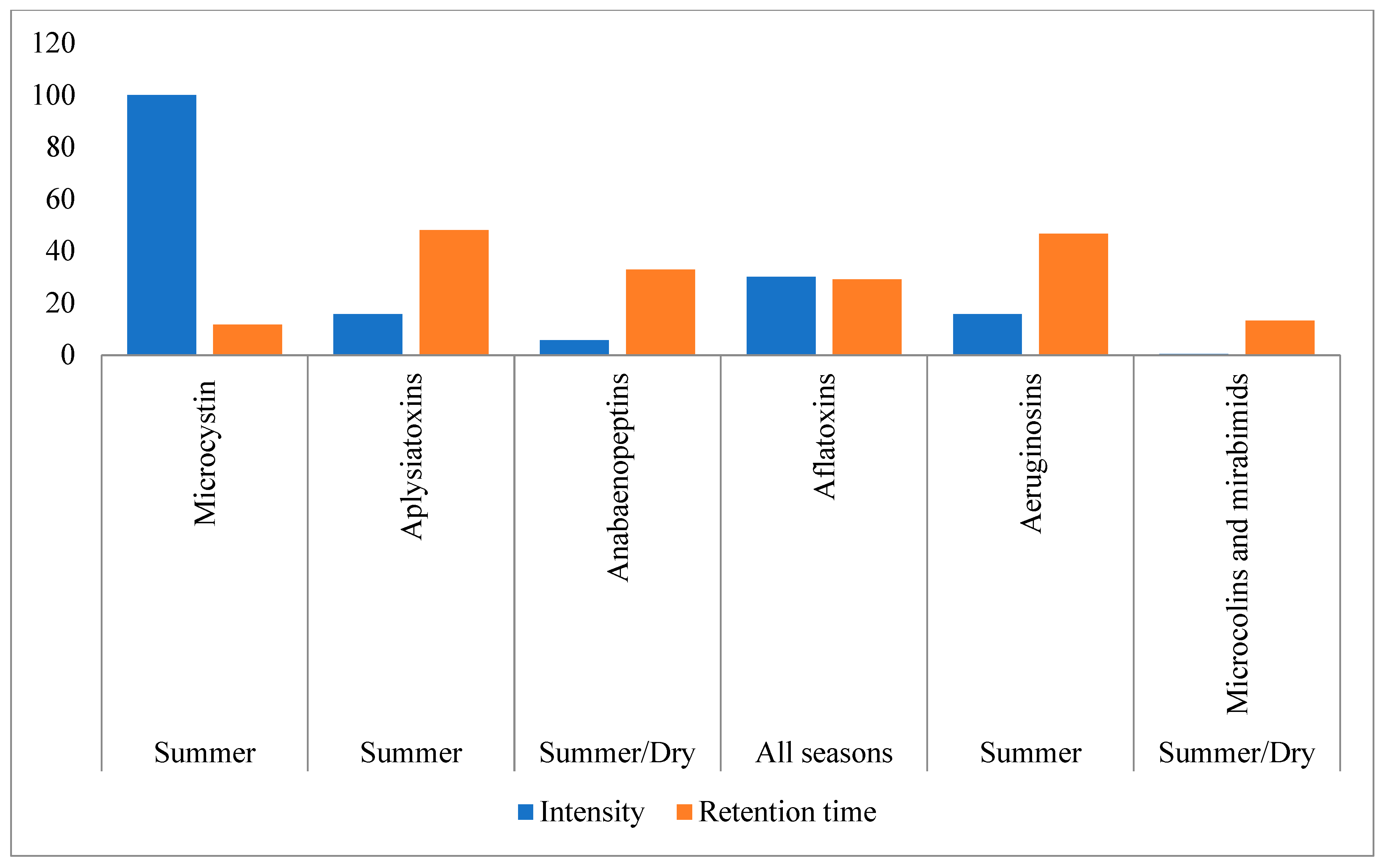
3.3. Seasonal and Regional Dynamics of Cyanotoxins in Fishponds
4. Implication
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ciebiada, M.; Kubiak, K.; Daroch, M. Modifying the Cyanobacterial Metabolism as a Key to Efficient Biopolymer Production in Photosynthetic Microorganisms. Int. J. Mol. Sci. 2020, 21, 7204. [Google Scholar] [CrossRef]
- Huertas, M.J.; Mallén-Ponce, M.J. Dark side of cyanobacteria: Searching for strategies to control blooms. Microb. Biotechnol. 2022, 15, 1321–1323. [Google Scholar] [CrossRef]
- Zuo, Z. Emission of cyanobacterial volatile organic compounds and their roles in blooms. Front. Microbiol. 2023, 14, 1097712. [Google Scholar] [CrossRef]
- Bhardwaj, A.; Singh, P.; Gupta, N.; Bhattacharjee, S.; Srivastava, A.; Parida, A.; Mishra, A.K. Cyanobacteria: A Key Player in Nutrient Cycling. In Cyanobacteria; Academic Press: Cambridge, MA, USA, 2024; pp. 579–596. [Google Scholar] [CrossRef]
- Arora, K.; Kumar, P.; Bose, D.; Li, X.; Kulshrestha, S. Potential applications of algae in biochemical and bioenergy sector. 3 Biotech 2021, 11, 296. [Google Scholar] [CrossRef]
- Keller, R.J.; Porter, W.; Goli, K.; Rosenthal, R.; Butler, N.; Jones, J.A. Biologically-Based and Physiochemical Life Support and In Situ Resource Utilization for Exploration of the Solar System—Reviewing the Current State and Defining Future Development Needs. Life 2021, 11, 844. [Google Scholar] [CrossRef]
- Wang, Z.; Akbar, S.; Sun, Y.; Gu, L.; Zhang, L.; Lyu, K.; Huang, Y.; Yang, Z. Cyanobacterial dominance and succession: Factors, mechanisms, predictions, and managements. J. Environ. Manag. 2021, 297, 113281. [Google Scholar] [CrossRef]
- He, X.; Liu, Y.-L.; Conklin, A.; Westrick, J.; Weavers, L.K.; Dionysiou, D.D.; Lenhart, J.J.; Mouser, P.J.; Szlag, D.; Walker, H.W. Toxic cyanobacteria and drinking water: Impacts, detection, and treatment. Harmful Algae 2016, 54, 174–193. [Google Scholar] [CrossRef] [PubMed]
- Metcalf, J.S.; Codd, G.A. Co-Occurrence of Cyanobacteria and Cyanotoxins with Other Environmental Health Hazards: Impacts and Implications. Toxins 2020, 12, 629. [Google Scholar] [CrossRef]
