Antagonistic Activity of Macrolepiota sp. CS185 against Post-Harvest Fungi of Fig Fruits (Ficus carica L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Biological Material
2.3. Cell-Wall Degrading Enzymes Detection
2.4. Determination of Antagonistic Activity in Multiple Confrontations
2.5. Determination of the Antagonistic Activity of Cell-Free Supernatants
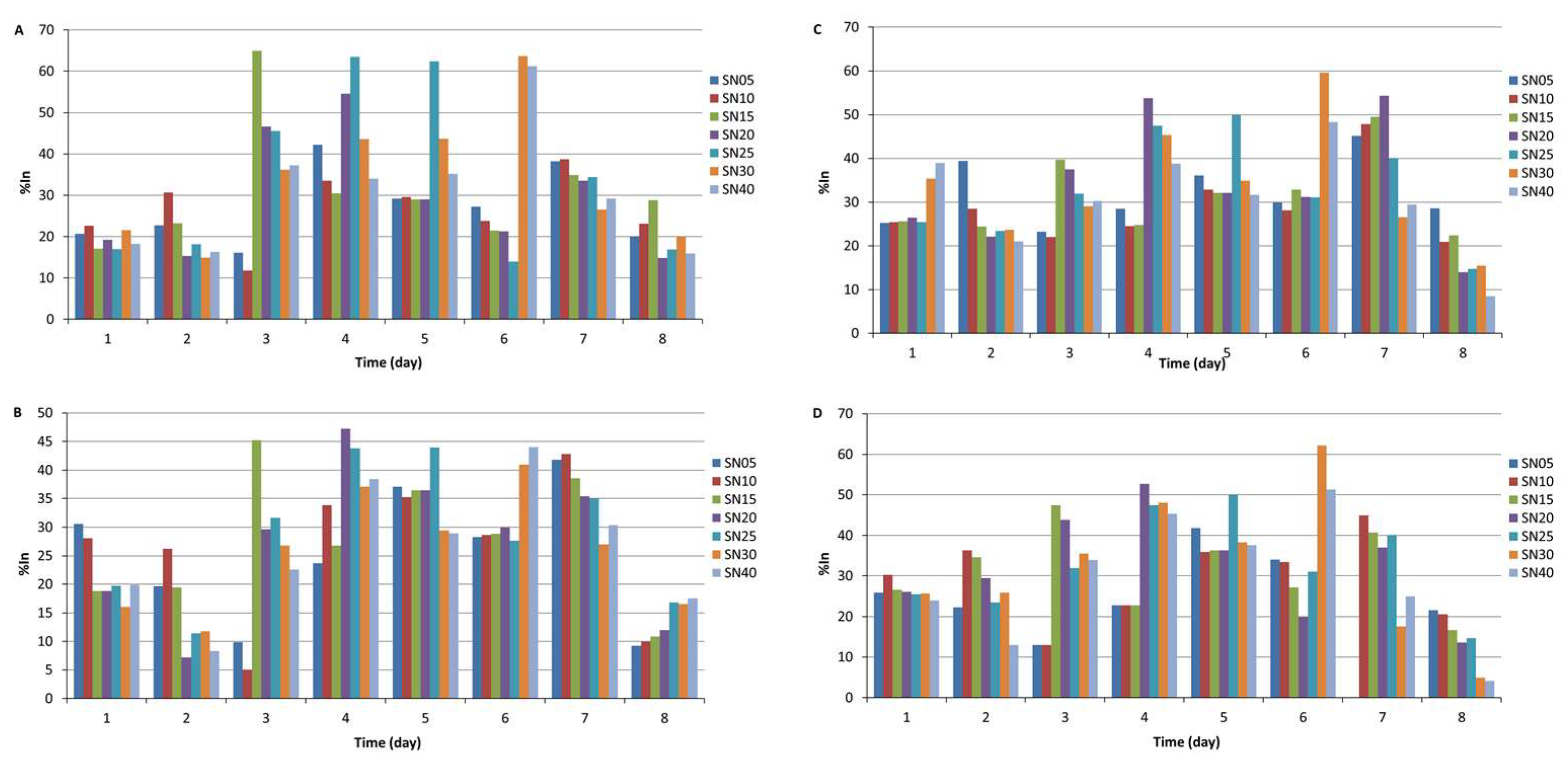
2.6. Production Kinetics of Antifungal Activity
2.7. Statistical Analysis
3. Results
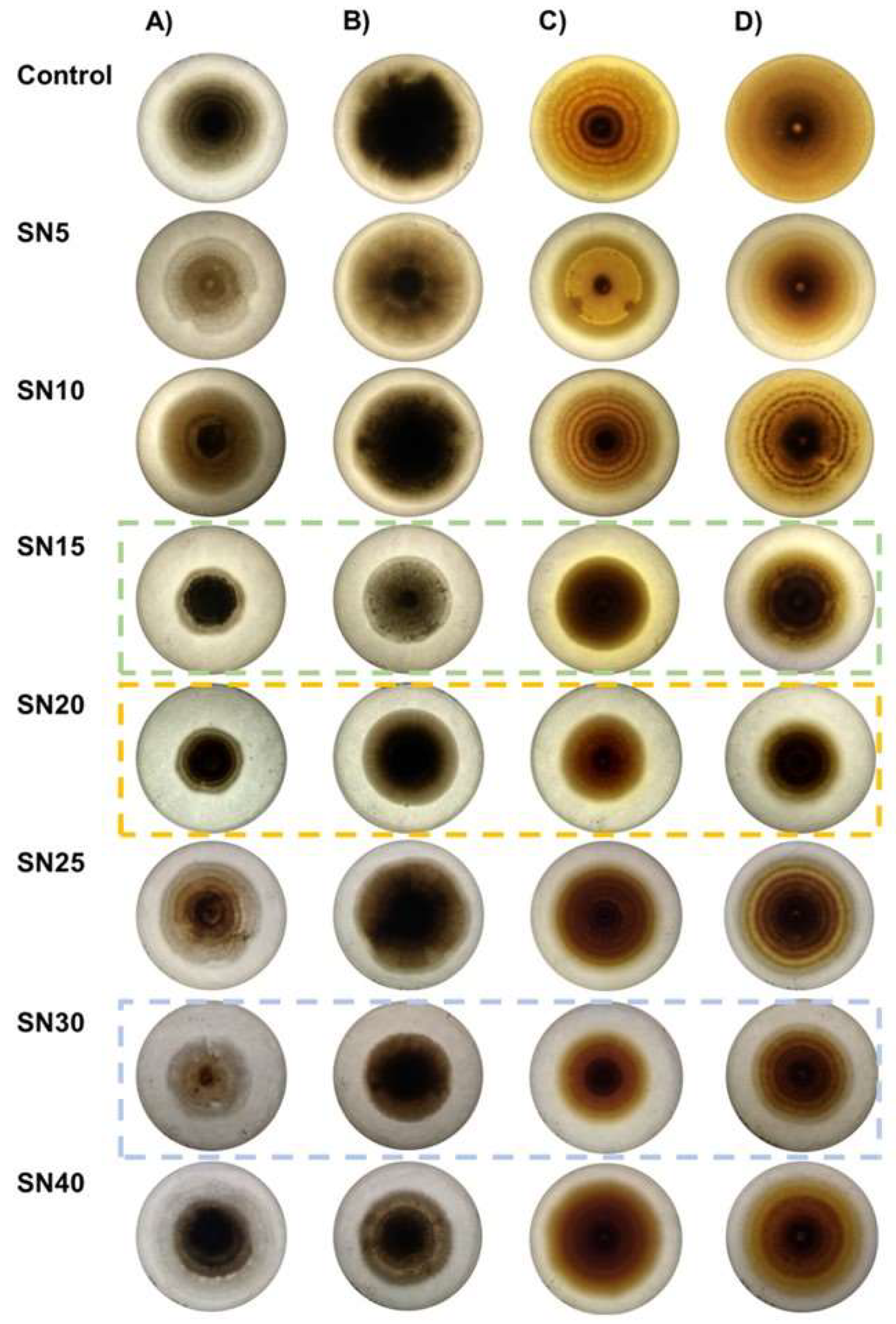
3.1. Determination of Antagonistic Activity in Multiple Confrontations
3.2. Determination of Antagonistic Activity in Multiple Confrontations
3.3. Production Kinetics of Antifungal Activity in Cell-Free Supernatants
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elik, A.; Yanik, D.K.; Istanbullu, Y.; Guzelsoy, N.A.; Yavuz, A.; Gogus, F. Strategies to reduce post-harvest losses for fruits and vegetables. Strategies 2019, 5, 29–39. [Google Scholar] [CrossRef]
- González-Estrada, R.; Blancas-Benítez, F.; Velázquez-Estrada, R.M.; Montaño-Leyva, B.; Ramos-Guerrero, A.; Aguirre-Güitrón, L.; Moreno-Hernández, C.; Coronado-Partida, L.; Herrera-González, J.A.; Rodríguez-Guzmán, C.A.; et al. Alternative Eco-Friendly Methods in the Control of Post-Harvest Decay of Tropical and Subtropical Fruits. In Modern Fruit Industry, 1st ed.; Kahramanoglu, I., Kafkas, N.E., Küden, A., Çömlekçioğlu, S., Eds.; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Carmona-Hernández, S.; Reyes-Pérez, J.J.; Chiquito-Contreras, R.G.; Rincon-Enriquez, G.; Cerdan-Cabrera, C.R.; Hernandez-Montiel, L.G. Biocontrol of postharvest fruit fungal diseases by bacterial antagonists: A review. Agronomy 2019, 9, 121. [Google Scholar] [CrossRef]
- Tzortzakis, N.; Chrysargyris, A. Postharvest ozone application for the preservation of fruits and vegetables. Food Rev. Int. 2017, 33, 270–315. [Google Scholar] [CrossRef]
- Ding, P.; Lee, Y.L. Use of essential oils for prolonging postharvest life of fresh fruits and vegetables. Int. Food Res. J. 2019, 26, 363–366. [Google Scholar]
- Arvaniti, O.S.; Samaras, Y.; Gatidou, G.; Thomaidis, N.S.; Stasinakis, A.S. Review on fresh and dried figs: Chemical analysis and occurrence of phytochemical compounds, antioxidant capacity and health effects. Food Res. Int. 2019, 119, 244–267. [Google Scholar] [CrossRef] [PubMed]
