Abstract
Among plant disease management strategies, biological control is a sustainable alternative to the use of chemicals for the control of vascular wilt caused by Fusarium oxysporum. Fusarium wilt is the most devastating disease affecting a wide variety of plants. Bacillus species are the most widely used biological control candidates for the control of these fungal diseases. This review describes the pathogenicity of F. oxysporum, its virulence mechanisms, and host plant–pathogen interactions. The control means deployed by Bacillus species inhibit or kill these phytopathogens. Bacillus spp. produce a wide range of secondary metabolites, including volatile and non-volatile organic compounds. Biocontrol potential is achieved through direct antimicrobial activity, the induction of the host plant’s immune response (Induced Systemic Resistance), and competition for nutrients and space. In addition, parameters governing the selection of effective biocontrol agents and their survival in plant microbial communities are discussed. The influence of the microbiota on the establishment and development of biocontrol agents can assess the potential of these treatments and facilitate the development of effective biopesticides during their field application.
1. Introduction
Plant diseases are caused by a variety of pathogens. These diseases are the major limiting factors in agricultural productivity. Plant pathogens cause approximately 10% of plant yield losses globally [1,2]. Many fungal plant pathogens can cause huge losses in the yield and quality of major crops each year [3]. Climate change could increase the abundance of these soil-borne pathogens, with potential negative impacts on food security and the global economy [4]. Worldwide, more than 19,000 genera of fungi are considered plant pests. These fungi cause different types of diseases in crops, like anthracnose, leaf spots, rusts, wilt, gall, and root rot [5].
The Fusarium genus is one of the most destructive and economically important fungi, severely affecting the quality and yield of plants worldwide. The genus includes at least 300 so far identified phylogenetically distinct species [6]. Fusarium oxysporum (Fo) is a plant pathogen that dwells within the soil, enabling it to inhabit diverse ecological environments across different geographical regions. It has a damaging effect on crops, attacks the plant during all phases of growth, and survives on plant debris for long periods. The pathogen encompasses several special forms depending on its host range [7]. Fo causes vascular wilt or root rot in plants. The symptoms of this involve leaf chlorosis, stunted growth, the discoloration of vascular vessels, and wilting leading to death [8].
Controlling soil-borne plant diseases is difficult because no curative treatment is available once the plant is infected [9]. In particular, chemicals are currently being used to combat these diseases [10]. However, their use can pose problems for the environment and human and animal health [11]. Likewise, the resistance of some pathogens to these agrochemicals has increased due to their overuse [12]. The biological control of plant diseases is considered as a viable alternative for treating plant diseases. Using beneficial organism populations to suppress plant pathogenic diseases is a sustainable and environmentally friendly approach [13]. The use of microbial biopesticides can make a significant contribution to global food security [14]. Beneficial bacteria include Gram-positive and spore-forming bacteria that tolerate harsh environments; produce secondary metabolites, enzymes, and other bioactive compounds; are effective against pathogens; and have beneficial effects on plants through various mechanisms [15].
Bacillus is one of the most studied biological control agents (BCA). Their effectiveness is associated with their antagonistic and competitive properties in the rhizosphere [16]. For the inhibitory effect of Bacillus on pathogen growth, various mechanisms are involved, such as the competition for nutrients and space, antibiotic production, hydrolases, siderophores and/or the induction of systemic resistance (ISR) [17]. Many Bacillus species, including B. subtilis, B. licheniformis, B. pumilus, B. amyloliquefaciens, B. cereus, B. filamentous, and B. thuringiensis, are known to inhibit the growth of Fusarium and other phytopathogenic fungi [18].
Given the current situation and the limitations of using chemical crop protection products, a good solution is to develop and produce biopesticides of a natural origin. Therefore, in this review, we attempted to highlight the various biocontrol mechanisms exerted by Bacillus as a biocontrol agent against Fo-induced Fusarium wilt.
The pathogenicity of Fo and the identification of its virulence factors and the interaction between the pathogen and the host plant are also discussed.
Ultimately, this review provides a thorough evaluation of how BCA selection strategies and the soil rhizosphere microbiome impact the stability and efficiency of BCAs, underscoring their critical significance in achieving successful screening outcomes.
2. Fusarium Wilt
Fusarium oxysporum and Its Pathogenecity
Fusarium is a genus of filamentous fungi (Sordariomycetes: Hypocreales: Nectriaceae) that includes many species known as plant pathogens [19]. Fusarium oxysporum (Fo) is a notable species within the genus, causing vascular wilt, a disease that affects important crops and poses a global threat [20,21]. It ranks among the top ten fungal plant pathogens [7]. Fo exhibits high host specificity and is classified into different formae speciales based on the plant species it infects. Fusarium species are classified into over 120 formae speciales (f. sp.) and races [22,23,24]. For example, pathogenic isolates affecting banana are classified as F. oxysporum f. sp. cubense (Foc) [25], and all tomato pathogens are classified as F. oxysporum f. sp. lycopersici (Fol) [26]. F. oxysporum f. sp. albedinis (Foa) is responsible for the vascular fusariosis of date palm, commonly known as Bayoud [27].
Fo causes vascular blight. It produces spores and chlamydospores that can remain viable in the soil for long periods [22,28]. Fo infects plants by germinating spores in response to host signals and invading the root cortex until it reaches the xylem vessels [29]. Fo employs secretion systems, virulence factors such as mycotoxins and cell-wall-degrading enzymes, and effector proteins to invade host cells, penetrate roots, and suppress host defense responses [30,31]. The interactions between plants and their pathogens are constantly changing as pathogens develop new strategies to infect the plant’s vascular system. The process of vascular infection by Fo is intricate and involves sophisticated mechanisms that can sometimes outsmart the host’s defense mechanisms [32]. Several factors contribute to this, including the aggressiveness of the fungal strain, genetic vulnerability of the plant, favorable environmental conditions for infection, and additional stress factors that weaken the plant [33].
In summary, Fo is a fungal pathogen capable of circumventing the defense mechanisms of host plants, which can lead to severe infections. An integrated approach to disease management is essential in order to prevent and control these infections and minimize crop losses [34].
3. Fusarium Wilt Management
Fusarium wilt has been a problem for many years, and numerous strategies have been proposed to control this fungal pathogen. However, attempts to control the disease have shown certain limitations, mainly due to the emergence of new pathogenic strains [35]. The control of Fusarium infection is typically achieved by applying fungicides to the soil. Currently, there is a significant interest in the biological control of plant pathogens, driven by the need to introduce more environmentally friendly alternatives to the widespread use of conventional chemicals [36]. Microbial products currently dominate the biocontrol agents (BCAs) market. It is well established that biological control organisms are the natural enemies of plant pathogens. In this concept, bacteria that naturally live in a close association with plants, known as plant growth-promoting rhizobacteria (PGPR), are used as BCAs against plant pathogens [37]. PGPR are important members of the microbiome and constitute a diverse group of bacteria isolated from the rhizosphere, mainly belonging to the genera Pseudomonas, Streptomyces, Azospirillum, Paenibacillus, Rhizobium, and Bacillus [37,38]. Bacillus species are preferred and considered the most effective and environmentally friendly BCA due to their safety, viability in hostile environments through the formation of stress-resistant endospores, and ability to produce a wide range of antimicrobial compounds [37]. Like other PGPR, Bacillus spp. can enhance plant growth through nitrogen fixation, phosphate solubilization, the production of phytohormones, or alleviation of the impact of certain abiotic stress factors [39].
3.1. Biocontrol Mechanisms by Bacillus
Bacillus spp. is a phenotypically and genotypically heterogeneous group of Gram-positive bacteria that form endospores, and is one of the most common genera found in various environments [5]. Several species of Bacillus have been identified as plant growth-promoting bacteria (PGPB) and/or BCAs [12]. Their extensive use in multiple crops is based on their exceptional characteristics such as increased tolerance to stressful conditions, ability to form endospores, high root colonization capacity, and the production of numerous secondary metabolites, which gives them the potential to be used for biological control [40]. The suppression of plant pathogens by Bacillus involves various mechanisms such as antibiotic production, lytic enzyme secretion, competition for nutrients and space, siderophore production, and the induction of systemic resistance (ISR) (Table 1, Figure 1) [41].
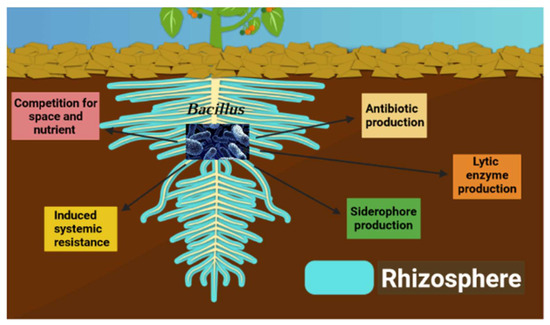
Figure 1.
Bacillus biocontrol mechanisms.
3.1.1. Antibiosis
Antibiosis is a biological process that relies on the release of antimicrobial secondary metabolites by biocontrol agents to confer protection against diseases caused by phytopathogenic agents [42]. The biological control activity of Bacillus spp. has been mainly linked to their ability to produce a wide range of chemically diverse compounds. Bacillus spp. produce a vast array of secondary metabolites, including volatile and non-volatile organic compounds. It has been reported that the portion of the genome involved in the synthesis of antimicrobial compounds and other bioactive secondary metabolites represents 4.5 to 15.4% of the entire genome, depending on the species and strain [37].
The production of secondary metabolites by Bacillus spp., possessing antibiotic properties, is mainly attributed to low-molecular-weight peptides produced through ribosomal processes (such as bacteriocins, which are peptides with antibacterial properties) or non-ribosomal processes (such as cyclic lipopeptides, small peptides, and polyketides) [43].