- Drobac, D.; Tokodi, N.; Lujić, J.; Marinović, Z.; Subakov-Simić, G.; Dulić, T.; Važić, T.; Nybom, S.; Meriluoto, J.; Codd, G.A.; et al. Cyanobacteria and cyanotoxins in fishponds and their effects on fish tissue. Harmful Algae 2016, 55, 66–76. [Google Scholar] [CrossRef]
- Chia, M.A.; Abdulwahab, R.; Ameh, I.; Balogun, J.K.; Auta, J. Farmed tilapia as an exposure route to microcystins in Zaria-Nigeria: A seasonal investigation. Environ. Pollut. 2021, 271, 116366. [Google Scholar] [CrossRef]
- de Almeida, C.; Passos, L.S.; de Freitas, P.N.N.; de Souza, A.O.; Pinto, E. Impacts of cyanobacterial metabolites on fish: Socioeconomic and environmental considerations. Rev. Aquac. 2024. [Google Scholar] [CrossRef]
- de Lima Pinheiro, M.M.; Santos, B.L.T.; Filho, J.V.D.; Pedroti, V.P.; Cavali, J.; dos Santos, R.B.; Nishiyama, A.C.O.C.; Guedes, E.A.C.; de Vargas Schons, S. First monitoring of cyanobacteria and cyanotoxins in freshwater from fish farms in Rondônia state, Brazil. Heliyon 2023, 9, e18518. [Google Scholar] [CrossRef]
- Vrba, J.; Šorf, M.; Nedoma, J.; Benedová, Z.; Kröpfelová, L.; Šulcová, J.; Tesařová, B.; Musil, M.; Pechar, L.; Potužák, J.; et al. Top-down and bottom-up control of plankton structure and dynamics in hypertrophic fishponds. Hydrobiologia 2023, 851, 1095–1111. [Google Scholar] [CrossRef]
- Jones, M.R.; Pinto, E.; Torres, M.A.; Dörr, F.; Mazur-Marzec, H.; Szubert, K.; Tartaglione, L.; Dell’Aversano, C.; Miles, C.O.; Beach, D.G.; et al. CyanoMetDB, a comprehensive public database of secondary metabolites from cyanobacteria. Water Res. 2021, 196, 117017. [Google Scholar] [CrossRef]
- Pei, Y.; Xu, R.; Hilt, S.; Chang, X. Effects of Cyanobacterial Secondary Metabolites on Phytoplankton Community Succession. In Co-Evolution of Secondary Metabolites; Springer: Berlin/Heidelberg, Germany, 2020; pp. 323–344. [Google Scholar] [CrossRef]
- Passos, L.S.; Jacinavicius, F.R.; Geraldes, V.; de Freitas, P.N.N.; Da Silva, G.H.; de Almeida, C.; do Carmo Alves, A.P.; Orlando, T.M.; da Silva Cerozi, B.; Martinez, D.S.T.; et al. Ecotoxicological assessment of guanitoxin-producing cyanobacteria in Danio rerio and Daphnia similis. Chemosphere 2023, 332, 138846. [Google Scholar] [CrossRef]
- Buratti, F.M.; Manganelli, M.; Vichi, S.; Stefanelli, M.; Scardala, S.; Testai, E.; Funari, E. Cyanotoxins: Producing organisms, occurrence, toxicity, mechanism of action and human health toxicological risk evaluation. Arch. Toxicol. 2017, 91, 1049–1130. [Google Scholar] [CrossRef]