- Çalişkan, O.; Polat, A.A. Phytochemical and antioxidant properties of selected fig (Ficus carica L.) accessions from the eastern Mediterranean region of Turkey. Sci. Hortic. 2011, 128, 473–478. [Google Scholar] [CrossRef]
- Solomon, A.; Golubowicz, S.; Yablowicz, Z.; Grossman, S.; Bergman, M.; Gottlieb, H.E.; Altman, Z.K.; Flaishman, M.A. Antioxidant activities and anthocyanin content of fresh fruits of common fig (Ficus carica L.). J. Agric. Food Chem. 2006, 54, 7717–7723. [Google Scholar] [CrossRef] [PubMed]
- Paolucci, M.; Di Stasio, M.; Sorrentino, A.; La Cara, F.; Volpe, M.G. Active edible polysaccharide-based coating for preservation of fresh figs (Ficus carica L.). Foods 2020, 9, 1793. [Google Scholar] [CrossRef]
- Abbas, E.; Osman, A.; Sitohy, M. Biochemical control of Alternaria tenuissima infecting post-harvest fig fruit by chickpea vicilin. J. Sci. Food Agric. 2020, 100, 2889–2897. [Google Scholar] [CrossRef]
- Hedawoo, G.B.; Bijwe, H.V.; Maggirwar, R.C. Occurrence of Mycobiota Associated with Ficus carica L. Weather 2017, 14, 38. [Google Scholar]
- Villalobos, M.C.; Serradilla, M.J.; Martín, A.; Hernández-León, A.; Ruíz-Moyano, S.; de Guía Córdoba, M. Characterization of microbial population of breba and main crops (Ficus carica) during cold storage: Influence of passive modified atmospheres (MAP) and antimicrobial extract application. Food Microbiol. 2017, 63, 35–46. [Google Scholar] [CrossRef]
- Stover, E.; Aradhya, M.; Ferguson, L.; Crisosto, C.H. The fig: Overview of an ancient fruit. HortScience 2007, 42, 1083–1087. [Google Scholar] [CrossRef]
- Hayati, R.; Rahmawaty, M.; Lestari, T.N. Low temperature and duration on quality of fig fruit (Ficus carica L.). In IOP Conference Series: Earth and Environmental Science; IOP Publishing: London, UK, 2021; Volume 667, p. 012080. [Google Scholar] [CrossRef]
- Ertan, B.; Görücüoğlu, P.; Dağ, S.; Göçmez, A.; Aksoy, U.; Ertan, E. A research on the freezing conservation of ‘Sarılop’ figs. In Proceedings of the XXX International Horticultural Congress IHC2018: International Symposium on Strategies and Technologies to Maintain Quality 1275, Istanbul, Turkey, 12–16 August 2018; pp. 269–276. [Google Scholar]