The main type of non-ribosomal peptide antibiotics are lipopeptides, which are cyclic lipopeptides synthesized by non-ribosomal peptide synthetases (NRPS) of large multi-enzymatic complexes. They are known for their antagonistic activity against a wide range of phytopathogens [44,45]. The most important and extensively studied cyclic lipopeptides in Bacillus spp. belong, based on their chemical structure, to the families of iturins, fengycins, and surfactins [44]. These cyclic lipopeptides act by disrupting the permeability and structure of the cell membrane of pathogens. They can also interact within cells by disrupting DNA [46].
On the other hand, fengycins and iturins are better characterized for their antifungal activities against a wide range of phytopathogenic agents. This is mainly due to their ability to disrupt the integrity of the fungal cell membrane, resulting in cytoplasm leakage and ultimately the death of hyphae with spore germination inhibition [47]. Bacillomycins are among the most well-known iturins for their antifungal activities. Indeed, B. subtilis and B. amyloliquefaciens synthesize bacillomycins L, and bacillomycins D, respectively, which have shown antifungal activities against various phytopathogenic fungi [48]. Zhao et al. [49] demonstrated that lipopeptides synthesized by B. amyloliquefaciens Q-426, such as bacillomycin D, fengycin A, and fengycin B, were responsible for inhibiting the growth of Fo f. sp. spinaciae (Table 1) [49]. Vacuolization was also observed when Foa was cultivated with B. amyloliquefaciens Ag1, and mycelial enlargement and cytoplasmic content leakage were also detected [37]. Fengycin from B. velezensis allowed for a better inhibition of Fusarium mycelial growth than chemical fungicides, and its activity remained stable even at high temperatures and low pH. Fengycin has been reported to increase the permeability and swelling of mycelial membranes with an accumulation of chitin and nucleic acids on the swollen side [50].
The surfactin family (pumilacidin, surfactin, lichenysin, bamilocin, and halobacillin) are cyclic heptapeptides. Surfactins have been identified in B. pumilus, B. subtilis, B. amyloliquefaciens, B. coagulans, and B. licheniformis. They act as both antifungal and antibacterial agents. The inhibitory effect of surfactins is likely not due to direct antagonism but rather due to interference with the pathogen colonization process [51]. Surfactins also have various distinct important elements, such as reducing surface tension and participating in signaling activities [52]. Due to their amphiphilic nature, surfactins can integrate into lipid layers and disrupt the cell membranes of other organisms [53]. Although surfactin may not be considered an antimicrobial molecule, it still has the ability to interact with biological membranes and induce structural modifications [54]. The antimicrobial activity of surfactin may be linked to a synergistic effect with other cyclic lipopeptides. In a study, the combination of surfactin with bacillomycin D or mycosubtilin resulted in more effective control of Fo f. sp. iridacearum. The combined use of surfactin and fengycin is effective against Fo f sp. iridacearum [55]. The co-production of two antifungal compounds by Bacillus strains enhances their antifungal properties. Surfactin and iturin are the most common lipopeptides in Bacillus. Surfactins are potent biological surfactants that increase biofilm formation capacity and swarming motility in Bacillus spp. Surfactins are also highly effective in combating various fungal and bacterial diseases. B. subtilis 9407 is known to produce surfactin and has been found to be highly effective in the biological control of melon seedlings in greenhouses, highlighting the importance of surfactin in biocontrol [56]. Other studies have shown that effective suppression of cucumber fusariosis is achieved through the colonization of the surfactin-producing strain B. subtilis B006 in the rhizosphere (Table 1) [51]. By contributing to motility and biofilm formation, surfactins are involved in the colonization of plant tissues, indirectly allowing Bacillus to outcompete phytopathogens for space and nutrients. Surfactins are also involved in molecular interactions with the host and are well-characterized as triggers of ISR in plants [47].
Other non-ribosomal lipopeptides include kurstakins, bacitracins, polymyxins, tyrocidines, and gramicidins. Kurstakins are cyclic or linear heptapeptides specific to B. thuringiensis and B. cereus, capable of destabilizing the biological membranes of bacteria and fungi [57]. Bacitracins are cyclic decapeptides produced by B. licheniformis, B. sonorensis, and B. subtilis. Polymyxins are cyclic decapeptides produced by P. polymyxa. Tyrocidines and gramicidins are cyclic decapeptides produced by Brevibacillus brevis. These lipopeptides have predominantly antibacterial activity, which can be narrow- or broad-spectrum, targeting a wide range of Gram-negative and Gram-positive bacteria [58].
Apart from lipopeptides, Bacillus spp. synthesize other non-ribosomal antibiotics, including peptides (bacilysin, rhizocticin, amicoumacin, mycobacillin, dodecylpiperazine) and polyketides (bacillene, dihydrobacillene, difficidin, macrolactin), which exhibit antifungal and antibacterial activities [43].
There are three main types of PK (polyketides) produced by B. subtilis species: bacillaene, difficidin, and macrolactin. Polyketides are known for their antibacterial activity, and structural variants can often be produced by the same strain. Difficidin exhibits antimicrobial activity depending on its structure. The derivative of oxydifficidin is approximately three times more active compared to difficidin [59]. Purified macrolactin has been reported to be active against Fo and R. solanacearum [60]. Bacillaene can protect Bacillus spp. against degradative enzymatic activity caused by competitors (Myxococcus xanthus and Streptomyces sp. Mg1) [61,62].
Several ribosomally synthesized antimicrobial peptides produced by Bacillus spp. are effective bacteriocins as antibacterial agents. Bacteriocins exhibit a broad spectrum of antibacterial activity by causing cell lysis, forming pores in the cell membrane, or inhibiting cell wall biosynthesis [63,64]. B. subtilis and related species produce bacteriocins, including lantibiotics, such as plantazolicin, subtilin, ericin, mersacidin, amylolysin, subtilosin, and amylocyclicin [63]. The activity of bacteriocins has occasionally been reported against plant pathogens [65].
Unlike ribosomally synthesized and post-translationally modified peptides, non-ribosomally synthesized molecules appear to be much more conserved within the species. These molecules are synthesized by large modular enzymatic complexes (non-ribosomal peptide synthetases and polyketide synthases) [64]. Due to the modular organization of non-ribosomal peptide synthetases, an increasing number of studies are dedicated to engineering these enzymes to create new lipopeptides with improved properties that could overcome the potential resistance mechanisms of pathogens [66].
In addition to these antimicrobial compounds, Bacillus spp. produce volatile organic compounds (VOCs), which can inhibit the growth and germination of spores of several phytopathogenic fungi [67].
Li et al. [68] proved that among the volatile organic compounds released by B. velezensis CT32, benzothiazole and 2,4-dimethyl-6-tert-butylphenol were the most effective against Fo and the causative agents of strawberry vascular wilt. Similar studies by Yuan et al. [69] demonstrated that the benzothiazoles phenol and 2,3,6-trimethylphenol are among the 12 benzene VOCs produced by B. amyloliquefaciens NJN-6 that could inhibit the activity of Foc. Studies have shown that B. amyloliquefaciens strain L3 exhibits strong antifungal activity against Fon, the causative agent of Fusarium wilt in watermelon, due to the release of 2-nonanone and 2-heptanone [67]. Another study revealed that the antifungal activity of ketones emitted by B. amyloliquefaciens NJN-6 against Foc was negatively correlated with the number of carbon atoms in the ketones. The ketones 2-nonanone (nine carbon atoms) and 2-decanone (ten carbon atoms) exhibited 100% inhibition of the pathogen, while 2-tetradecanone (14 carbon atoms) and 2-pentadecanone (15 carbon atoms) showed weak antifungal effects [69]. The significance of ketones in biocontrol and the negative correlation between the number of carbon atoms and their antifungal activity were also addressed in the study by Poulaki, and Tjamos [70].
Volatiles act as elicitors by activating host plant defense genes to inhibit pathogen growth [71]. B. subtilis isolates release a wide variety of volatile compounds of various chemical nature, and approximately 231 volatile substances have been described to date. Recent studies have thus shown the effectiveness of volatile metabolites released by the B. subtilis GB03 for growth stimulation and systemic resistance induction in Arabidopsis thaliana [72]. On the other hand, B. megaterium produces ammonia which has been shown to be very effective in inhibiting pathogenic Fo species [73].

Table 1.
Biocontrol mechanisms exhibited by Bacillus species against Fusarium oxysporum.
Table 1.
Biocontrol mechanisms exhibited by Bacillus species against Fusarium oxysporum.