- Hilborn, E.D.; Roberts, V.A.; Backer, L.; DeConno, E.; Egan, J.S.; Hyde, J.B.; Nicholas, D.C.; Wiegert, E.J.; Billing, L.M.; DiOrio, M.; et al. Algal bloom-associated disease outbreaks among users of freshwater lakes-United States, 2009–2010. Centers for Disease Control and Prevention (CDC). Morb. Mortal. Wkly Rep. 2014, 10, 11–15. [Google Scholar]
- Lad, A.; Breidenbach, J.D.; Su, R.C.; Murray, J.; Kuang, R.; Mascarenhas, A.; Najjar, J.; Patel, S.; Hegde, P.; Youssef, M.; et al. As We Drink and Breathe: Adverse Health Effects of Microcystins and Other Harmful Algal Bloom Toxins in the Liver, Gut, Lungs and Beyond. Life 2022, 12, 418. [Google Scholar] [CrossRef]
- Niture, S.; Gadi, S.; Qi, Q.; Rios-Colon, L.; Khatiwada, S.; Vandana; Fernando, R.A.; Levine, K.E.; Kumar, D. Cyanotoxins Increase Cytotoxicity and Promote Nonalcoholic Fatty Liver Disease Progression by Enhancing Cell Steatosis. Toxins 2023, 15, 411. [Google Scholar] [CrossRef]
- Funari, E.; Testai, E. Human Health Risk Assessment Related to Cyanotoxins Exposure. Crit. Rev. Toxicol. 2008, 38, 97–125. [Google Scholar] [CrossRef]
- Lee, J.; Lee, S.; Jiang, X. Cyanobacterial Toxins in Freshwater and Food: Important Sources of Exposure to Humans. Annu. Rev. Food Sci. Technol. 2017, 8, 281–304. [Google Scholar] [CrossRef]
- Gärtner, G.; Stoyneva-Gärtner, M.; Uzunov, B. Algal Toxic Compounds and Their Aeroterrestrial, Airborne and other Extremophilic Producers with Attention to Soil and Plant Contamination: A Review. Toxins 2021, 13, 322. [Google Scholar] [CrossRef]
- Chia, M.A.; Kwaghe, M.J. Microcystins contamination of surface water supply sources in Zaria-Nigeria. Environ. Monit. Assess. 2015, 187, 606. [Google Scholar] [CrossRef]
- Chia, A.M.; Oniye, S.J.; Ladan, Z.; Lado, Z.; Pila, A.E.; Inekwe, V.U.; Mmerole, J.U. A Survey for the Presence of Microcystins in Aquaculture ponds in Zaria, Northern-Nigeria: Possible Public Health Implication. Afr. J. Biotechnol. 2009, 8, 22. [Google Scholar] [CrossRef]
- Prasannan, C.B.; Jaiswal, D.; Davis, R.; Wangikar, P.P. An improved method for extraction of polar and charged metabolites from cyanobacteria. PLoS ONE 2018, 13, e0204273. [Google Scholar] [CrossRef] [PubMed]