- Martinez-Damian, M.T.; Omegar, C.A.; Oscar, C.A. Effect of modified atmosphere packaging on nutraceutical quality and overall appearance of figs stored at 1 °C. Not. Bot. Horti Agrobot. Cluj-Napoca 2020, 48, 2292–2305. [Google Scholar] [CrossRef]
- Irfan, P.K.; Vanjakshi, V.; Prakash, M.K.; Ravi, R.; Kudachikar, V.B. Calcium chloride extends the keeping quality of fig fruit (Ficus carica L.) during storage and shelf-life. Postharvest Biol. Technol. 2013, 82, 70–75. [Google Scholar] [CrossRef]
- Usberti, F.C.S.; Ferraz, A.C.D.O. UV-C radiation on fresh fig quality. Sci. Agric. 2020, 78, 1–5. [Google Scholar] [CrossRef]
- Chen, C.; Cai, N.; Chen, J.; Peng, X.; Wan, C. Chitosan-based coating enriched with hairy fig (Ficus hirta Vahl.) fruit extract for “Newhall” navel orange preservation. Coatings 2018, 8, 445. [Google Scholar] [CrossRef]
- Khaliq, G.; Mohamed, M.T.M.; Ali, A.; Ding, P.; Ghazali, H.M. Effect of gum arabic coating combined with calcium chloride on physico-chemical and qualitative properties of mango (Mangifera indica L.) fruit during low temperature storage. Sci. Hortic. 2015, 190, 187–194. [Google Scholar] [CrossRef]
- Saki, M.; ValizadehKaji, B.; Abbasifar, A.; Shahrjerdi, I. Effect of chitosan coating combined with thymol essential oil on physicochemical and qualitative properties of fresh fig (Ficus carica L.) fruit during cold storage. J. Food Meas. Charact. 2019, 13, 1147–1158. [Google Scholar] [CrossRef]
- Moccia, S.; La Cara, F.; Cervellera, C.; Russo, G.L.; Volpe, M.G. Active Edible Coating to Preserve Fresh Figs. Chem. Eng. Trans. 2021, 87, 181–186. [Google Scholar] [CrossRef]
- Allegra, A.; Sortino, G.; Inglese, P.; Settanni, L.; Todaro, A.; y Gallotta, A. La eficacia del recubrimiento comestible de mucílago de Opuntia ficus-indica en el mantenimiento poscosecha de la fruta ‘Dottato’ fig (Ficus carica L.). Envas. Aliment. Vida Útil. 2017, 12, 135–141. [Google Scholar]
- Lakshmi, S.J.; Roopa Bai, R.S.; Sharanagouda, H.; Ramachandra, C.T.; Nadagouda, S.; Nidoni, U. Effect of biosynthesized zinc oxide nanoparticles coating on quality parameters of fig (Ficus carica L.) fruit. J. Pharmacogn. Phytochem. 2018, 7, 10–14. [Google Scholar]