| Bacillus Species | Mechanism(s) | Pathogen(s) | Plant Disease | References |
|---|---|---|---|---|
| B. amyloliquefaciens Q-426 | Bacillomycine D, Fengycine A | Fusarium oxysporum f. sp. spinaciae | Fusarium wilt of spinach | [49] |
| B. subtilis B006 | Surfactine | F. oxysporum f. sp. cucumerinum | Fusarium wilt of cucumber | [51] |
| B. amyloliquefaciens NJN-6 | Benzothiazoles phenol, 2,3,6-trimethylphenol | F. oxysporum f. sp. cubens | Fusarium wilt of Banana | [69] |
| B. subtilis B8, Bacillus sp. B5 | Chitinase | F. oxysporum | Plant wilt | [74] |
| Bacillus sp. B44 | β-1,3-glucanase, chitinase, protease, volatile and non-volatile metabolites | F. oxysporum f. sp. lycopersici | Fusarium wilt of tomato | [75] |
| B. subtilis YB-04 | Protease, amylase, celullase, β-1,3-glucanase, siderophore | F. oxysporum f. sp. cucumerinum | Fusarium wilt of cucumber | [50] |
| Bacillus subtilis | Siderophore, chitinase, β-1,3-glucanase, protease | F. oxysporum f. sp. capsici | Fusarium wilt of pepper | [76] |
| B. subtilis EPCO16 | Siderophore, chitinase, β-1,3-glucanase, protease | F. oxysporum f. sp. lycopersici | Fusarium wilt of tomato | [77] |
| B. subtilis SQR 9 | Competition | F. oxysporum f. sp. cucumerinum | Fusarium wilt of cucumber | [78] |
| B. cereus MH778713 | Competition | F. oxysporum | Fusarium wilt | [12] |
| B. amyloliquefaciens Ag1 | Peroxidase, hydrocinnamic acid | F. oxysporum f. sp. albedinis | Fusarium wilt of date palm | [37] |
| B. subtilis | Peroxidase, polyphenol oxidase, phenylalanine ammonia-lyase | F. oxysporum f. sp. cucumerinum | Fusarium wilt of cucumber | [79] |
| B. licheniformis CSR-D4 | β-1,3 glucanase, peroxidase, chitinase, polyphenol oxidase, Phenyl-alanine ammonia-lyase | F. oxysporum f. sp. cubens | Fusarium wilt of Banana | [80] |
3.1.2. Production of Lytic Enzymes
The antagonistic activities of enzymes are an important strategy in the suppression of plant diseases. These enzymes are involved in degrading the cell wall of phytopathogenic microorganisms, being one of the most commonly reported biological control mechanisms, primarily against fungal pathogens. Among the lytic enzymes produced by the studied biological control agents are chitinases, cellulases, proteases, and β-1,3-glucanases, which degrade the structure of the cell wall [81,82]. Chitinases and glucanases have great potential for managing diseases caused by fungal phytopathogens [83]. Chitinases break down the glycosidic bonds of chitin, the main component of the fungal cell wall [84]. Many Bacillus spp. species, including B. cereus, B. thuringiensis, B. subtilis, B. licheniformis, B. amyloliquefaciens, B. safensis, B. pumilus, B. velezensis, etc., produce chitinases that have shown inhibitory effects on Fo. Another study identified the main antifungal component as a chitinase produced by B. subtilis [85]. Brzezinska et al. [74] demonstrated that both B. subtilis B8 and Bacillus sp. B5 strains inhibit the growth of Fo by exhibiting strong chitinolytic activity. A reduction of 20 to 35% in the number of diseased plants was observed in tomato seedlings in greenhouse and field trials, respectively, when inoculated with a chitinase-producing strain of B. subtilis [86].
On the other hand, β-glucanases hydrolyze the polysaccharide β-glucan into monomeric or oligomeric glucose saccharide. β-glucan is the second major polysaccharide in the cell wall of phytopathogenic fungi [87]. It has been demonstrated that B. subtilis W3.15, a producer of β-glucanase, shows a strong inhibition of Fo growth with a significant reduction in fungal biomass [88]. In addition to chitin and glucan, the fungal cell wall skeleton contains cellulose, lipids, and proteins. Bacterial cellulases, lipases, and proteases can therefore play an important role in the lysis of the cell wall that occurs during pathogen–bacteria interactions [81]. It has been reported that the purified alkaline protease from B. amyloliquefaciens SP1 inhibits the growth of Fo [89]. Jangir et al. [75] demonstrated that the control of Fol by Bacillus sp. B44 was associated with the production of β-1,3-glucanase, chitinase, protease, and volatile and non-volatile metabolites. Furthermore, the secretion of extracellular enzymes such as protease, amylase, cellulase, glucanase, and the production of siderophores by B. subtilis YB-04 showed great effectiveness in the biological control of Foc, the causal agent of cucumber Fusarium wilt [50]. Mixtures of hydrolytic enzymes with complementary modes of action may be necessary for maximum effectiveness, and proper combinations of enzymes can enhance antifungal activity [90,91]. Overall, these studies clearly indicate that strains of Bacillus spp. with these hydrolytic enzymes are an important tool in the effective management of fungal diseases in plants.
3.1.3. Production of Siderophore
Iron (Fe) deficiency is common and is associated with high pH and calcareous soils, which cover approximately 39% of the Earth’s crust. Siderophores are low-molecular-weight organic compounds with high affinity that can bind certain elements, such as Fe3+ and other metal ions, and increase their availability in soil [92]. Siderophore-mediated iron competition is one of the mechanisms for antagonistic activity [44]. In fact, siderophores significantly reduced the amount of iron ions available to certain rhizosphere microbial communities and inhibited the growth of phytopathogenic fungi. On the contrary, siderophores can be used in agricultural disease management and in the promotion of plant growth [93].
According to their structure, siderophores are classified into three functional groups including hydroxamate, catecholate, and carboxylate [94]. Catecholate is the main siderophore produced by Bacillus sp. like bacillibactin [95]. Bacillibactins with built-in threonine are known for their highest affinity for iron (Fe3+) and have been described as the most dominant extracellular iron scavengers of B. subtilis under iron-limited conditions [96]. In addition to bacillibactin, B. subtilis, B. amyloliquefaciens, B. cereus, and B. anthracis produce other siderophores such as schizokinene and petrobactin which are also used for the biocontrol of foliar and post-harvest soil fungal pathogens [97].
The genus Bacillus produce siderophores that are related to the suppression of various plant diseases (Table 1). Ghosh et al. [76] reported that siderophores produced by B. subtilis play an important role in the biological control of F. oxysporum Schl. f.sp. capsici cause the Fusarium wilt of pepper. Other results published by Ramyabharathi and Raguchander [77] found that B. subtilis EPCO16 was able to inhibit Fol growth and reduce the occurrence of tomato wilt by producing siderophores and lytic enzymes (chitinase, β-1,3-glucanase, protease).
In addition to iron chelation, siderophores may have other functions, such as the transport of nonferrous metals, sequestration of toxic metals, signaling, protection against oxidative stress, and antibiotic activity [98].
The use of siderophore-producing endophytes as biological control agents is considered a promising solution for overcoming plant diseases. For example, in a study by Yu et al. [99], the siderophore-producing B. subtilis CAS15, capable of controlling Fusarium wilt and improving pepper growth, was reported.
3.1.4. Competition for Space and Nutrients
Biological control occurs when pathogens and nonpathogenic organisms compete for space and nutrients around a host plant. The surfaces of plants and soil contain finite amounts of nutrients. Therefore, to effectively colonize plant surfaces, pathogenic and nonpathogenic microorganisms must compete to meet their nutritional requirements [83]. The bacterial colonization of plant roots is a critical step in the interaction of microorganisms with plants and thus appears to be important for their growth-promoting and biocontrolling effects. Thus, successful root colonization depends on bacterial properties, including motility and biofilm formation, as well as signaling interactions with the plant [100].
Root exudates consist of amino acids, carbohydrates, and carboxylic acids that provide a food source and signals that attract microorganisms to the rhizosphere, known as bacterial chemotaxis. Chemotaxis is necessary to establish the initial interaction between the host and microorganisms, which is a fundamental step towards the root system [101].
The effectiveness of Bacillus in biocontrol, and its persistence in highly competitive niches in the rhizosphere, derives from its great potential to synthesize various VOCs and soluble bioactive secondary metabolites. The latter are linked to the process of root colonization which indirectly protects the plant by reducing the space for pathogens and the availability of nutrients, as they are involved in the development of Bacillus motility and biofilm formation [47]. In their study, Cao et al. [78] demonstrated the successful colonization and survival of B. subtilis strain SQR 9 within the rhizosphere of cucumber plants. They further found that this strain exhibited inhibitory effects on cucumber wilt by impeding the growth of the fungal pathogen Foc in the rhizosphere. Therefore, the inoculation of tomato plants with B. cereus MH778713 can reduce and control Fo-induced Fusarium wilt [12].
3.1.5. Induced Systemic Resistance (ISR)
Induced Systemic Resistance is one of the biocontrol mechanisms by which PGPB can cause physiological changes and induce host defenses, thereby conferring protection against a variety of pathogens [102]. PGPB produce various chemical triggers such as lipopolysaccharides, siderophores, cyclic lipopeptides, DAPG, acyl homoserine lactones, and volatile compounds [103].
Unlike SAR, the ISR signaling pathway does not depend on the accumulation of salicylic acid SA and is completely dependent on jasmonic acid (JA) and ethylene [104]. ISR is also accompanied by the increased expression of PR genes and plant defensin 1.2 (PDF1.2) [105] and enhanced activity of defense substances such as phenylalanine ammonia lyase, polyphenol oxidase, peroxidase, β-1,3-glucanase, and chitinase, with ROS accumulation [33].
Previous studies have evaluated the effectiveness of several Bacillus strains in reducing plant disease by ISR in greenhouse and field trials [106]. An ISR state has been reported to be induced in plants when certain Bacillus root endophytes are applied to seeds or seedlings. This protects plants from various biotic and abiotic stresses [12]. To mitigate the adverse effects of infections caused by pathogens, Bacillus spp. reduce lipid peroxidation, increase ascorbate peroxidase, catalase, peroxidase, glutathione peroxidase, polyphenol oxidase, and other antioxidant enzymes, thereby increasing the production of other defense enzymes such as phenylalanine ammonia lyase, chitinase, β-1,3-glucanase, and phenolic acids [107].
The activity of various antioxidant enzymes helps plants to suppress the levels of ROS, a source of oxidative stress during pathogen infection. Thus, antioxidant enzymes were found to be responsible for the production of phenolic compounds that help to strengthen the cellular barrier. Indeed, specific phenolic compounds and flavonoids induce resistance in host plants after inoculation with pathogens [108].
Dimkic et al. [37] reported that B. amyloliquefaciens Ag1 increased peroxidase activity and induced the synthesis of hydrocinnamic acid derivatives in date palm roots before and after Foa infection. The authors proposed that increased peroxidase activity is responsible for cell wall strengthening and the inhibition of pathogens from the necrotic zone of date palm roots, and that phenolic components increase the disease resistance of host plants. Additional work by Jinal et al. [109] demonstrated that the B. paralicheniformis (EAL) strain was effective in enhancing the antioxidant enzyme (peroxidase, superoxide dismutase, phenylalanine ammonia lyase, polyphenol oxidase, and proline) production potential of Fo-infected tomato plants compared to control plants (not treated with B. paralicheniformis).
