Tannin-Tolerant Saccharomyces cerevisiae Isolated from Traditional Fermented Tea (Miang) of Northern Thailand and Its Feasible Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation and Screening of Tannin-Tolerant Yeast Capable of Ethanol Production
2.2. Molecular Identification
2.3. Effect of Carbon Sources on Ethanol Production
2.4. Effect of pH and Temperature on Ethanol Production
2.5. Effect of Tannin and Sugar Concentrations on Ethanol Production
2.6. Ethanol Fermentation by Selected Tannin-Tolerant Yeast
2.7. Java Plum Fruit Wine Fermentation
2.8. Analytical Methods
2.8.1. Ethanol and Glucose Analysis
2.8.2. Total Sugar Determination
2.8.3. Total Polyphenol, Total Tannin, and Total Flavonoid Contents
2.8.4. 1,1-Diphenyl-2-picrylhydrazyl (DPPH) Radical Scavenging Activity
2.9. Statistical Analysis
3. Results and Discussion
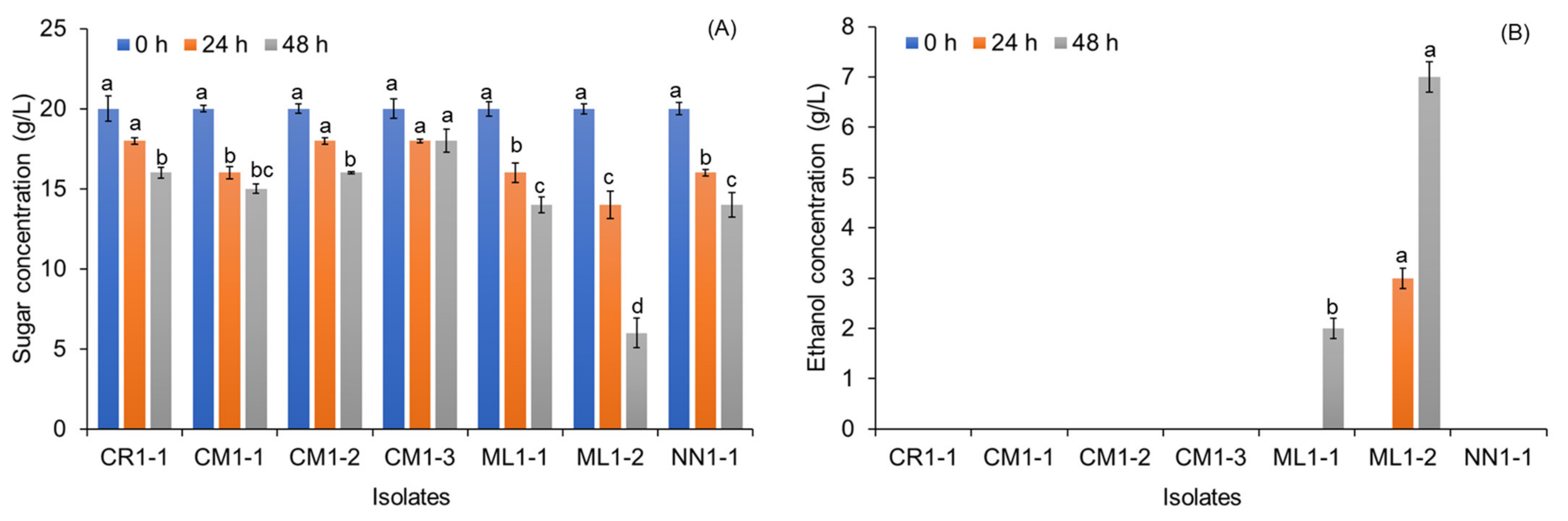
3.1. Isolation and Screening of Tannin-Tolerant Yeast Capable of Ethanol Production
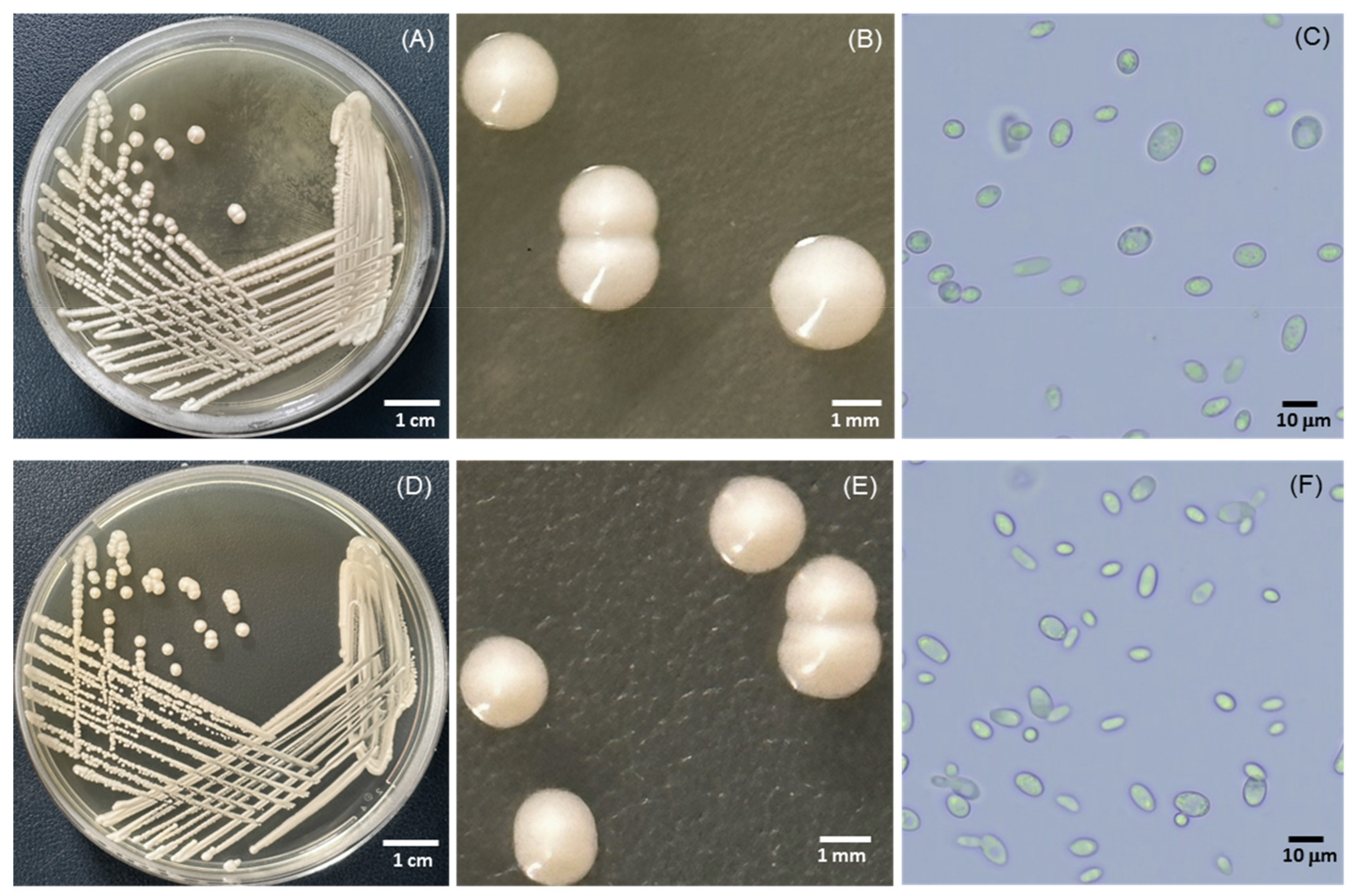
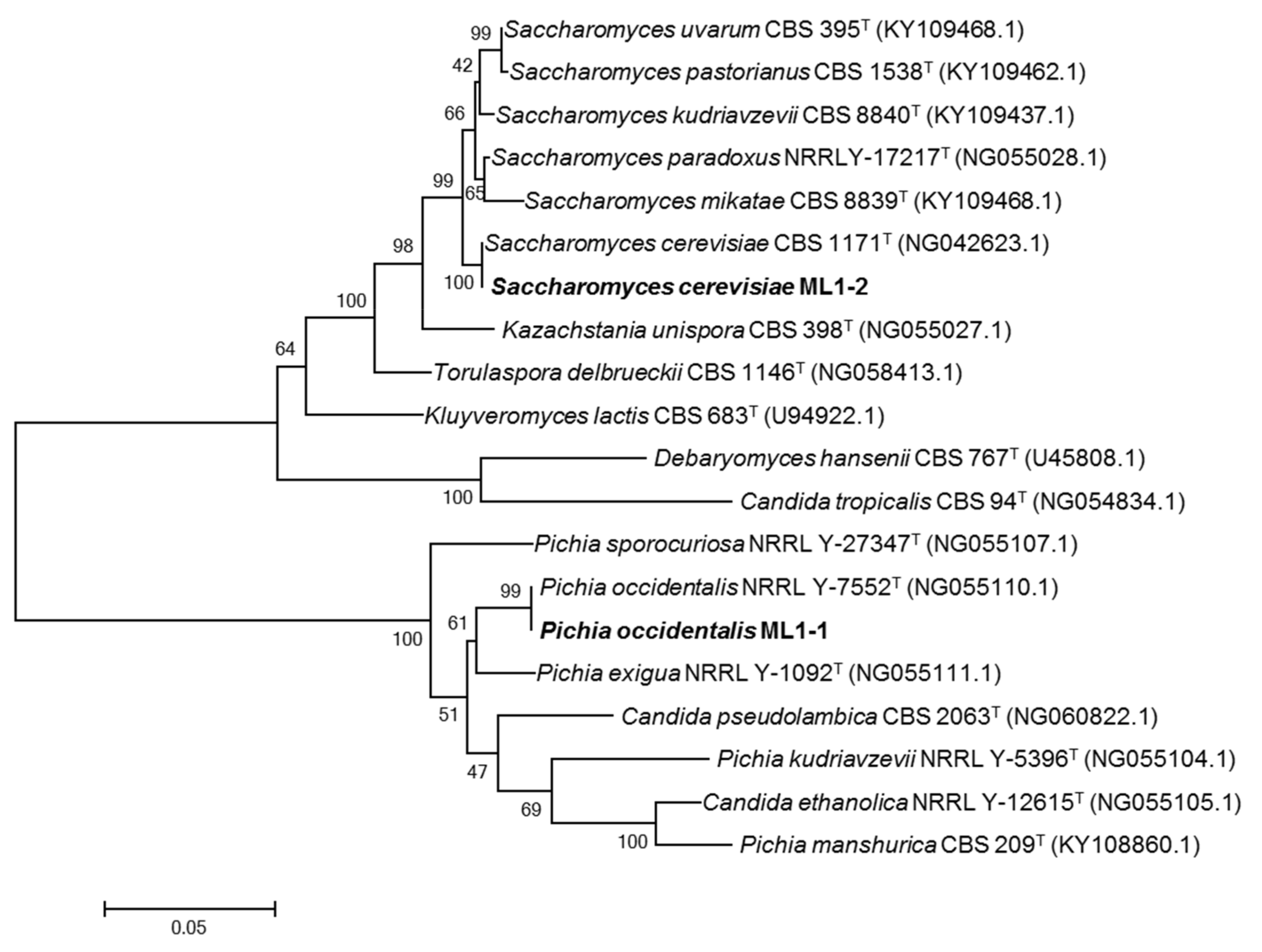
3.2. Identification and Phylogenetic Analyses of Yeasts
3.3. Effects of Carbon Sources on Ethanol Production
3.4. Effects of pH and Temperature on Ethanol Production
3.5. Effects of Tannin Supplementation and Sugar Concentration on Ethanol Production
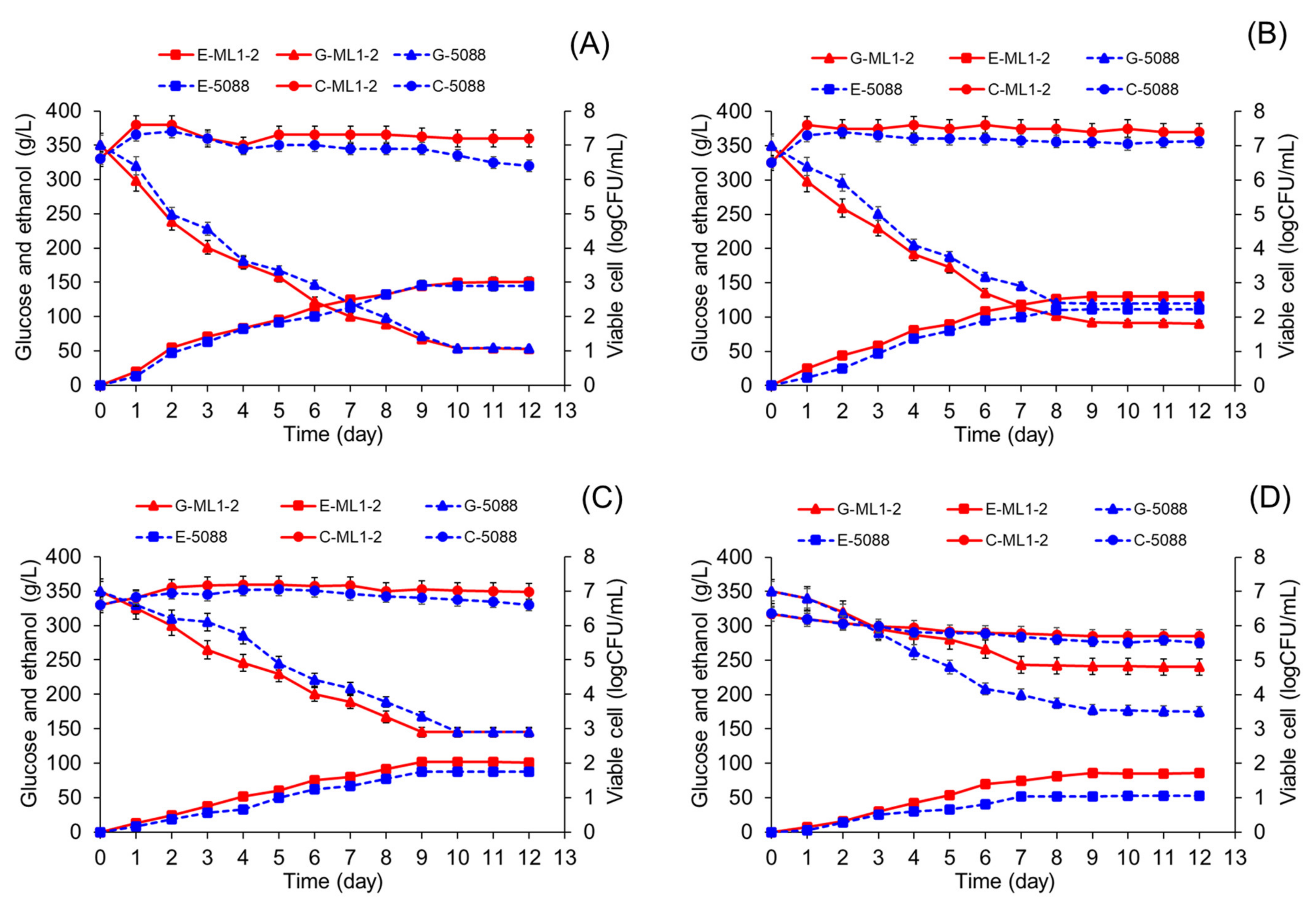
3.6. Ethanol Fermentation by Tannin-Tolerant Selected Yeast
3.7. Java Plum Fruit Wine Fermentation by Tannin-Tolerant Selected Yeast
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Walker, G.M.; Stewart, G.G. Saccharomyces cerevisiae in the production of fermented beverages. Beverages 2016, 2, 30. [Google Scholar] [CrossRef]
- Walker, G.M.; Walker, R.S. Enhancing yeast alcoholic fermentations. Adv. Appl. Microbiol. 2018, 105, 87–129. [Google Scholar] [PubMed]