- Sidorova, I.; Voronina, E. Bioactive secondary metabolites of basidiomycetes and its potential for agricultural plant growth promotion. In Secondary Metabolites of Plant Growth Promoting Rhizomicroorganisms; Springer: Singapore, 2019; pp. 3–26. [Google Scholar]
- Schüffler, A. Secondary metabolites of basidiomycetes. In Physiology and Genetics; Springer: Cham, Switzerland, 2018; pp. 231–275. [Google Scholar]
- Fukushima-Sakuno, E. Bioactive small secondary metabolites from the mushrooms Lentinula edodes and Flammulina velutipes. J. Antibiot. 2020, 73, 687–696. [Google Scholar] [CrossRef]
- Priya, K.; Thiribhuvanamala, G.; Kamalakannan, A.; Krishnamoorthy, A.S. Antimicrobial activity of biomolecules from mushroom fungi against Colletotrichum capsici (Syd.) Butler and Bisby, the fruit rot pathogen of Chilli. Int. J. Curr. Microbiol. A Sci. 2019, 8, 1172–1186. [Google Scholar] [CrossRef]
- Dutta, S.; Woo, E.E.; Yu, S.M.; Nagendran, R.; Yun, B.S.; Lee, Y.H. Control of anthracnose and gray mold in pepper plants using culture extract of white-rot fungus and active compound schizostatin. Mycobiology 2019, 47, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Ochoa, J.S.; Levin, L.N.; Hernández-Luna, C.E.; Contreras-Cordero, J.F.; Niño-Medina, G.; Chávez-Montes, A.; López-Sandin, I.; Gutiérrez-Soto, G. Antagonistic potential of Macrolepiota sp. against Alternaria Solani as causal agent of early blight disease in tomato plants. Gesunde Pflanz. 2020, 72, 69–76. [Google Scholar] [CrossRef]
- Aqueveque, P.; Céspedes, C.L.; Becerra, J.; Aranda, M.; Sterner, O. Antifungal activities of secondary metabolites isolated from liquid fermentations of Stereum hirsutum (Sh134-11) against Botrytis cinerea (grey mould agent). Food Chem. Toxicol. 2017, 109, 1048–1054. [Google Scholar] [CrossRef]
- Han, J.W.; Oh, M.; Lee, Y.J.; Choi, J.; Choi, G.J.; Kim, H. Crinipellins A and I, two diterpenoids from the basidiomycete fungus Crinipellis rhizomaticola, as potential natural fungicides. Molecules 2018, 23, 2377. [Google Scholar] [CrossRef]
- Nofiani, R.; de Mattos-Shipley, K.; Lebe, K.E.; Han, L.C.; Iqbal, Z.; Bailey, A.M.; Cox, R.J. Strobilurin biosynthesis in Basidiomycete fungi. Nat. Commun. 2018, 9, 3940. [Google Scholar] [CrossRef]