Other work by Chen et al. [79] found that B. subtilis was able to reduce the incidence of Foc-induced bacterial wilt, promote seedling growth, and increase the activities of antioxidant enzymes in cucumber seedlings. Thus, it was shown that banana plants inoculated with B. licheniformis CSR-D4, producing β-1,3-glucanase, peroxidase, chitinase, polyphenol oxidase, and phenylalanine ammonia enzymes, have resistance to Foc Tropical Race 4 (FOC TR4) compared to untreated plants (Table 1) [80].
3.2. In Situ BCA Responses
For the biological control of this Fusarium wilt, the selection of microorganisms that have an antagonistic effect on the pathogen is a very important criterion. Antagonists must be effective in situ with the ability to compete or provide antibiosis [11], and be able to rapidly colonize plant roots [110]. Therefore, the development of an effective biocontrol agent requires a selection strategy that must take into account several parameters [111].
To optimize the selection of disease-specific BCA, it is crucial to focus on the rhizomicrobiome of infected plants. It is advisable to conduct sampling in ecological niches that accurately represent the specific agricultural area where the BCA will ultimately be utilized. Within this zone, both the pathogen and its antagonist coexist, making it an ideal location for sampling. Thus, BCA must be based on autochthonous bacterial strains because the introduction of allochthonous bacteria could become ineffective. BCA from the rhizosphere of diseased plants showed high root colonization and biocontrol capacity, which appeared to be a result of their adaptation to the environment of ubiquitous pathogens [112]. The theory that diseased plants or soil are good sources of potential BCA is well supported [113]. BCA develop specific survival strategies in response to the proliferation of pathogens, making them competitive candidates.
Most screenings for biocontrol organisms are based on the release of diffusible antimicrobial metabolites (antibiotics, lipopeptides, etc.). The conventional method for the in vitro screening of isolates is a dual culture assay against the pathogen of interest [114] and against other pathogens to select a BCA with a broad spectrum of activity [113,115]. Bacillus species are very efficient producers of antibiotic molecules and their inhibitory activity against plant pathogens via antibacterial action is the best-known mechanism [37]. Numerous critical studies and reviews have been devoted to elucidating the remarkable effects of Bacillus metabolites on plant pathogens [38,48,116]. However, other performances are required for BCA efficiency. They involve the production of other metabolites, volatile (VOC, HCN, NH3) or not (lytic enzymes; chitinases, protease, cellulases, siderophores) [117,118]. This arsenal of metabolites released by Bacillus species contribute effectively to the performance of disease control by inhibiting the growth of phytopathogens and by activating plant immunity against various stressors [119]. Despite the existence of all these potentialities, the capacity for root colonization and competition for space and nutrients remain the critical points of BCA competence. These properties can be examined in vivo by inoculation into a workable gnotobiotic system on model plants or on the target plant under controlled conditions [120,121]. In this case, the interaction of the BCA of the plant and the pathogen are the only parameters to be controlled. Although several studies have mentioned the effectiveness of BCA following metabolite production on a medium, few studies have compared the results of different screening strategies and concluded that whole plant testing was the most effective strategy [113,122]. Screening methods require multifactorial or plant-mediated control mechanisms. Not only nutrient sources but also signaling molecules and chemoattractants are to be considered, often very specific for certain species or even bacterial strains, which could improve the effectiveness of a BCA against the host pathogens of the plants [123]. In addition, BCA was tested for its PGP activity as an alternative to chemical pesticides, but in addition to promoting plant growth and alleviating various natural environmental stressors. Thus, multiple desirable properties present in a single inoculant or population of bacteria can be successfully exploited.
On the other hand, the behavior of BCA incorporated into the soil and its interaction with plant microbial communities requires additional measures. Once inoculated into soil, BCA encounters and interacts with distinct microbial communities depending on the plant species and environmental factors. Therefore, the survival and availability of BCAs in soil are essential. Understanding the structure and function of the microbial communities could enable the potential of these bacterial inoculants to be appreciated and emphasized. Bacterial inoculants must be present in the plant rhizosphere at sufficient biomass density and for a sufficient length of time to have an active effect, especially in crop protection. This depends on the ability of the bacterial inoculant to proliferate and effectively colonize the root system, which is widely considered to be the rate-limiting step in biological control. Biological control can be ineffective in situ due to the poor colonization of bacteria in the rhizosphere [124]. For this, PGPR must have specific properties that enable them to be used effectively. They must be able to survive in the soil, be compatible with the crop on which they are inoculated, and interact with the native soil microflora and abiotic factors [125,126]. The intrinsic productive capacity of a strain is affected by environmental factors. Once a beneficial microbial strain is able to colonize a host plant, it may be able to synthesize a variety of activities that contribute to plant fitness [104].
The inoculation of external strains can alter the structure of the microbial community. It should be noted that the inoculated PGPB positively influenced the rhizosphere by stimulating its beneficial bacterial community [127,128,129]. Bio-inoculants can alter the balance of bacterial communities to select for beneficial populations [127]. Inoculated bacteria may induce growth hormones and other metabolites that promote the proliferation of other native bacteria. Interactions between the microbiota and BCA will affect pathogen control and pathogenesis. Thus, these bacterial species can be used to reinforce or activate the defense systems of plant species due to additive or synergistic effects between strains [130,131]. Functional microbiota can be inherited vertically through the seed [132] or horizontally through the environment. Bacterial diversification stems from their ability to perform horizontal gene transfer between different phylogenetic groups [133].
Therefore, manipulating plant microbiota by using PGPB and exploiting its physiological properties is one of the possible strategies to modify the rhizosphere and reduce chemical usage in agriculture. Therefore, the increased rigor in the selection of antagonists in order to obtain strains having all the abilities to eliminate the fungus, and their persistence in the soil after inoculation are decisive for their successful colonization and population establishment in the rhizosphere [120].
4. Conclusions and Perspectives
Bacillus has become the leading candidate for combating plant diseases. Its beneficial use as a BCA in agricultural applications makes it possible to reduce the use of pesticides, which can have harmful effects on human, animal, and environmental health. Bacillus possesses various biological control mechanisms, such as the production of numerous secondary metabolites, lytic enzymes, and siderophores, and stimulates systemic resistance in plants. Bacillus acts by inhibiting the growth of harmful microorganisms through their ability to produce lipopeptides, which form the basis of their beneficial effects. They have stimulating effects on plant growth. They promote seed germination, increase plant resistance to environmental stress, improve nutrient absorption, and strengthen the plant immune system. LPs act by forming a protective barrier around plant roots, preventing the adhesion and colonization of pathogens. The result is healthier crops, higher productivity, and reduced losses due to plant diseases. In addition, LPs are environmentally friendly and pose no major risks to human or animal health. They degrade rapidly in the environment and do not accumulate toxic residues. Moreover, the ability of Bacillus to form endospores is a feature of particular interest, as it enables them to survive under conditions of abiotic stress, facilitating their production and storage over long periods. On the other hand, after inoculation with these biopesticides, it is possible to observe a dual effect on agricultural crops due to the action of biological control, attenuating phytopathogens and indirectly promoting plant growth with improved health.
This study also provides an improved strategy for optimizing the selection of antagonistic microorganisms to combat plant diseases. It is essential to study the complex interactions that occur between plants and pathogens. Exogenous inoculants and microbiota can be identified and analyzed to improve the efficacy of Bacillus as a BCA. A better understanding of the colonization capacity of Bacillus species, as well as their mechanisms of action, is necessary for this future research.
In addition, there are promising prospects for the development and application of Bacillus as a biopesticide using bioformulation techniques. These strategies can modify the rhizosphere and reduce the dependence on chemical inputs in agriculture.
The current global trend is to move away from the use of chemicals, which can have harmful effects on the health of consumers and the farmers who use them. The growing supply of biopesticides and biofertilizers on the market meets the global demand to reduce chemical pesticides and promote organic farming. In addition to the environmental benefits, these biological solutions also offer developing countries cheaper alternatives that can help to alleviate food shortages and promote sustainable development in these regions.
Biological control is an attractive and constantly evolving option for the development of sustainable agriculture. The intensification of agriculture, climate change, and changes in land use pose a threat to the biodiversity and stability of microbial communities. As a result, it is becoming essential to intensify the application of biological control methods, improve their efficacy, and develop Bacillus-based products. These efforts are both promising and of great importance.
In summary, the use of Bacillus as a biological control agent offers potential benefits for agriculture, notably by reducing the use of chemical agents and promoting more sustainable practices. The Bacillus subtilis species is one of the most well-studied and widely used. It is generally recognized as safe (GRAS) by regulatory authorities. Their broad spectrum of antimicrobial activity, their ability to stimulate plant growth, and their environmental safety make them valuable allies in developing effective bioformulation techniques for cleaner and more environmentally friendly agriculture in the years to come.