- Saranraj, P.; Sivasakthivelan, P.; Naveen, M. Fermentation of fruit wine and its quality analysis: A review. Aust. J. Sci. Technol. 2017, 1, 85–97. [Google Scholar]
- Matallana, E.; Aranda, A. Biotechnological impact of stress response on wine yeast. Lett. Appl. Microbiol. 2017, 64, 103–110. [Google Scholar] [CrossRef]
- Abdel-Banat, B.M.; Hoshida, H.; Ano, A.; Nonklang, S.; Akada, R. High-temperature fermentation: How can processes for ethanol production at high temperatures become superior to the traditional process using mesophilic yeast? Appl. Microbiol. Biotechnol. 2010, 85, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Nuanpeng, S.; Thanonkeo, S.; Klanrit, P.; Thanonkeo, P. Ethanol production from sweet sorghum by Saccharomyces cerevisiae DBKKUY-53 immobilized on alginate-loofah matrices. Braz. J. Microbiol. 2018, 49, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, A.; Blaiotta, G.; Aponte, M.; Moio, L. Effect of yeast strain and some nutritional factors on tannin composition and potential astringency of model wines. Food Microbiol. 2016, 53, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Bindon, K.A.; Kassara, S.; Solomon, M.; Bartel, C.; Smith, P.A.; Barker, A.; Curtin, C. Commercial Saccharomyces cerevisiae yeast strains significantly impact Shiraz tannin and polysaccharide composition with implications for wine colour and astringency. Biomolecules 2019, 9, 466. [Google Scholar] [CrossRef]
- Kanpiengjai, A.; Chui-Chai, N.; Chaikaew, S.; Khanongnuch, C. Distribution of tannin-tolerant yeasts isolated from Miang, a traditional fermented tea leaf (Camellia sinensis var. assamica) in northern Thailand. Int. J. Food Microbiol. 2016, 238, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Taskin, M. Co-production of tannase and pectinase by free and immobilized cells of the yeast Rhodotorula glutinis MP-10 isolated from tannin-rich persimmon (Diospyros kaki L.) fruits. Bioprocess Biosyst. Eng. 2013, 36, 165–172. [Google Scholar] [CrossRef]