- Dührkop, K.; Shen, H.; Meusel, M.; Rousu, J.; Böcker, S. Searching molecular structure databases with tandem mass spectra using CSI:FingerID. Proc. Natl. Acad. Sci. USA 2015, 112, 12580–12585. [Google Scholar] [CrossRef] [PubMed]
- Dührkop, K.; Fleischauer, M.; Ludwig, M.; Aksenov, A.A.; Melnik, A.V.; Meusel, M.; Dorrestein, P.C.; Rousu, J.; Böcker, S. SIRIUS 4: A rapid tool for turning tandem mass spectra into metabolite structure information. Nat. Methods 2019, 16, 299–302. [Google Scholar] [CrossRef]
- Dührkop, K.; Nothias, L.-F.; Fleischauer, M.; Reher, R.; Ludwig, M.; Hoffmann, M.A.; Petras, D.; Gerwick, W.H.; Rousu, J.; Dorrestein, P.C.; et al. Systematic classification of unknown metabolites using high-resolution fragmentation mass spectra. Nat. Biotechnol. 2021, 39, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; Wang, M.; Leber, C.A.; Nothias, L.F.; Reher, R.; Kang, K.B.; van der Hooft, J.J.J.; Dorrestein, P.C.; Gerwick, W.H.; Cottrell, G.W. NPClassifier: A Deep Neural Network-Based Structural Classification Tool for Natural Products. J. Nat. Prod. 2021, 84, 2795–2807. [Google Scholar] [CrossRef] [PubMed]
- Damiani, T.; Heuckeroth, S.; Smirnov, A.; Mokshyna, O.; Brungs, C.; Korf, A.; Smith, J.; Stincone, P.; Dreolin, N.; Nothias, L.-F.; et al. Mass spectrometry Data Processing in MZmine 3: Feature detection and annotation. ChemRxiv 2023. [Google Scholar] [CrossRef]
- Libis, V.; Antonovsky, N.; Zhang, M.; Shang, Z.; Montiel, D.; Maniko, J.; Ternei, M.A.; Calle, P.Y.; Lemetre, C.; Owen, J.G.; et al. Uncovering the biosynthetic potential of rare metagenomic DNA using co-occurrence network analysis of targeted sequences. Nat. Commun. 2019, 10, 3848. [Google Scholar] [CrossRef]
- Narduzzi, L.; Stanstrup, J.; Mattivi, F. Comparing Wild American Grapes with Vitis vinifera: A Metabolomics Study of Grape Composition. J. Agric. Food Chem. 2015, 63, 6823–6834. [Google Scholar] [CrossRef]
- Ibrahim, M.; Chen, D.; Ibrahim, S.S.; Danjaji, H.A.; Tian, R. Microalgal bloom and cyanotoxin proliferation in freshwaters: Cycle and dynamics, impacts and remediation strategies. IRESPUB J. Environ. Mater. Sci. 2022, 2, 1–10. [Google Scholar]
- Ali, O.; Szabó, A. Review of Eukaryote Cellular Membrane Lipid Composition, with Special Attention to the Fatty Acids. Int. J. Mol. Sci. 2023, 24, 15693. [Google Scholar] [CrossRef]