Author Contributions
S.B., H.C.-S., A.S., L.L., F.N.A. and L.B.; methodology, S.B., H.C.-S., A.S. and L.B.; software, S.B., H.C.-S., L.L., F.N.A. and A.S.; validation, S.B., H.C.-S., A.S. and L.B.; formal analysis, S.B., H.C.-S., A.S., A.C.B. and L.B.; investigation, S.B., H.C.-S., A.S., A.C.B., L.L., F.N.A. and L.B.; resources, H.C.-S., A.C.B. and L.B.; data curation, S.B. and H.C.-S.; writing—original draft preparation, S.B., H.C.-S., A.S. and L.B.; writing—review and editing, S.B., H.C.-S., A.S. and L.B.; visualization, S.B., H.C.-S., A.S., A.C.B. and L.B.; supervision, H.C.-S., A.S. and L.B.; project administration, H.C.-S., A.S. and L.B.; funding acquisition, H.C.-S. and A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Savary, S.; Willocquet, L.; Pethybridge, S.J.; Esker, P.; McRoberts, N.; Nelson, A. The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol. 2019, 3, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Verma, J.P.; Liu, H.; Wang, J.; Batista, B.D.; Kaur, S.; de Araujo Pereira, A.P.; Macdonald, C.A.; Trivedi, P.; Weaver, T.; et al. Response of the plant core microbiome to Fusarium oxysporum infection and identification of the pathobiome. Environ. Microbiol. 2022, 24, 4652–4669. [Google Scholar] [CrossRef] [PubMed]
- Pandaranayaka, E.P.; Frenkel, O.; Elad, Y.; Prusky, D.; Harel, A. Network analysis exposes core functions in major lifestyles of fungal and oomycete plant pathogens. BMC Genom. 2019, 20, 445. [Google Scholar] [CrossRef]
- Yang, J.; Hsiang, T.; Bhadauria, V.; Chen, X.L.; Li, G. Plant fungal pathogenesis. BioMed Res. Int. 2017, 2017, 9724283. [Google Scholar] [CrossRef]
- Jain, A.; Sarsaiya, S.; Wu, Q.; Lu, Y.; Shi, J. A review of plant leaf fungal diseases and its environment speciation. Bioengineered 2019, 10, 409–424. [Google Scholar] [CrossRef]
- Rampersad, S.N. Pathogenomics and management of Fusarium diseases in plants. Pathogens 2020, 9, 340. [Google Scholar] [CrossRef] [PubMed]
- Dean, R.; Van Kan, J.A.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef] [PubMed]
- Soleha, S.; Muslim, A.; Suwandi, S.; Kadir, S.; Pratama, R. The identification and pathogenicity of Fusarium oxysporum causing acacia seedling wilt disease. J. For. Res. 2022, 33, 711–719. [Google Scholar] [CrossRef]
- de Lamo, F.J.; Šimkovicová, M.; Fresno, D.H.; de Groot, T.; Tintor, N.; Rep, M.; Takken, F.L. Pattern-triggered immunity restricts host colonization by endophytic fusaria, but does not affect endophyte-mediated resistance. Mol. Plant Pathol. 2021, 22, 204–215. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Han, M.; Hu, Y.; Li, Z.; Liu, C.; Wang, X.; Tian, Q.; Jiao, W.; Hu, J.; Liu, L.; et al. Effects of continuous cropping of sweet potato on the fungal community structure in rhizospheric soil. Front. Microbiol. 2019, 10, 2269. [Google Scholar] [CrossRef]
- Carmona-Hernandez, S.; Reyes-Pérez, J.J.; Chiquito-Contreras, R.G.; Rincon-Enriquez, G.; Cerdan-Cabrera, C.R.; Hernandez-Montiel, L.G. Biocontrol of postharvest fruit fungal diseases by bacterial antagonists: A review. Agronomy 2019, 9, 121. [Google Scholar] [CrossRef]
- Ramírez, V.; Martínez, J.; Bustillos-Cristales, M.D.R.; Catañeda-Antonio, D.; Munive, J.A.; Baez, A. Bacillus cereus MH778713 elicits tomato plant protection against Fusarium oxysporum. J. Appl. Microbiol. 2022, 132, 470–482. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Purvis, B.; Mao, Y.; Robinson, D. Three pillars of sustainability: In search of conceptual origins. Sustain. Sci. 2019, 14, 681–695. [Google Scholar] [CrossRef]
- Tzipilevich, E.; Russ, D.; Dangl, J.L.; Benfey, P.N. Plant immune system activation is necessary for efficient root colonization by auxin-secreting beneficial bacteria. Cell Host Microbe 2021, 29, 1507–1520. [Google Scholar] [CrossRef] [PubMed]
- Grosu, A.I.; Sicuia, O.A.; Dobre, A.; Voaideş, C.; Cornea, C.P. Evaluation of Some Bacillus spp. Strains for the Biocontrol of Fusarium graminearum and F. culmorum in Wheat. Agric. Agric. Sci. Procedia 2015, 6, 559–566. [Google Scholar] [CrossRef]
- Miljaković, D.; Marinković, J.; Balešević-Tubić, S. The significance of Bacillus spp. in disease suppression and growth promotion of field and vegetable crops. Microorganisms 2020, 8, 1037. [Google Scholar] [CrossRef]
- Castillo, H.F.D.; Reyes, C.F.; Morales, G.G.; Herrera, R.R.; Aguilar, C. Biological control of root pathogens by plant growth promoting Bacillus spp. In Weed and Pest Control-Conventional and New Challenges; Soloneski, S., Larramendy, M., Eds.; IntechOpen: London, UK, 2013; pp. 79–103. [Google Scholar]
- Ma, L.J.; Geiser, D.M.; Proctor, R.H.; Rooney, A.P.; O’Donnell, K.; Trail, F.; Gardiner, D.M.; Manners, J.M.; Kazan, K. Fusarium pathogenomics. Annu. Rev. Microbiol. 2013, 67, 399–416. [Google Scholar] [CrossRef] [PubMed]
- Al-Hatmi, A.M.; Meis, J.F.; de Hoog, G.S. Fusarium: Molecular diversity and intrinsic drug resistance. PLoS Pathog. 2016, 12, e1005464. [Google Scholar] [CrossRef]
- Burgess, L.W.; Summerell, B.A. Mycogeography of Fusarium: Survey of Fusarium species in subtropical and semi-arid grassland soils from Queensland, Australia. Mycol. Res. 1992, 96, 780–784. [Google Scholar] [CrossRef]
- De Lamo, F.J.; Takken, F.L. Biocontrol by Fusarium oxysporum using endophyte-mediated resistance. Front. Plant Sci. 2020, 11, 37. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, G.M.; Armstrong, J.K. Another approach to race classification of Fusarium oxysporum f. sp. pisi. Phytopathology 1981, 71, 474–478. [Google Scholar] [CrossRef]
- Taylor, A.; Vágány, V.; Jackson, A.C.; Harrison, R.J.; Rainoni, A.; Clarkson, J.P. Identification of pathogenicity-related genes in Fusarium oxysporum f. sp. cepae. Mol. Plant Pathol. 2016, 17, 1032–1047. [Google Scholar] [CrossRef]
- Maryani, N.; Lombard, L.; Poerba, Y.S.; Subandiyah, S.; Crous, P.W.; Kema, G.H.J. Phylogeny and genetic diversity of the banana Fusarium wilt pathogen Fusarium oxysporum f. sp. cubense in the Indonesian center of origin. Stud. Mycol. 2018, 91, 79–99. [Google Scholar]
- Srinivas, C.; Devi, D.N.; Murthy, K.N.; Mohan, C.D.; Lakshmeesha, T.R.; Singh, B.; Kalagatur, N.L.; Niranjana, S.R.; Hashem, A.; Alqarawi, A.A.; et al. Fusarium oxysporum f. sp. lycopersici causal agent of vascular wilt disease of tomato: Biology to diversity—A review. Saudi J. Biol. Sci. 2019, 26, 1315–1324. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, Y.; Hussien, A.; El-badry, N.; Soliman, M.S. Validation of a Diagnostic Protocol for the Detection of Fusarium oxysporum f.sp. albedinis, the Causal Agent of Bayoud Disease of Date Palm. Egypt. J. Phytopathol. 2019, 47, 297–312. [Google Scholar]
- Crous, P.W.; Sandoval-Denis, M.; Costa, M.M.; Groenewald, J.Z.; van Iperen, A.L.; Starink-Willemse, M.; Hernández-Restrepo, M.; Kandemir, H.; Ulaszewski, B.; de Boer, W.; et al. Fusarium and allied fusarioid taxa (FUSA). 1. Fungal Syst. Evol. 2022, 9, 161–200. [Google Scholar] [CrossRef] [PubMed]
- Perez-Nadales, E.; Nogueira, M.F.A.; Baldin, C.; Castanheira, S.; El Ghalid, M.; Grund, E.; Lengeler, K.; Marchegiani, E.; Mehrotra, P.V.; Moretti, M.; et al. Fungal model systems and the elucidation of pathogenicity determinants. Fungal Genet. Biol. 2014, 70, 42–67. [Google Scholar] [CrossRef]
- Zuriegat, Q.; Zheng, Y.; Liu, H.; Wang, Z.; Yun, Y. Current progress on pathogenicity-related transcription factors in Fusarium oxysporum. Mol. Plant Pathol. 2021, 22, 882–895. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, R.; Viljoen, A.; Myburg, A.A.; van den Berg, N. Pathogenicity associated genes in Fusarium oxysporum f. sp. cubense race 4. S. Afr. J. Sci. 2013, 109, 10. [Google Scholar] [CrossRef]
- Husaini, A.M.; Sakina, A.; Cambay, S.R. Host–pathogen interaction in Fusarium oxysporum infections: Where do we stand? Mol. Plant-Microbe Interact. 2018, 31, 889–898. [Google Scholar] [CrossRef] [PubMed]