- Iqbal, Z.; Han, L.C.; Soares-Sello, A.M.; Nofiani, R.; Thormann, G.; Zeeck, A.; Simpson, T.J. Investigations into the biosynthesis of the antifungal strobilurins. Org. Biomol. Chem. 2018, 16, 5524–5532. [Google Scholar] [CrossRef]
- Chen, L.; Zhu, Y.J.; Fan, Z.J.; Guo, X.F.; Zhang, Z.M.; Xu, J.H.; Song, Y.Q.; Yurievich, M.Y.; Belskaya, N.P.; Bakulev, V.A. Synthesis of 1, 2, 3-thiadiazole and thiazole-based strobilurins as potent fungicide candidates. J. Agric. Food Chem. 2017, 65, 745–751. [Google Scholar] [CrossRef]
- Erbiai, E.H.; Pinto da Silva, L.; Saidi, R.; Lamrani, Z.; Esteves da Silva, J.C.G.; Maouni, A. Chemical Composition, Bioactive Compounds and Antioxidant Activity of Two Wild Edible Mushrooms Armillaria mellea and Macrolepiota procera from Two Countries (Morocco and Portugal). Biomolecules 2021, 11, 575. [Google Scholar] [CrossRef] [PubMed]
- Adamska, I.; Tokarczyk, G. Possibilities of Using Macrolepiota procera in the Production of Prohealth Food and in Medicine. Int. J. Food Sci. 2022, 2022, 5773275. [Google Scholar] [CrossRef] [PubMed]
- UNAM. Hongos Comestibles de México. Repositorio Digital Multimedia Para la Determinación de Hongos Comestibles y Tóxicos de México. Available online: https://hongoscomestiblesytoxicos.ib.unam.mx/fichas/Macrolepiota_procera.html (accessed on 30 December 2023).
- Vellinga, E.C.; de Kok, R.P.; Bruns, T.D. Phylogeny and taxonomy of Macrolepiota (Agaricaceae). Mycologia 2003, 95, 442–456. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Luna, C.E.; Gutiérrez-Soto, G.; Salcedo-Martínez, S.M. Screening for decolorizing basidiomycetes in Mexico. World J. Microbiol. Biotechnol. 2008, 24, 465–473. [Google Scholar] [CrossRef]
- Sin, M.K.; Hyde, K.D.; Pointing, S.B. Comparative enzyme production by fungi from diverse lignocellulosic substrates. J. Microbiol. 2002, 40, 241–244. [Google Scholar]
- Gutiérrez-Soto, G.; Medina-Gonzalez, G.E.; Treviño-Ramírez, J.E.; Hernández-Luna, C.E. Native macrofungi that produce lignin-modifying enzymes, cellulases, and xylanases with potential biotechnological applications. BioResources 2015, 10, 6676–6689. [Google Scholar] [CrossRef]
- SADER. 2022. Available online: https://www.gob.mx/agricultura/articulos/que-hay-detras-de-la-produccion-de-higo?idiom=es (accessed on 30 December 2023).