- Khanongnuch, C.; Unban, K.; Kanpiengjai, A.; Saenjum, C. Recent research advances and ethno-botanical history of miang, a traditional fermented tea (Camellia sinensis var. assamica) of Northern Thailand. J. Ethn. Foods 2017, 4, 135–144. [Google Scholar] [CrossRef]
- Hamilton-Miller, J. Antimicrobial properties of tea (Camellia sinensis L.). Antimicrob. Agents Chemother. 1995, 39, 2375–2377. [Google Scholar] [CrossRef] [PubMed]
- Abdullahi, A.D.; Kodchasee, P.; Unban, K.; Pattananandecha, T.; Saenjum, C.; Kanpiengjai, A.; Shetty, K.; Khanongnuch, C. Comparison of phenolic contents and scavenging activities of Miang extracts derived from filamentous and non-filamentous fungi-based fermentation processes. Antioxidants 2021, 10, 1144. [Google Scholar] [CrossRef]
- Unban, K.; Khatthongngam, N.; Pattananandecha, T.; Saenjum, C.; Shetty, K.; Khanongnuch, C. Microbial community dynamics during the non-filamentous fungi growth-based fermentation process of Miang, a traditional fermented tea of north Thailand and their product characterizations. Front. Microbiol. 2020, 11, 1515. [Google Scholar] [CrossRef]
- Kodchasee, P.; Nain, K.; Abdullahi, A.D.; Unban, K.; Saenjum, C.; Shetty, K.; Khanongnuch, C. Microbial dynamics-linked properties and functional metabolites during Miang fermentation using the filamentous fungi growth-based process. Food Biosci. 2021, 41, 100998. [Google Scholar] [CrossRef]
- Kurtzman, C.; Robnett, C. Identification of clinically important ascomycetous yeasts based on nucleotide divergence in the 5′end of the large-subunit (26S) ribosomal DNA gene. J. Clin. Microbiol. 1997, 35, 1216–1223. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Dudley, J.; Nei, M.; Kumar, S. MEGA4: Molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol. Biol. Evol. 2007, 24, 1596–1599. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Sánchez-Moreno, C.; Larrauri, J.A.; Saura-Calixto, F. A procedure to measure the antiradical efficiency of polyphenols. J. Sci. Food Agric. 1998, 76, 270–276. [Google Scholar] [CrossRef]
- Chaikaew, S.; Baipong, S.; Sone, T.; Kanpiengjai, A.; Chui-chai, N.; Asano, K.; Khanongnuch, C. Diversity of lactic acid bacteria from Miang, a traditional fermented tea leaf in northern Thailand and their tannin-tolerant ability in tea extract. J. Microbiol. 2017, 55, 720–729. [Google Scholar] [CrossRef]
- Li, Q.; Huang, J.; Li, Y.; Zhang, Y.; Luo, Y.; Chen, Y.; Lin, H.; Wang, K.; Liu, Z. Fungal community succession and major components change during manufacturing process of Fu brick tea. Sci. Rep. 2017, 7, 6947. [Google Scholar] [CrossRef] [PubMed]
- Okada, S.; Takahashi, N.; Ohara, N.; Uchimura, T.; Kozaki, M. Microorganisms involving in the fermentation of Japanese fermented tea leaves, 2: Microorganisms in fermentation of Goishi-cha, Japanese fermented tea leaves. J. Jpn. Soc. Food. Sci. Technol. 1996, 43, 1019–1027. [Google Scholar] [CrossRef]
- Bo, B.; Kim, S.A.; Han, N.S. Bacterial and fungal diversity in Laphet, traditional fermented tea leaves in Myanmar, analyzed by culturing, DNA amplicon-based sequencing, and PCR-DGGE methods. Int. J. Food Microbiol. 2020, 320, 108508. [Google Scholar] [CrossRef]
- Vicente, J.; Calderón, F.; Santos, A.; Marquina, D.; Benito, S. High potential of Pichia kluyveri and other Pichia species in wine technology. Int. J. Mol. Sci. 2021, 22, 1196. [Google Scholar] [CrossRef]
- Arroyo-López, F.; Querol, A.; Bautista-Gallego, J.; Garrido-Fernández, A. Role of yeasts in table olive production. Int. J. Food Microbiol. 2008, 128, 189–196. [Google Scholar] [CrossRef]