- Vlot, A.C.; Sales, J.H.; Lenk, M.; Bauer, K.; Brambilla, A.; Sommer, A.; Chen, Y.; Wenig, M.; Nayem, S. Systemic propagation of immunity in plants. New Phytol. 2021, 229, 1234–1250. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Liu, L.; Wang, L.; Wang, Q. Indole primes plant defense against necrotrophic fungal pathogen infection. PLoS ONE 2018, 13, e0207607. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.C.; Bettiol, W. Potential of non-pathogenic Fusarium oxysporum isolates for control of Fusarium wilt of tomato. Fitopatol. Brasil. 2005, 30, 409–412. [Google Scholar] [CrossRef]
- Gan, B.H.; Gaynord, J.; Rowe, S.M.; Deingruber, T.; Spring, D.R. The multifaceted nature of antimicrobial peptides: Current synthetic chemistry approaches and future directions. Chem. Soc. Rev. 2021, 50, 7820–7880. [Google Scholar] [CrossRef]
- Dimkić, I.; Janakiev, T.; Petrović, M.; Degrassi, G.; Fira, D. Plant-associated Bacillus and Pseudomonas antimicrobial activities in plant disease suppression via biological control mechanisms—A review. Physiol. Mol. Plant Pathol. 2022, 117, 101754. [Google Scholar] [CrossRef]
- Bakkera, M.G.; Gloverb, J.D.; Maib, J.G.; Kinkela, L.L. Plant community effects on the diversity and pathogen suppressive activity of soil Streptomycetes. Appl. Soil Ecol. 2010, 46, 35–42. [Google Scholar] [CrossRef]
- Cherif-Silini, H.; Silini, A.; Yahiaoui, B.; Ouzari, I.; Boudabous, A. Phylogenetic and plant-growth-promoting characteristics of Bacillus isolated from the wheat rhizosphere. Ann. Microbiol. 2016, 66, 1087–1097. [Google Scholar] [CrossRef]
- Samaras, A.; Roumeliotis, E.; Ntasiou, P.; Karaoglanidis, G. Bacillus subtilis MBI600 promotes growth of tomato plants and induces systemic resistance contributing to the control of soilborne pathogens. Plants 2021, 10, 1113. [Google Scholar] [CrossRef]
- Beneduzi, A.; Ambrosini, A.; Passaglia, L.M. Plant growth-promoting rhizobacteria (PGPR): Their potential as antagonists and biocontrol agents. Genet. Mol. Biol. 2012, 35, 1044–1051. [Google Scholar] [CrossRef]
- Latha, P.; Karthikeyan, M.; Rajeswari, E. Endophytic bacteria: Prospects and applications for the plant disease management. In Plant Health under Biotic Stress; Ansari, R., Mahmood, I., Eds.; Springer: Singapore, 2019; pp. 1–50. [Google Scholar] [CrossRef]
- Wang, T.; Liang, Y.; Wu, M.; Chen, Z.; Lin, J.; Yang, L. Natural products from Bacillus subtilis with antimicrobial properties. Chin. J. Chem. Eng. 2015, 23, 744–754. [Google Scholar] [CrossRef]
- Wang, X.Q.; Zhao, D.L.; Shen, L.L.; Jing, C.L.; Zhang, C.S. Application and mechanisms of Bacillus subtilis in biological control of plant disease. In Role of Rhizospheric Microbes in Soil; Meena, V., Ed.; Springer: Singapore, 2018; pp. 225–250. [Google Scholar] [CrossRef]
- Luo, C.; Liu, X.; Zhou, H.; Wang, X.; Chen, Z. Non-ribosomal peptide synthase gene clusters for lipopeptide biosynthesis in Bacillus subtilis 916 and their phenotypic functions. Appl. Environ. Microbiol. 2015, 81, 422–431. [Google Scholar] [CrossRef]
- Etesami, H.; Jeong, B.R.; Glick, B.R. Biocontrol of plant diseases by Bacillus spp. Physiol. Mol. Plant Pathol. 2023, 126, 102048. [Google Scholar] [CrossRef]
- Andrić, S.; Meyer, T.; Ongena, M. Bacillus responses to plant-associated fungal and bacterial communities. Front. Microbiol. 2020, 11, 1350. [Google Scholar] [CrossRef]
- Kaspar, F.; Neubauer, P.; Gimpel, M. Bioactive secondary metabolites from Bacillus subtilis: A comprehensive review. J. Nat. Prod. 2019, 82, 2038–2053. [Google Scholar] [CrossRef]
- Zhao, P.; Quan, C.; Wang, Y.; Wang, J.; Fan, S. Bacillus amyloliquefaciens Q-426 as a potential biocontrol agent against Fusarium oxysporum f. sp. spinaciae. J. Basic Microbiol. 2014, 54, 448–456. [Google Scholar] [CrossRef]
- Xu, W.; Yang, Q.; Yang, F.; Xie, X.; Goodwin, P.H.; Deng, X.; Tian, B.; Yang, L. Evaluation and genome analysis of Bacillus subtilis YB-04 as a potential biocontrol agent against Fusarium wilt and growth promotion agent of Cucumber. Front. Microbiol. 2022, 13, 885430. [Google Scholar] [CrossRef] [PubMed]
- Jia, K.; Gao, Y.H.; Huang, X.Q.; Guo, R.J.; Li, S.D. Rhizosphere inhibition of cucumber Fusarium wilt by different surfactin-excreting strains of Bacillus subtilis. Plant Pathol. J. 2015, 31, 140. [Google Scholar] [CrossRef] [PubMed]
- Sansinenea, E.; Ortiz, A. Secondary metabolites of soil Bacillus spp. Biotechnol. Lett. 2011, 33, 1523–1538. [Google Scholar] [CrossRef] [PubMed]
- Ongena, M.; Jacques, P. Bacillus lipopeptides: Versatile weapons for plant disease biocontrol. Trends Microbiol. 2008, 16, 115–125. [Google Scholar] [CrossRef]
- Deleu, M.; Lorent, J.; Lins, L.; Brasseur, R.; Braun, N.; El Kirat, K.; Nylander, T.; Dufrêne, Y.F.; Mingeot-Leclercq, M.P. Effects of surfactin on membrane models displaying lipid phase separation. Biochim. Biophys. Acta (BBA)-Biomembr. 2013, 1828, 801–815. [Google Scholar] [CrossRef]
- Mihalache, G.; Balaes, T.; Gostin, I.; Stefan, M.; Coutte, F.; Krier, F. Lipopeptides produced by Bacillus subtilis as new biocontrol products against fusariosis in ornamental plants. Environ. Sci. Pollut. Res. 2018, 25, 29784–29793. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Zhang, Z.; Li, Y.; Zhang, X.; Duan, Y.; Wang, Q.I. Biocontrol of bacterial fruit blotch by Bacillus subtilis 9407 via surfactin-mediated antibacterial activity and colonization. Front. Microbiol. 2017, 8, 1973. [Google Scholar] [CrossRef] [PubMed]
- Gélis-Jeanvoine, S.; Canette, A.; Gohar, M.; Caradec, T.; Lemy, C.; Gominet, M.; Jacques, P.; Lereclus, D.; Slamti, L. Genetic and functional analyses of krs, a locus encoding kurstakin, a lipopeptide produced by Bacillus thuringiensis. Res. Microbiol. 2017, 168, 356–368. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Stanovych, A.; Gori, D.; Zirah, S.; Kouklovsky, C.; Alezra, V. β, γ-diamino acids as building blocks for new analogues of Gramicidin S: Synthesis and biological activity. Eur. J. Med. Chem. 2018, 149, 122–128. [Google Scholar] [CrossRef]
- Im, S.M.; Yu, N.H.; Joen, H.W.; Kim, S.O.; Park, H.W.; Park, A.R.; Kim, J.C. Biological control of tomato bacterial wilt by oxydifficidin and difficidin-producing Bacillus methylotrophicus DR-08. Pestic. Biochem. Physiol. 2020, 163, 130–137. [Google Scholar] [CrossRef]
- Yuan, J.; Li, B.; Zhang, N.; Waseem, R.; Shen, Q.; Huang, Q. Production of bacillomycin-and macrolactin-type antibiotics by Bacillus amyloliquefaciens NJN-6 for suppressing soilborne plant pathogens. J. Agric. Food Chem. 2012, 60, 2976–2981. [Google Scholar] [CrossRef]
- Barger, S.R.; Hoefler, B.C.; Cubillos-Ruiz, A.; Russell, W.K.; Russell, D.H.; Straight, P.D. Imaging secondary metabolism of Streptomyces sp. Mg1 during cellular lysis and colony degradation of competing Bacillus subtilis. Antonie Leeuwenhoek 2012, 102, 435–445. [Google Scholar] [CrossRef]
- Müller, S.; Strack, S.N.; Ryan, S.E.; Kearns, D.B.; Kirby, J.R. Predation by Myxococcus xanthus induces Bacillus subtilis to form spore-filled megastructures. Appl. Environ. Microbiol. 2015, 81, 203–210. [Google Scholar] [CrossRef]
- Abriouel, H.; Franz, C.M.; Omar, N.B.; Gálvez, A. Diversity and applications of Bacillus bacteriocins. FEMS Microbiol. Rev. 2011, 35, 201–232. [Google Scholar] [CrossRef]
- Bozhüyük, K.A.; Linck, A.; Tietze, A.; Kranz, J.; Wesche, F.; Nowak, S.; Fleischhacker, F.; Shi, Y.N.; Grün, P.; Bode, H.B. Modification and de novo design of non-ribosomal peptide synthetases using specific assembly points within condensation domains. Nat. Chem. 2019, 11, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Lajis, A.F.B. Biomanufacturing process for the production of bacteriocins from Bacillaceae family. Bioresour. Bioprocess. 2020, 7, 8. [Google Scholar] [CrossRef]
- Noman, M.; Ahmed, T.; Ijaz, U.; Shahid, M.; Azizullah; Li, D.; Manzoor, I.; Song, F. Plant–Microbiome crosstalk: Dawning from composition and assembly of microbial community to improvement of disease resilience in plants. Int. J. Mol. Sci. 2021, 22, 6852. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhou, J.; Li, C.; Ma, Y. Antifungal and plant growth promotion activity of volatile organic compounds produced by Bacillus amyloliquefaciens. Microbiol. Open 2019, 8, e00813. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Shi, X.; Wang, B.; Li, M.; Wang, Q.; Zhang, S. Antifungal effect of volatile organic compounds from Bacillus velezensis CT32 against Verticillium dahliae and Fusarium oxysporum. Processes 2020, 8, 1674. [Google Scholar] [CrossRef]