- Contreras Saavedra, S. Antifungal Activity of a Biodegradable Coating for Post-Harvest Handling of Ficus carica. Master’s Thesis, Instituto Politécnico Nacional, Mexico, Mexico, 2019. [Google Scholar]
- Anand, T.; Bhaskaran, R.; Karthikeyan, T.G.; Rajesh, M.; Senthilraja, G. Production of cell wall degrading enzymes and toxins by Colletotrichum capsici and Alternaria alternata causing fruit rot of chillies. J. Plant Prot. Res. 2008, 48, 438–451. [Google Scholar] [CrossRef]
- Brazkova, M.; Koleva, R.; Angelova, G.; Yemendzhiev, H. Ligninolytic enzymes in Basidiomycetes and their application in xenobiotics degradation. BIO Web Conf. 2022, 45, 02009. [Google Scholar] [CrossRef]
- Wang, Z.X.; Feng, X.L.; Liu, C.; Gao, J.M.; Qi, J. Diverse Metabolites and Pharmacological Effects from the Basidiomycetes Inonotus hispidus. Antibiotics 2022, 11, 1097. [Google Scholar] [CrossRef]
- Wang, Q.; Cao, R.; Zhang, Y.; Qi, P.; Wang, L.; Fan, S. Biosynthesis and regulation of terpenoids from basidiomycetes: Exploration of new research. AMB Express 2021, 11, 150. [Google Scholar] [CrossRef]
- Ragupathi, K.P.; Renganayaki, P.R.; Sundareswaran, S.; Kumar, S.M.; Kamalakannan, A. Mycomolecules against Alternaria solani causing Early blight of tomato. J. Entomol. Zool. Stud. 2020, 9, 101–104. [Google Scholar] [CrossRef]
- Thangaraj, P.; Subbiah, K.A.; Uthandi, S.; Amirtham, D. Antifungal volatiles from macrobasidiomycetes inhibits Fusarium oxysporum f. sp. lycopersici. Madras Agric. J. 2021, 108, 38–43. [Google Scholar] [CrossRef]
- Ikeda, K.; Park, P.; Nakayashiki, H. Cell biology in phytopathogenic fungi during host infection: Commonalities and differences. J. Gen. Plant Pathol. 2019, 85, 163–173. [Google Scholar] [CrossRef]
- Adachi, Y.; Watanabe, H.; Tsuge, T. Relationships between genetic polymorphisms and fungicide resistance within Alternaria alternata. Phytopathology 1996, 86, 1248–1254. [Google Scholar] [CrossRef]
- Alizadeh-Moghaddam, G.; Rezayatmand, Z.; Nasr-Esfahani, M.; Khozaei, M. Bio-genetic analysis of resistance in tomato to early blight disease, Alternaria alternata. Phytochemistry 2020, 179, 112486. [Google Scholar] [CrossRef] [PubMed]
- Flores, C.; Vidal, C.; Trejo-Hernández, M.R.; Galindo, E.; Serrano-Carreón, L. Selection of Trichoderma strains capable of increasing laccase production by Pleurotus ostreatus and Agaricus bisporus in dual cultures. J. Appl. Microbiol. 2009, 106, 249–257. [Google Scholar] [CrossRef]
- Frljak, J.; Mulabećirović, A.H.; Isaković, S.; Karahmet, E.; Toroman, A. Biological active components of selected medical fungi. Open J. Prev. Med. 2021, 11, 9–22. [Google Scholar] [CrossRef]
- Sivanandhan, S.; Khusro, A.; Paulraj, M.G.; Ignacimuthu, S.; Al-Dhabi, N.A. Biocontrol properties of basidiomycetes: An overview. J. Fungi 2017, 3, 2. [Google Scholar] [CrossRef]