- Okamoto, K.; Nakagawa, S.; Kanawaku, R.; Kawamura, S. Ethanol production from cheese whey and expired milk by the brown rot fungus Neolentinus lepideus. Fermentation 2019, 5, 49. [Google Scholar] [CrossRef]
- Li, J.-Y.; Du, G.; Yang, X.; Huang, W.-D. Effect of proanthocyanidins on yeast metabolism, H+-ATPase activity, and wine fermentation. Am. J. Enol. Vitic. 2011, 62, 512–518. [Google Scholar] [CrossRef]
- Li, J.; Zhao, H.; Huang, W. Mechanism of proanthocyanidins-induced alcoholic fermentation enhancement in Saccharomyces cerevisiae. J. Ind. Microbiol. Biotechnol. 2014, 41, 1793–1802. [Google Scholar] [CrossRef]
- Salmon, J.M.; Mauricio, J.C. Relationship between sugar uptake kinetics and total sugar consumption in different industrial Saccharomyces cerevisiae strains during alcoholic fermentation. Biotechnol. Lett. 1994, 16, 89–94. [Google Scholar] [CrossRef]
- Bauer, F.; Pretorius, I.S. Yeast stress response and fermentation efficiency: How to survive the making of wine. S. Afr. J. Enol. Vitic. 2000, 21, 27–51. [Google Scholar] [CrossRef]
- Attfield, P.V. Stress tolerance: The key to effective strains of industrial baker’s yeast. Nat. Biotechnol. 1997, 15, 1351–1357. [Google Scholar] [CrossRef]
- Dodić, S.; Popov, S.; Dodić, J.; Ranković, J.; Zavargo, Z.; Mučibabić, R.J. Bioethanol production from thick juice as intermediate of sugar beet processing. Biomass Bioenergy 2009, 33, 822–827. [Google Scholar] [CrossRef]
- Phukoetphim, N.; Salakkam, A.; Laopaiboon, P.; Laopaiboon, L. Improvement of ethanol production from sweet sorghum juice under batch and fed-batch fermentations: Effects of sugar levels, nitrogen supplementation, and feeding regimes. Electron. J. Biotechnol. 2017, 26, 84–92. [Google Scholar] [CrossRef]
- Joannis-Cassan, C.; Riess, J.; Jolibert, F.; Taillandier, P. Optimization of very high gravity fermentation process for ethanol production from industrial sugar beet syrup. Biomass Bioenergy 2014, 70, 165–173. [Google Scholar] [CrossRef]