- Yuan, J.; Raza, W.; Shen, Q.; Huang, Q. Antifungal activity of Bacillus amyloliquefaciens NJN-6 volatile compounds against Fusarium oxysporum f. sp. cubense. Appl. Environ. Microbiol. 2012, 78, 5942–5944. [Google Scholar] [CrossRef]
- Poulaki, E.G.; Tjamos, S.E. Bacillus species: Factories of plant protective volatile organic compounds. J. Appl. Microbiol. 2023, 134, lxad037. [Google Scholar] [CrossRef]
- Tahir, H.A.S.; Gu, Q.; Wu, H.; Raza, W.; Hanif, A.; Wu, L.; Colman, M.V.; Gao, X. Plant Growth Promotion by Volatile Organic Compounds Produced by Bacillus subtilis SYST2. Front. Microbiol. 2017, 8, 171. [Google Scholar] [CrossRef]
- Kai, M. Diversity and distribution of volatile secondary metabolites throughout Bacillus subtilis isolates. Front. Microbiol. 2020, 11, 559. [Google Scholar] [CrossRef]
- Shobha, G.; Kumudini, B.S. Antagonistic effect of the newly isolated PGPR Bacillus spp. on Fusarium oxysporum. Int. J. Appl. Sci. Eng. Res. 2012, 1, 463–474. [Google Scholar]
- Brzezinska, M.S.; Kalwasińska, A.; Świątczak, J.; Żero, K.; Jankiewicz, U. Exploring the properties of chitinolytic Bacillus isolates for the pathogens biological control. Microb. Pathog. 2020, 148, 104462. [Google Scholar] [CrossRef] [PubMed]
- Jangir, M.; Pathak, R.; Sharma, S.; Sharma, S. Biocontrol mechanisms of Bacillus sp., isolated from tomato rhizosphere, against Fusarium oxysporum f. sp. lycopersici. Biol. Control. 2018, 123, 60–70. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Bera, T.; Chakrabarty, A.M. Microbial siderophore–A boon to agricultural sciences. Biol. Control. 2020, 144, 104214. [Google Scholar] [CrossRef]
- Ramyabharathi, S.A.; Raguchander, T. Mode of action of Bacillus subtilis EPCO16 against tomato Fusarium wilt. Biochem. Cell. Arch. 2014, 14, 47–50. [Google Scholar]
- Cao, Y.; Zhang, Z.; Ling, N.; Yuan, Y.; Zheng, X.; Shen, B.; Shen, Q. Bacillus subtilis SQR 9 can control Fusarium wilt in cucumber by colonizing plant roots. Biol. Fertil. Soils. 2011, 47, 495–506. [Google Scholar] [CrossRef]
- Chen, F.; Wang, M.; Zheng, Y.; Luo, J.; Yang, X.; Wang, X. Quantitative changes of plant defense enzymes and phytohormone in biocontrol of cucumber Fusarium wilt by Bacillus subtilis B579. World J. Microbiol. Biotechnol. 2010, 26, 675–684. [Google Scholar] [CrossRef]
- Yadav, K.; Damodaran, T.; Dutt, K.; Singh, A.; Muthukumar, M.; Rajan, S.; Gopal, R.; Sharma, P.C. Effective biocontrol of banana Fusarium wilt tropical race 4 by a Bacillus rhizobacteria strain with antagonistic secondary metabolites. Rhizosphere 2021, 18, 100341. [Google Scholar] [CrossRef]
- Kenneth, O.C.; Nwadibe, E.C.; Kalu, A.U.; Unah, U.V. Plant growth promoting rhizobacteria (PGPR): A novel agent for sustainable food production. Am. J. Agric. Biol. Sci. 2019, 14, 35–54. [Google Scholar] [CrossRef]
- Flores-Gallegos, A.C.; Nava-Reyna, E. Plant growth-promoting microbial enzymes. In Enzymes in Food Biotechnology; Kuddus, M., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 521–534. [Google Scholar]
- Shafi, J.; Tian, H.; Ji, M. Bacillus species as versatile weapons for plant pathogens: A review. Biotechnol. Biotechnol. Equip. 2017, 31, 446–459. [Google Scholar] [CrossRef]
- Jadhav, H.P.; Shaikh, S.S.; Sayyed, R.Z. Role of Hydrolytic Enzymes of Rhizoflora in Biocontrol of Fungal Phytopathogens: An Overview. In Rhizotrophs: Plant Growth Promotion to Bioremediation; Microorganisms for Sustainability; Mehnaz, S., Ed.; Springer: Singapore, 2017; Volume 2. [Google Scholar] [CrossRef]
- Akeed, Y.; Atrash, F.; Naffaa, W. Partial purification and characterization of chitinase produced by Bacillus licheniformis B307. Heliyon 2020, 6, e03858. [Google Scholar] [CrossRef]
- Yan, L.; Jing, T.; Yujun, Y.; Bin, L.I.; Hui, L.I.; Chun, L.I. Biocontrol efficiency of Bacillus subtilis SL-13 and characterization of an antifungal chitinase. Chin. J. Chem. Eng. 2011, 19, 128–134. [Google Scholar]
- Dewi, R.T.K.; Mubarik, N.R.; Suhartono, M.T. Medium optimization of β-glucanase production by Bacillus subtilis SAHA 32.6 used as biological control of oil palm pathogen. Emir. J. Food Agric. 2016, 28, 116–125. [Google Scholar] [CrossRef]
- Putri, R.E.; Mubarik, N.R.; Ambarsari, L.; Wahyudi, A.T. Antagonistic activity of glucanolytic bacteria Bacillus subtilis W3. 15 against Fusarium oxysporum and its enzyme characterization. Biodiversitas J. Biol. Divers. 2021, 22, 4067–4077. [Google Scholar] [CrossRef]
- Guleria, S.; Walia, A.; Chauhan, A.; Shirkot, C.K. Molecular characterization of alkaline protease of Bacillus amyloliquefaciens SP1 involved in biocontrol of Fusarium oxysporum. Int. J. Food Microbiol. 2016, 232, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Etesami, H.; Alikhani, H.A. Rhizosphere and endorhiza of oilseed rape (Brassica napus L.) plant harbor bacteria with multifaceted beneficial effects. Biol. Control 2016, 94, 11–24. [Google Scholar] [CrossRef]
- Mardanova, A.M.; Hadieva, G.F.; Lutfullin, M.T.; Khilyas, I.V.E.; Minnullina, L.F.; Gilyazeva, A.G.; Bogomolnaya, L.M.; Sharipova, M.R. Bacillus subtilis strains with antifungal activity against the phytopathogenic fungi. Agric. Sci. 2016, 8, 1–20. [Google Scholar] [CrossRef]
- Rasouli-Sadaghiani, M.; Malakouti, M.J.; Khavazi, K.; Miransari, M. Siderophore Efficacy of Fluorescent Pseudomonades Affecting Labeled Iron (59Fe) Uptake by Wheat (Triticum aestivum L.) Genotypes Differing in Fe Efficiency. In Use of Microbes for the Alleviation of Soil Stresses; Miransari, M., Ed.; Springer: New York, NY, USA, 2014. [Google Scholar] [CrossRef]
- Patil, S.; Shivannavar, C.T.; Bheemaraddi, M.C.; Gaddad, S.M. Antiphytopathogenic and plant growth promoting attributes of Bacillus strains isolated from rhizospheric soil of chickpea. J. Agric. Sci. Technol. 2015, 17, 1365–1377. [Google Scholar]
- Sah, S.; Singh, R. Siderophore: Structural and functional characterization—A comprehensive review. Agric. (Pol’nohospodárstvo) 2015, 61, 97–114. [Google Scholar] [CrossRef]
- Dimopoulou, A.; Theologidis, I.; Benaki, D.; Koukounia, M.; Zervakou, A.; Tzima, A.; Diallinas, G.; Hatzinikolaou, D.G.; Skandalis, N. Direct antibiotic activity of bacillibactin broadens the biocontrol range of Bacillus amyloliquefaciens MBI600. Msphere 2021, 6, e00376-21. [Google Scholar] [CrossRef]
- Nithyapriya, S.; Lalitha, S.; Sayyed, R.Z.; Reddy, M.S.; Dailin, D.J.; El Enshasy, H.A.; Luh Suriani, N.; Herlambang, S. Production, Purification, and Characterization of Bacillibactin Siderophore of Bacillus subtilis and Its Application for Improvement in Plant Growth and Oil Content in Sesame. Sustainability 2021, 13, 5394. [Google Scholar] [CrossRef]
- Khan, A.; Doshi, H.V.; Thakur, M.C. Bacillus spp.: A Prolific Siderophore Producer. In Bacilli and Agrobiotechnology; Islam, M., Rahman, M., Pandey, P., Jha, C., Aeron, A., Eds.; Springer: Cham, Switzerland, 2016. [Google Scholar] [CrossRef]
- Legein, M.; Smets, W.; Vandenheuvel, D.; Eilers, T.; Muyshondt, B.; Prinsen, E.; Samson, R.; Lebeer, S. Modes of action of microbial biocontrol in the phyllosphere. Front. Microbiol. 2020, 11, 1619. [Google Scholar] [CrossRef]
- Yu, X.; Ai, C.; Xin, L.; Zhou, G. The siderophore-producing bacterium, Bacillus subtilis CAS15, has a biocontrol effect on Fusarium wilt and promotes the growth of pepper. Eur. J. Soil Biol. 2011, 47, 138–145. [Google Scholar] [CrossRef]
- Blake, C.; Christensen, M.N.; Kovács, Á.T. Molecular aspects of plant growth promotion and protection by Bacillus subtilis. Mol. Plant-Microbe Interact. 2021, 34, 15–25. [Google Scholar] [CrossRef]
- Jin, Y.; Zhu, H.; Luo, S.; Yang, W.; Zhang, L.; Li, S.; Jin, Q.; Cao, Q.; Sun, S.; Xiao, M. Role of maize root exudates in promotion of colonization of Bacillus velezensis strain S3-1 in rhizosphere soil and root tissue. Curr. Microbiol. 2019, 76, 855–862. [Google Scholar] [CrossRef]
- Jain, S.; Varma, A.; Tuteja, N.; Choudhary, D.K. Bacteria-Mediated Elicitation of Induced Resistance in Plants upon Fungal Phytopathogen. In Plant-Microbe Interaction: An Approach to Sustainable Agriculture; Choudhary, D., Varma, A., Tuteja, N., Eds.; Springer: Singapore, 2016. [Google Scholar] [CrossRef]