- Shen, T.; Morlock, G.; Zorn, H. Production of cyathane type secondary metabolites by submerged cultures of Hericium erinaceus and evaluation of their antibacterial activity by direct bioautography. Fungal Biol. Biotechnol. 2015, 2, 8. [Google Scholar] [CrossRef]
- Mustafin, K.; Bisko, N.; Blieva, R.; Al-Maali, G.; Krupodorova, T.; Narmuratova, Z.; Saduyeva, Z.; Zhakipbekova, A. Antioxidant and antimicrobial potential of Ganoderma lucidum and Trametes versicolor. Turk. J. Biochem. 2022, 47, 483–489. [Google Scholar] [CrossRef]
- Costa, T.M.; Kaufmann, V.; Paganelli, C.J.; Siebert, D.A.; Micke, G.A.; Alberton, M.D.; Ballod Tavares, L.B.; de Oliveira, D. Kinetic identification of phenolic compounds and potential production of caffeic acid by Ganoderma lipsiense in solid-state fermentation. Bioprocess Biosyst. Eng. 2019, 42, 1325–1332. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.Q.; Wang, F.; Bian, X.Y.; Liu, J.K. Rufuslactone, a new antifungal sesquiterpene from the fruiting bodies of the basidiomycete Lactarius rufus. J. Antibiot. 2005, 58, 456–459. [Google Scholar] [CrossRef] [PubMed]
- Perera, W.H.; Meepagala, K.M.; Wedge, D.E.; Duke, S.O. Sesquiterpenoids from culture of the fungus Stereum complicatum (Steraceae): Structural diversity, antifungal and phytotoxic activities. Phytochem. Lett. 2020, 37, 51–58. [Google Scholar] [CrossRef]

| Isolate | Day | p-Value | |||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||
| A. alternata 1 | 53.0 aA | 58.6 aA | 60.3 abA | 56.1 bcdA | 0.88 |
| A. alternata 2 | 43.7 aA | 46.2 abA | 49.8 bcA | 52.4 cdA | 0.13 |
| A. alternata 3 | 38.8 abC | 48.0 abBC | 62.9 abAB | 66.9 abcA | 0.003 |
| A. alternata 4 | 51.9 aB | 53.6 abAB | 62.9 abAB | 66.0 abcA | 0.02 |
| Colletotrichum sp. 1 | 55.2 aB | 57.8 aB | 63.5 abAB | 68.8 abA | 0.005 |
| Colletotrichum sp. 2 | 63.1 aC | 63.7 aBC | 72.0 aAB | 73.3 aA | 0.01 |
| Fusarium oxysporum | 15.3 bcC | 27.0 bcBC | 46.6 bcAB | 52.9 cdA | 0.005 |
| F. solani | 0.0 cB | 8.1 cB | 30.7 cA | 43.2 dA | 0.0003 |
| p-value | 0.001 | 0.002 | 0.001 | 0.001 | |
| Isolate * | Cellulases | Xylanases | Pectinases | Laccase | LME |
|---|---|---|---|---|---|
| DR (%) | |||||
| Alternaria alternata 1 | 1.07 bc | 0.96 a | 0.00 b | 0.00 b | 0.00 |
| A. alternata 2 | 1.16 ab | 0.75 c | 0.00 b | 0.00 b | 0.00 |
| A. alternata 3 | 1.26 a | 0.78 bc | 0.00 b | 0.00 b | 0.00 |
| A. alternata 4 | 1.12 abc | 0.98 a | 0.00 b | 0.00 b | 0.00 |
| Colletotrichum sp. 1 | 0.983 cd | 0.71 c | 0.00 b | 0.00 b | 0.00 |
| Colletotrichum sp. 2 | 1.17 ab | 0.92 ab | 0.00 b | 2.32 a | 0.00 |
| Fusarium oxysporum | 0.91 d | 0.81 bc | 1.10 a | 0.00 b | 0.00 |
| F. solani | 1.22 a | 0.77 c | 0.00 b | 0.00 b | 0.00 |
| p-value | 0.001 | 0.001 | 0.001 | 0.001 | sd |
| Post-Harvest Fungi * | 0% | 15% | 30% | 50% | ||||
|---|---|---|---|---|---|---|---|---|
| MGR (mm/d) | RPI (%) | MGR (mm/d) | RPI (%) | MGR (mm/d) | RPI (%) | MGR (mm/d) | RPI (%) | |