- Swiegers, J.; Bartowsky, E.; Henschke, P.; Pretorius, I. Yeast and bacterial modulation of wine aroma and flavour. Aust. J. Grape Wine Res. 2005, 11, 139–173. [Google Scholar] [CrossRef]
- Ning, Z.; Jiang, J.-C.; Jing, Y.; Min, W.; Jian, Z.; Hao, X.; Xie, J.-C.; Tong, Y.-J. Ethanol production from acorn starch by tannin tolerance mutant Pachysolen tannophilus. Energy Sources 2018, 40, 572–578. [Google Scholar] [CrossRef]
- Kanpiengjai, A.; Khanongnuch, C.; Lumyong, S.; Haltrich, D.; Nguyen, T.-H.; Kittibunchakul, S. Co-production of gallic acid and a novel cell-associated tannase by a pigment-producing yeast, Sporidiobolus ruineniae A45. 2. Microb. Cell Fact. 2020, 19, 95. [Google Scholar] [CrossRef] [PubMed]
- Das, G.; Nath, R.; Das Talukdar, A.; Ağagündüz, D.; Yilmaz, B.; Capasso, R.; Shin, H.-S.; Patra, J.K. Major bioactive compounds from java plum seeds: An investigation of its extraction procedures and clinical effects. Plants 2023, 12, 1214. [Google Scholar] [CrossRef]
- Baliga, M.S.; Bhat, H.P.; Baliga, B.R.V.; Wilson, R.; Palatty, P.L. Phytochemistry, traditional uses and pharmacology of Eugenia jambolana Lam.(black plum): A review. Food Res. Int. 2011, 44, 1776–1789. [Google Scholar] [CrossRef]
- Tak, Y.; Kaur, M.; Jain, M.C.; Samota, M.K.; Meena, N.K.; Kaur, G.; Kumar, R.; Sharma, D.; Lorenzo, J.M.; Amarowicz, R. Jamun seed: A review on bioactive constituents, nutritional value and health benefits. Pol. J. Food Nutr. Sci. 2022, 72, 211–228. [Google Scholar] [CrossRef]
- Raharjo, S.; Falah, I.; Santoso, U. Methanolic extract of Java Plum (Syzygium cumini Linn.) seeds as a natural antioxidant on lipid oxidation of oil-in-water emulsions. Int. Food Res. J. 2017, 24, 1636. [Google Scholar]