- Verma, M.; Mishra, J.; Arora, N.K. Plant Growth-Promoting Rhizobacteria: Diversity and Applications. In Environmental Biotechnology: For Sustainable Future; Sobti, R., Arora, N., Kothari, R., Eds.; Springer: Singapore, 2019. [Google Scholar] [CrossRef]
- Meena, M.; Swapnil, P.; Zehra, A.; Aamir, M.; Dubey, M.A.; Goutam, J.; Upadhyay, R.S. Beneficial Microbes for Disease Suppression and Plant Growth Promotion. In Plant-Microbe Interactions in Agro-Ecological Perspectives; Singh, D., Singh, H., Prabha, R., Eds.; Springer: Singapore, 2017. [Google Scholar] [CrossRef]
- Jiang, C.; Fan, Z.; Li, Z.; Niu, D.; Li, Y.; Zheng, M.; Wang, Q.; Jin, H.; Guo, J. Bacillus cereus AR156 triggers induced systemic resistance against Pseudomonas syringae pv. tomato DC3000 by suppressing miR472 and activating CNLs-mediated basal immunity in Arabidopsis. Mol. Plant Pathol. 2020, 21, 854–870. [Google Scholar] [CrossRef]
- Kloepper, J.W.; Ryu, C.M.; Zhang, S. Induced systemic resistance and promotion of plant growth by Bacillus spp. Phytopathology 2004, 94, 1259–1266. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Hashem, A.; Abd_Allah, E.F. Bacillus: A biological tool for crop improvement through bio-molecular changes in adverse environments. Front. Physiol. 2017, 8, 667. [Google Scholar] [CrossRef] [PubMed]
- Rais, A.; Jabeen, Z.; Shair, F.; Hafeez, F.Y.; Hassan, M.N. Bacillus spp., a bio-control agent enhances the activity of antioxidant defense enzymes in rice against Pyricularia oryzae. PLoS ONE 2017, 12, e0187412. [Google Scholar] [CrossRef] [PubMed]
- Jinal, N.H.; Amaresan, N. Evaluation of biocontrol Bacillus species on plant growth promotion and systemic-induced resistant potential against bacterial and fungal wilt-causing pathogens. Arch. Microbiol. 2020, 202, 1785–1794. [Google Scholar] [CrossRef] [PubMed]
- Castillo, J.D.; Lawrence, K.S.; Kloepper, J.W. Biocontrol of the reniform nematode by Bacillus firmus GB-126 and Paecilomyces lilacinus 251 on cotton. Plant Dis. 2013, 97, 967–976. [Google Scholar] [CrossRef]
- Cherif-Silini, H.; Silini, A.; Chenari Bouket, A.; Alenezi, F.N.; Luptakova, L.; Bouremani, N.; Nowakowska, J.A.; Oszako, T.; Belbahri, L. Tailoring next generation plant growth promoting microorganisms as versatile tools beyond soil desalinization: A road map towards field application. Sustainability 2021, 13, 4422. [Google Scholar] [CrossRef]
- Huang, J.; Wei, Z.; Tan, S.; Mei, X.; Yin, S.; Shen, Q.; Xu, Y. The rhizosphere soil of diseased tomato plants as a source for novel microorganisms to control bacterial wilt. Appl. Soil Ecol. 2013, 72, 79–84. [Google Scholar] [CrossRef]
- Boulahouat, S.; Cherif-Silini, H.; Silini, A.; Chenari Bouket, A.; Luptakova, L.; Saadaoui, N.; Alenezi, F.N.; Belbahri, L. Critical Evaluation of Biocontrol Ability of Bayoud Infected Date Palm Phyllospheric Bacillus spp. Suggests That In Vitro Selection Does Not Guarantee Success in Planta. Agronomy 2022, 12, 2403. [Google Scholar] [CrossRef]
- Raymaekers, K.; Ponet, L.; Holtappels, D.; Berckmans, B.; Cammue, B.P. Screening for novel biocontrol agents applicable in plant disease management—A review. Biol. Control. 2020, 144, 104240. [Google Scholar] [CrossRef]
- Slama, H.; Cherif-Silini, H.; Chenari Bouket, A.; Qader, M.; Silini, A.; Yahiaoui, B.; Alenezi, F.N.; Luptakova, L.; Triki, M.A.; Vallat, A.; et al. Screening for Fusarium Antagonistic Bacteria From Contrasting Niches Designated the Endophyte Bacillus halotolerans as Plant Warden against Fusarium. Front. Microbiol. 2019, 9, 3236. [Google Scholar] [CrossRef]
- Raaijmakers, J.M.; De Bruijn, I.; Nybroe, O.; Ongena, M. Natural functions of lipopeptides from Bacillus and Pseudomonas: More than surfactants and antibiotics. FEMS Microbiol. Rev. 2010, 34, 1037–1062. [Google Scholar] [CrossRef]
- Mefteh, F.B.; Daoud, A.; Chenari Bouket, A.; Thissera, B.; Kadri, Y.; Cherif-Silini, H.; Eshelli, M.; Alenezi, F.N.; Vallat, A.; Oszako, T.; et al. Date Palm Trees Root-Derived Endophytes as Fungal Cell Factories for Diverse Bioactive Metabolites. Int. J. Mol. Sci. 2018, 19, 1986. [Google Scholar] [CrossRef]
- Díaz-Valle, A.; López-Calleja, A.C.; Alvarez-Venegas, R. Enhancement of pathogen resistance in common bean plants by inoculation with Rhizobium etli. Front. Plant Sci. 2019, 10, 1317. [Google Scholar] [CrossRef]
- Alenezi, F.N.; Slama, H.B.; Chenari Bouket, A.; Cherif-Silini, H.; Silini, A.; Luptakova, L.; Nowakowska, J.A.; Oszako, T.; Belbahri, L. Bacillus velezensis: A treasure house of bioactive compounds of medicinal, biocontrol and environmental importance. Forests 2021, 12, 1714. [Google Scholar] [CrossRef]
- Kerbab, S.; Silini, A.; Chenari Bouket, A.; Cherif-Silini, H.; Eshelli, M.; El Houda Rabhi, N.; Belbahri, L. Mitigation of NaCl Stress in Wheat by Rhizosphere Engineering Using Salt Habitat Adapted PGPR Halotolerant Bacteria. Appl. Sci. 2021, 11, 1034. [Google Scholar] [CrossRef]
- Rabhi, N.E.H.; Cherif-Silini, H.; Silini, A.; Alenezi, F.N.; Chenari Bouket, A.; Oszako, T.; Belbahri, L. Alleviation of Salt Stress via Habitat-Adapted Symbiosis. Forests 2022, 13, 586. [Google Scholar] [CrossRef]
- Daayf, F.; Adam, L.; Fernando, W.G.D. Comparative screening of bacteria for biocontrol of potato late blight (strain US-8), using in-vitro, detached-leaves, and whole plant testing systems. Can. J. Plant Pathol. 2003, 25, 276–284. [Google Scholar] [CrossRef]
- Chen, L.; Shi, H.; Heng, J.; Wang, D.; Bian, K. Antimicrobial, plant growth-promoting and genomic properties of the peanut endophyte Bacillus velezensis LDO2. Microbiol. Res. 2019, 218, 41–48. [Google Scholar] [CrossRef]
- de Souza, R.S.C.; Armanhi, J.S.L.; Damasceno, N.d.B.; Imperial, J.; Arruda, P. Genome sequences of a plant beneficial synthetic bacterial community reveal genetic features for successful plant colonization. Front. Microbiol. 2019, 10, 1779. [Google Scholar] [CrossRef]
- Basu, A.; Prasad, P.; Das, S.N.; Kalam, S.; Sayyed, R.Z.; Reddy, M.S.; El Enshasy, H. Plant growth promoting rhizobacteria (PGPR) as green bioinoculants: Recent developments, constraints, and prospects. Sustainability 2021, 13, 1140. [Google Scholar] [CrossRef]
- Ahmed, A.; He, P.; He, Y.; Singh, B.K.; Wu, Y.; Munir, S.; He, P. Biocontrol of plant pathogens in omics era—With special focus on endophytic bacilli. Crit. Rev. Biotechnol. 2023, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Roesti, D.; Gaur, R.; Johri, B.; Imfeld, G.; Sharma, S.; Kawaljeet, K.; Aragno, M. Plant growth stage, fertiliser management and bio-inoculation of arbuscular mycorrhizal fungi and plant growth promoting rhizobacteria affect the rhizobacterial community structure in rain-fed wheat fields. Soil Biol. Biochem. 2006, 38, 1111–1120. [Google Scholar] [CrossRef]
- Glick, B.R. Beneficial Plant-Bacterial Interactions; Springer: Berlin/Heidelberg, Germany, 2015; p. 383. [Google Scholar]
- Saadaoui, N.; Silini, A.; Cherif-Silini, H.; Chenari Bouket, A.; Alenezi, F.N.; Luptakova, L.; Boulahouat, S.; Belbahri, L. Semi-Arid-Habitat-Adapted Plant-Growth-Promoting Rhizobacteria Allows Efficient Wheat Growth Promotion. Agronomy 2022, 12, 2221. [Google Scholar] [CrossRef]
- Rojas-Solís, D.; Hernández-Pacheco, C.E.; Santoyo, G. Evaluation of Bacillus and Pseudomonas to colonize the rhizosphere and their effect on growth promotion in tomato (Physalis ixocarpa Brot. ex Horm.). Rev. Chapingo Ser. Hortic. 2016, 22, 45–57. [Google Scholar] [CrossRef]
- Timm, C.M.; Pelletier, D.A.; Jawdy, S.S.; Gunter, L.E.; Henning, J.A.; Engle, N.; Lu, T.Y. Two poplar-associated bacterial isolates induce additive favorable responses in a constructed plant-microbiome system. Front. Plant Sci. 2016, 7, 497. [Google Scholar] [CrossRef]
- Lemanceau, P.; Blouin, M.; Muller, D.; Moënne-Loccoz, Y. Let the Core Microbiota Be Functional. Trends Plant Sci. 2017, 22, 583–595. [Google Scholar] [CrossRef] [PubMed]
- McDonald, B.R.; Currie, C.R. Lateral gene transfer dynamics in the ancient bacterial genus Streptomyces. mBio 2017, 8, e00644-17. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).