- Wardhan, R.; Mudgal, P. Introduction to Biomembranes. In Textbook of Membrane Biology; Springer: Singapore; pp. 1–28. [CrossRef]
- Gull, M.; Pasek, M.A. The Role of Glycerol and Its Derivatives in the Biochemistry of Living Organisms, and Their Prebiotic Origin and Significance in the Evolution of Life. Catalysts 2021, 11, 86. [Google Scholar] [CrossRef]
- Summers, K.M.; Krempa, H.M.; Garrett, J.D. Central Midwest Water Science Center—Harmful Algal Blooms team. US Geol. Surv. Fact Sheet 2022, 3011, 4. [Google Scholar] [CrossRef]
- Marie, B.; Gallet, A. Fish metabolome from sub-urban lakes of the Paris area (France) and potential influence of noxious metabolites produced by cyanobacteria. Chemosphere 2022, 296, 134035. [Google Scholar] [CrossRef] [PubMed]
- Zastepa, A.; Westrick, J.A.; Liang, A.; Birbeck, J.A.; Furr, E.; Watson, L.C.; Stockdill, J.L.; Ramakrishna, B.S.; Crevecoeur, S. Broad screening of toxic and bioactive metabolites in cyanobacterial and harmful algal blooms in Lake of the Woods (Canada and USA), 2016–2019. J. Great Lakes Res. 2023, 49, 134–146. [Google Scholar] [CrossRef]
- Kust, A.; Řeháková, K.; Vrba, J.; Maicher, V.; Mareš, J.; Hrouzek, P.; Chiriac, M.-C.; Benedová, Z.; Tesařová, B.; Saurav, K. Insight into Unprecedented Diversity of Cyanopeptides in Eutrophic Ponds Using an MS/MS Networking Approach. Toxins 2020, 12, 561. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.D.; Franklin, R.B.; Garman, G.; McIninch, S.; Porter, A.J.; Bukaveckas, P.A. Exposure to the Cyanotoxin Microcystin Arising from Interspecific Differences in Feeding Habits among Fish and Shellfish in the James River Estuary, Virginia. Environ. Sci. Technol. 2014, 48, 5194–5202. [Google Scholar] [CrossRef] [PubMed]
- Chorus, I.; Fastner, J.; Welker, M. Cyanobacteria and Cyanotoxins in a Changing Environment: Concepts, Controversies, Challenges. Water 2021, 13, 2463. [Google Scholar] [CrossRef]
- Massey, I.Y.; Al Osman, M.; Yang, F. An overview on cyanobacterial blooms and toxins production: Their occurrence and influencing factors. Toxin Rev. 2022, 41, 326–346. [Google Scholar] [CrossRef]