- VenuGopal, K.; Anu-Appaiah, K. Seed incorporation during vinification and its impact on chemical and organoleptic properties in Syzygium cumini wine. Food Chem. 2017, 237, 693–700. [Google Scholar] [CrossRef]

| Provinces (Code) | No. of Miang Samples | No. of Gas Formation | No. of Yeast Isolates |
|---|---|---|---|
| Chiang Rai (CR) | 4 | 2 | 1 |
| Chiang Mai (CM) | 10 | 8 | 3 |
| Lampang (LP) | 2 | 1 | 0 |
| Phrae (ML) | 17 | 9 | 2 |
| Nan (NN) | 10 | 5 | 1 |
| Total | 43 | 25 | 7 |
| Parameters | Java Plum Fruit Wine Fermented without Ground Seed | Java Plum Fruit Wine Fermented with Ground Seed | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Before Inoculating | S. cerevisiae ML1-2 | S. cerevisiae TISTR 5088 | Before Inoculating | S. cerevisiae ML1-2 | S. cerevisiae TISTR 5088 | |||||
| 6 Days | 12 Days | 6 Days | 12 Days | 6 Days | 12 Days | 6 Days | 12 Days | |||
| Total sugar (g/L) | 350 ± 0.58 a | 145.9 ± 0.92 b | 88.9 ± 0.29 d | 140.8 ± 0.94 c | 90.1 ± 0.73 d | 350 ± 0.31 a | 108.7 ± 0.78 c | 15.1 ± 0.66 e | 193.7 ± 0.35 b | 20.2 ± 0.62 d |
| Ethanol (g/L) | - | 70.5 ± 0.36 c | 132.8 ± 0.34 a | 69.4 ± 0.28 d | 130.7 ± 0.24 b | - | 122.7 ± 0.51 c | 168.6 ± 0.38 a | 80.2 ± 0.62 d | 165.3 ± 0.82 b |
| Viable cell (logCFU/mL) | - | 8.23 ± 0.08 a | 7.75 ± 0.05 c | 7.98 ± 0.05 b | 7.67 ± 0.04 c | - | 8.02 ± 0.06 a | 7.61 ± 0.03 c | 7.78 ± 0.08 b | 7.64 ± 0.05 c |
| pH | 3.63 ± 0.01 ab | 3.56 ± 0.03 bc | 3.67 ± 0.11 a | 3.49 ± 0.02 c | 3.73 ± 0.02 a | 3.67 ± 0.05 c | 3.83 ± 0.04 b | 3.98 ± 0.03 a | 3.79 ± 0.01 b | 3.96 ± 0.02 a |
| Total polyphenol (mg GAE/mL) | 0.13 ± 0.01 e | 0.32 ± 0.02 c | 0.67 ± 0.05 a | 0.21 ± 0.01 d | 0.55 ± 0.05 b | 1.72 ± 0.07 c | 3.32 ± 0.02 a | 3.54 ± 0.13 a | 2.53 ± 0.25 b | 2.65 ± 0.19 b |
| Total tannin (mg TAE/mL) | 0.02 ± 0.00 d | 0.02 ± 0.01 d | 0.09 ± 0.00 a | 0.04 ± 0.02 c | 0.07 ± 0.00 b | 2.21 ± 0.03 bc | 2.54 ± 0.11 a | 2.34 ± 0.07 ab | 2.04 ± 0.21 c | 2.11 ± 0.08 c |