| A. alternata 1 | 4.44 aA | 0 aD | 3.99 aB | 10.01 eC | 3.62 aC | 18.47 eB | 3.30 aD | 25.71 dA |
| A. alternata 2 | 2.58 eA | 0 aD | 2.25 eB | 12.83 dC | 1.96 eD | 23.83 cdA | 2.06 cdC | 19.99 eB |
| A. alternata 3 | 2.80 dA | 0 aD | 2.49 cB | 11.01 deC | 2.19 dC | 21.81 dB | 2.02 cdD | 27.98 dA |
| A. alternata 4 | 4.00 bA | 0 aD | 3.05 bB | 23.83 bC | 3.02 bB | 24.61 cB | 2.75 bC | 31.30 cA |
| Colletotrichum sp. 1 | 3.02 cA | 0 aD | 2.42 dB | 19.92 cC | 2.17 dC | 28.05 bB | 1.88 dD | 37.65 bA |
| Colletotrichum sp. 2 | 4.34 aA | 0 aD | 3.09 bB | 28.83 aC | 2.42 cC | 44.17 aB | 2.20 cD | 49.27 aA |
| Tt | A. alternata 1 | A. alternata 4 | Colletotrichum sp. 1 | Colletotrichum sp. 2 | ||||
|---|---|---|---|---|---|---|---|---|
| MGR (mm/d) | RPI (%) | MGR (mm/d) | RPI (%) | MGR (mm/d) | RPI (%) | MGR (mm/d) | RPI (%) | |
| Control * | 4.50 a | 0.00 d | 4.56 a | 0.00 c | 4.88 a | 0.00 e | 4.67 a | 0.00 e |
| SN05 | 4.97 | 0.57 d | 4.95 a | 0.00 c | 4.16 ab | 14.87 d | 4.56 a | 2.48 de |
| SN10 | 3.87 ab | 22.57 b | 4.66 a | 0.00 c | 3.92 bc | 19.72 c | 4.49 a | 3.94 d |
| SN15 | 3.25 b | 34.94 a | 3.40 a | 12.33 a | 3.01 d | 38.27 a | 3.10 a | 33.66 a |
| SN20 | 3.25 b | 34.94 a | 3.40 a | 12.33 a | 3.01 d | 38.27 a | 3.10 a | 33.66 a |
| SN25 | 3.35 b | 32.91 a | 3.40 a | 12.33 a | 3.55 bcd | 27.27 b | 3.55 a | 24.05 b |
| SN30 | 3.39 b | 32.08 a | 4.11 a | 9.97 b | 3.06 cd | 37.29 a | 3.03 a | 35.21 a |
| SN40 | 4.57 ab | 8.62 c | 5.11 a | 0.00 c | 4.01 ab | 17.79 c | 3.84 a | 17.86 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutiérrez-Soto, G.; López-Sandin, I.; Hernández Ochoa, J.S.; Hernadez-Luna, C.E.; Contreras-Cordero, J.F.; Hernández-Martínez, C.A. Antagonistic Activity of Macrolepiota sp. CS185 against Post-Harvest Fungi of Fig Fruits (Ficus carica L.). Microbiol. Res. 2024, 15, 371-384. https://doi.org/10.3390/microbiolres15010025
Gutiérrez-Soto G, López-Sandin I, Hernández Ochoa JS, Hernadez-Luna CE, Contreras-Cordero JF, Hernández-Martínez CA. Antagonistic Activity of Macrolepiota sp. CS185 against Post-Harvest Fungi of Fig Fruits (Ficus carica L.). Microbiology Research. 2024; 15(1):371-384. https://doi.org/10.3390/microbiolres15010025
Chicago/Turabian StyleGutiérrez-Soto, Guadalupe, Iosvany López-Sandin, Jesús Salvador Hernández Ochoa, Carlos Eduardo Hernadez-Luna, Juan Francisco Contreras-Cordero, and Carlos Alberto Hernández-Martínez. 2024. "Antagonistic Activity of Macrolepiota sp. CS185 against Post-Harvest Fungi of Fig Fruits (Ficus carica L.)" Microbiology Research 15, no. 1: 371-384. https://doi.org/10.3390/microbiolres15010025
APA StyleGutiérrez-Soto, G., López-Sandin, I., Hernández Ochoa, J. S., Hernadez-Luna, C. E., Contreras-Cordero, J. F., & Hernández-Martínez, C. A. (2024). Antagonistic Activity of Macrolepiota sp. CS185 against Post-Harvest Fungi of Fig Fruits (Ficus carica L.). Microbiology Research, 15(1), 371-384. https://doi.org/10.3390/microbiolres15010025