- Filatova, D.; Picardo, M.; Núñez, O.; Farré, M. Analysis, levels and seasonal variation of cyanotoxins in freshwater ecosystems. Trends Environ. Anal. Chem. 2020, 26, e00091. [Google Scholar] [CrossRef]
- Burford, M.; Carey, C.; Hamilton, D.; Huisman, J.; Paerl, H.; Wood, S.; Wulff, A. Perspective: Advancing the research agenda for improving understanding of cyanobacteria in a future of global change. Harmful Algae 2020, 91, 101601. [Google Scholar] [CrossRef]
- Mohamed, Z.; Ahmed, Z.; Bakr, A.; Hashem, M.; Alamri, S. Detection of free and bound microcystins in tilapia fish from Egyptian fishpond farms and its related public health risk assessment. J. Consum. Prot. Food Saf. 2020, 15, 37–47. [Google Scholar] [CrossRef]
- Azevedo, S.M.F.O.; Carmichael, W.W.; Jochimsen, E.M.; Rinehart, K.L.; Lau, S.; Shaw, G.R.; Eaglesham, G.K. Human intoxication by microcystins during renal dialysis treatment in Caruaru—Brazil. Toxicology 2002, 181–182, 441–446. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Cyanobacterial Toxins: Microcystins; World Health Organization: Geneve, Switzerland, 2020. [Google Scholar]
- Mutoti, M.; Gumbo, J.; Jideani, A.I.O. Occurrence of cyanobacteria in water used for food production: A review. Phys. Chem. Earth Parts A/B/C 2022, 125, 103101. [Google Scholar] [CrossRef]

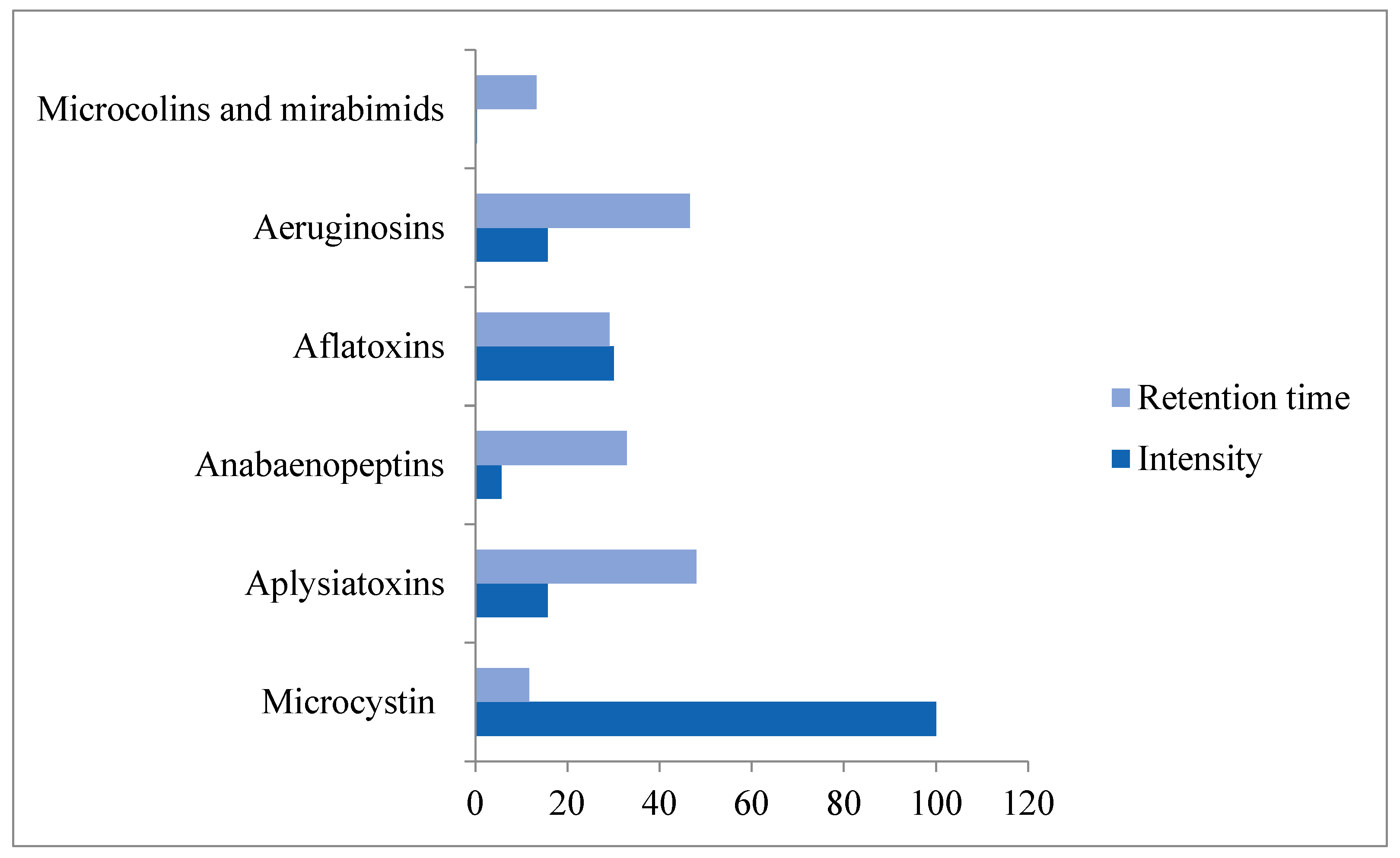
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bassey, O.J.; Gumbo, J.R.; Mujuru, M.; Adeyemi, A.; Dondofema, F. Comparative Analysis of Cyanotoxins in Fishponds in Nigeria and South Africa. Microbiol. Res. 2024, 15, 447-456. https://doi.org/10.3390/microbiolres15020030
Bassey OJ, Gumbo JR, Mujuru M, Adeyemi A, Dondofema F. Comparative Analysis of Cyanotoxins in Fishponds in Nigeria and South Africa. Microbiology Research. 2024; 15(2):447-456. https://doi.org/10.3390/microbiolres15020030
Chicago/Turabian StyleBassey, Odo J., Jabulani R. Gumbo, Munyaradzi Mujuru, Adeeyo Adeyemi, and Farai Dondofema. 2024. "Comparative Analysis of Cyanotoxins in Fishponds in Nigeria and South Africa" Microbiology Research 15, no. 2: 447-456. https://doi.org/10.3390/microbiolres15020030
APA StyleBassey, O. J., Gumbo, J. R., Mujuru, M., Adeyemi, A., & Dondofema, F. (2024). Comparative Analysis of Cyanotoxins in Fishponds in Nigeria and South Africa. Microbiology Research, 15(2), 447-456. https://doi.org/10.3390/microbiolres15020030