| Total flavonoid (μg QE/mL) | 9.05 ± 0.15 a | 9.32 ± 0.19 a | 9.45 ± 0.56 a | 9.87 ± 0.23 a | 9.54 ± 0.82 a | 45.11 ± 0.73 a | 45.34 ± 0.11 a | 43.21 ± 0.27 b | 42.33 ± 0.21 c | 41.21 ± 0.38 d |
| 1/IC50 | 0.17 ± 0.02 d | 0.32 ± 0.01 b | 0.41 ± 0.01 a | 0.25 ± 0.02 c | 0.35 ± 0.03 b | 1.67 ± 0.22 e | 8.92 ± 0.09 b | 9.54 ± 0.10 a | 3.54 ± 0.02 d | 4.42 ± 0.17 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Unban, K.; Muangkajang, N.; Kodchasee, P.; Kanpiengjai, A.; Shetty, K.; Khanongnuch, C. Tannin-Tolerant Saccharomyces cerevisiae Isolated from Traditional Fermented Tea (Miang) of Northern Thailand and Its Feasible Applications. Microbiol. Res. 2023, 14, 1969-1983. https://doi.org/10.3390/microbiolres14040133
Unban K, Muangkajang N, Kodchasee P, Kanpiengjai A, Shetty K, Khanongnuch C. Tannin-Tolerant Saccharomyces cerevisiae Isolated from Traditional Fermented Tea (Miang) of Northern Thailand and Its Feasible Applications. Microbiology Research. 2023; 14(4):1969-1983. https://doi.org/10.3390/microbiolres14040133
Chicago/Turabian StyleUnban, Kridsada, Natchanon Muangkajang, Pratthana Kodchasee, Apinun Kanpiengjai, Kalidas Shetty, and Chartchai Khanongnuch. 2023. "Tannin-Tolerant Saccharomyces cerevisiae Isolated from Traditional Fermented Tea (Miang) of Northern Thailand and Its Feasible Applications" Microbiology Research 14, no. 4: 1969-1983. https://doi.org/10.3390/microbiolres14040133
APA StyleUnban, K., Muangkajang, N., Kodchasee, P., Kanpiengjai, A., Shetty, K., & Khanongnuch, C. (2023). Tannin-Tolerant Saccharomyces cerevisiae Isolated from Traditional Fermented Tea (Miang) of Northern Thailand and Its Feasible Applications. Microbiology Research, 14(4), 1969-1983. https://doi.org/10.3390/microbiolres14040133







