Novel Bioformulations with Trichoderma lixii to Improve the Growth Dynamics and Biocontrol of the Cowpea Damping-Off Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungi Used and Culture Conditions
2.2. Agricultural Wastes Used and Their Chemical Properties
2.3. Conidia Production Statistical Optimization under SSF Conditions
2.3.1. Using Two Levels
2.3.2. Using Three Levels
2.4. Assessment of Shelf Life and Viability
2.5. Effect of T. viride Bioformulations Generated on Plant Growth in a Cowpea Gnotobiotic Clay System
2.6. Cowpea Pot Experiments
2.6.1. The Incidence of the Disease
2.6.2. Vegetative Growth
2.6.3. Physiological Features
Photosynthetic Pigments
TSS
2.6.4. Antioxidant Enzymes
Peroxidase Activity (PO)
Polyphenol Oxidase Assay (PPO)
Phenylalanine Ammonia Lyase Assay (PAL)
Total Phenolic Content (TPC)
2.7. Chemical Contents of Leaves
2.8. Statistical Analyses
3. Results
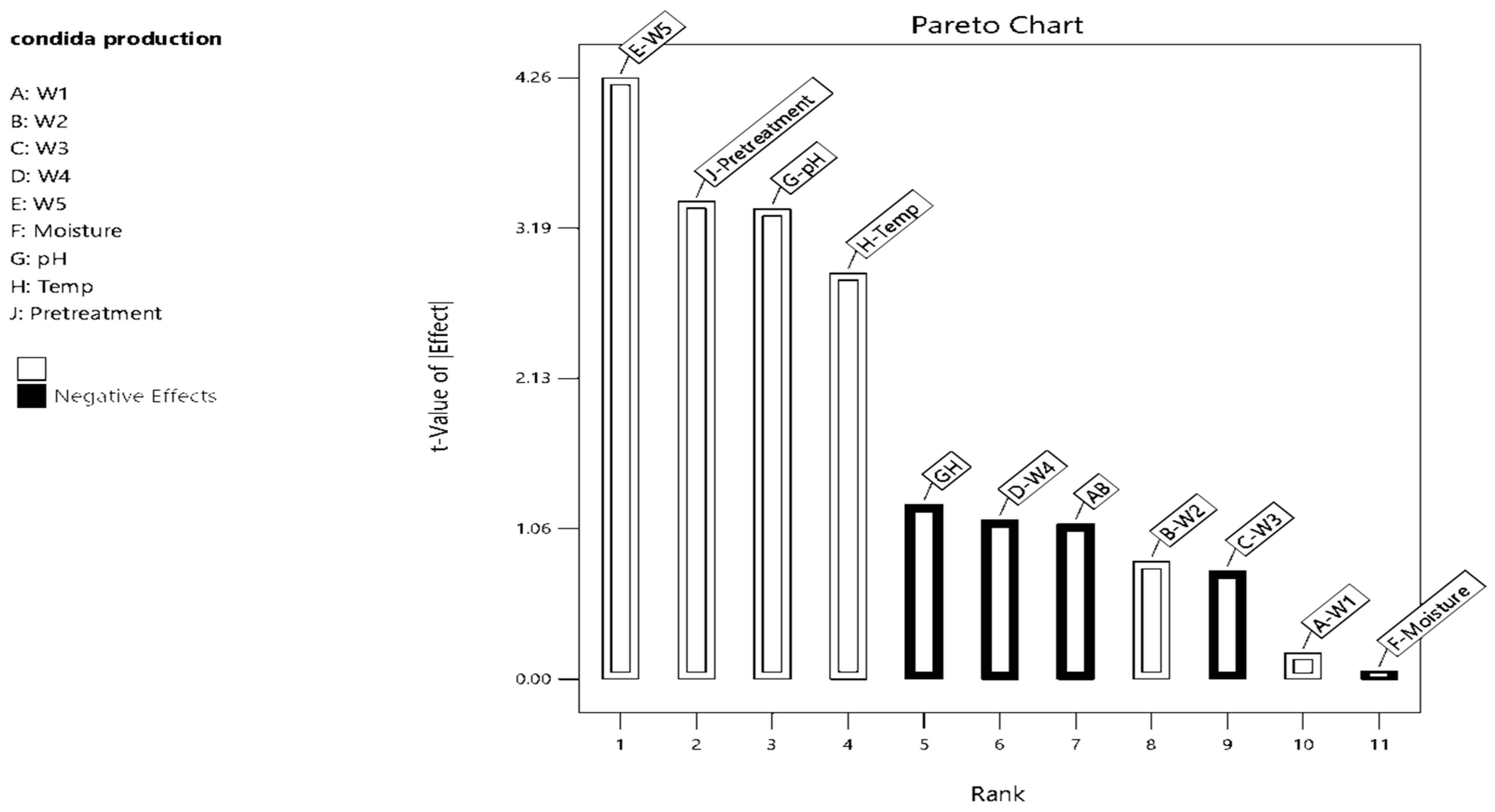
3.1. Optimization of the Culture Conditions Using Response Surface Design
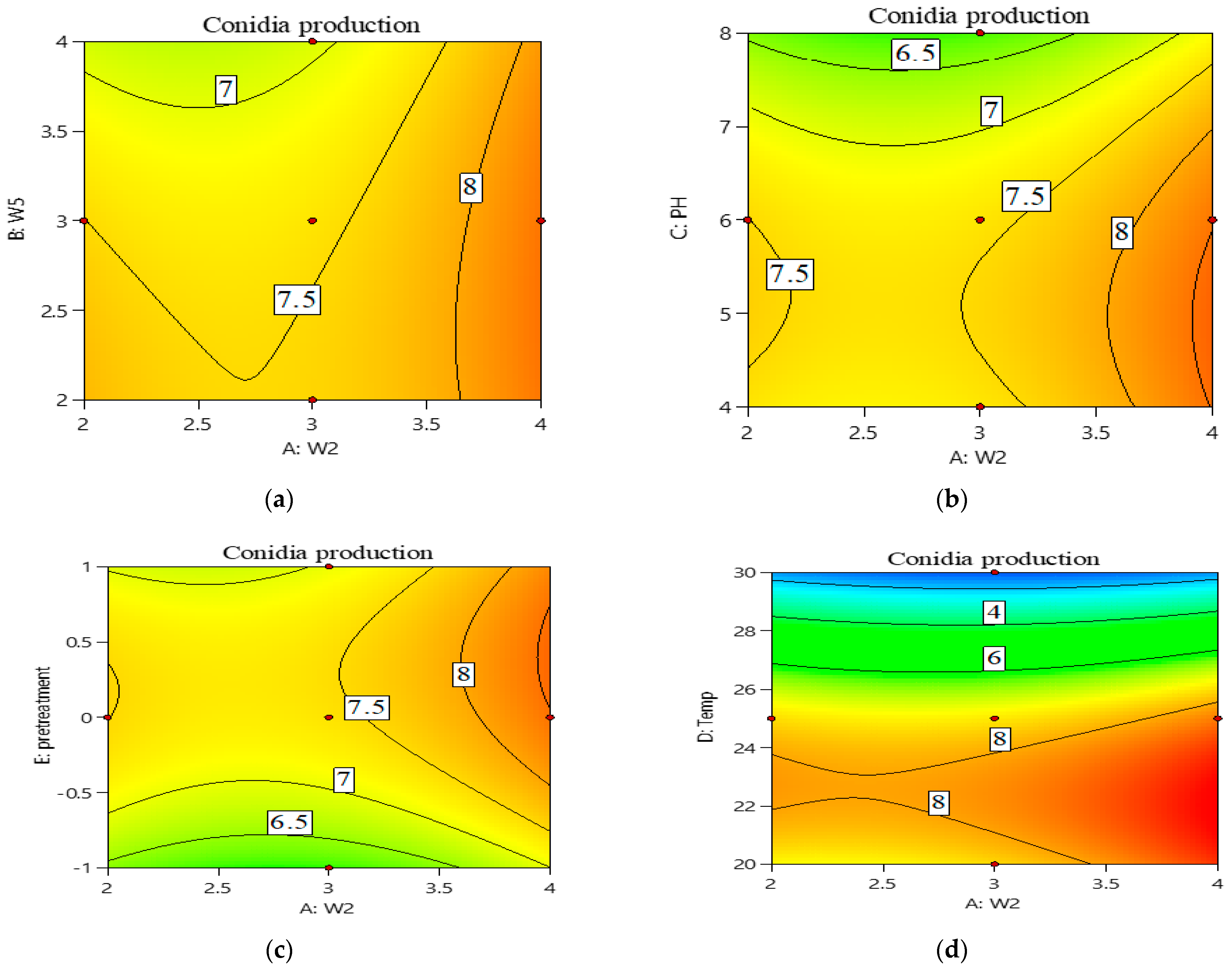
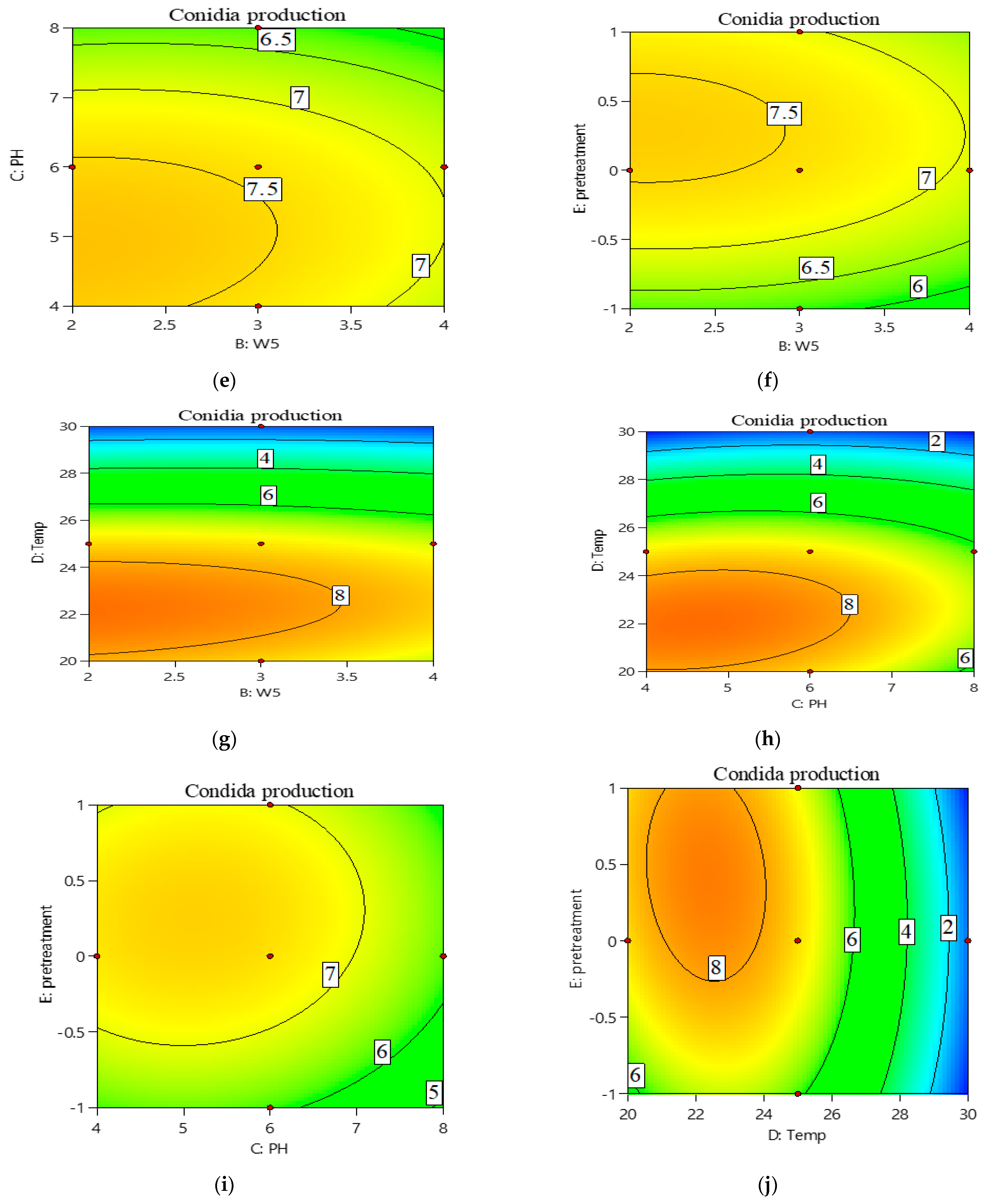
3.2. Central Composite Design (CCD) Approach for Optimization of Conidia Production
3.3. Shelf Life and Viability of Bio-Fungicides
3.4. Gnotobiotic System Experiment
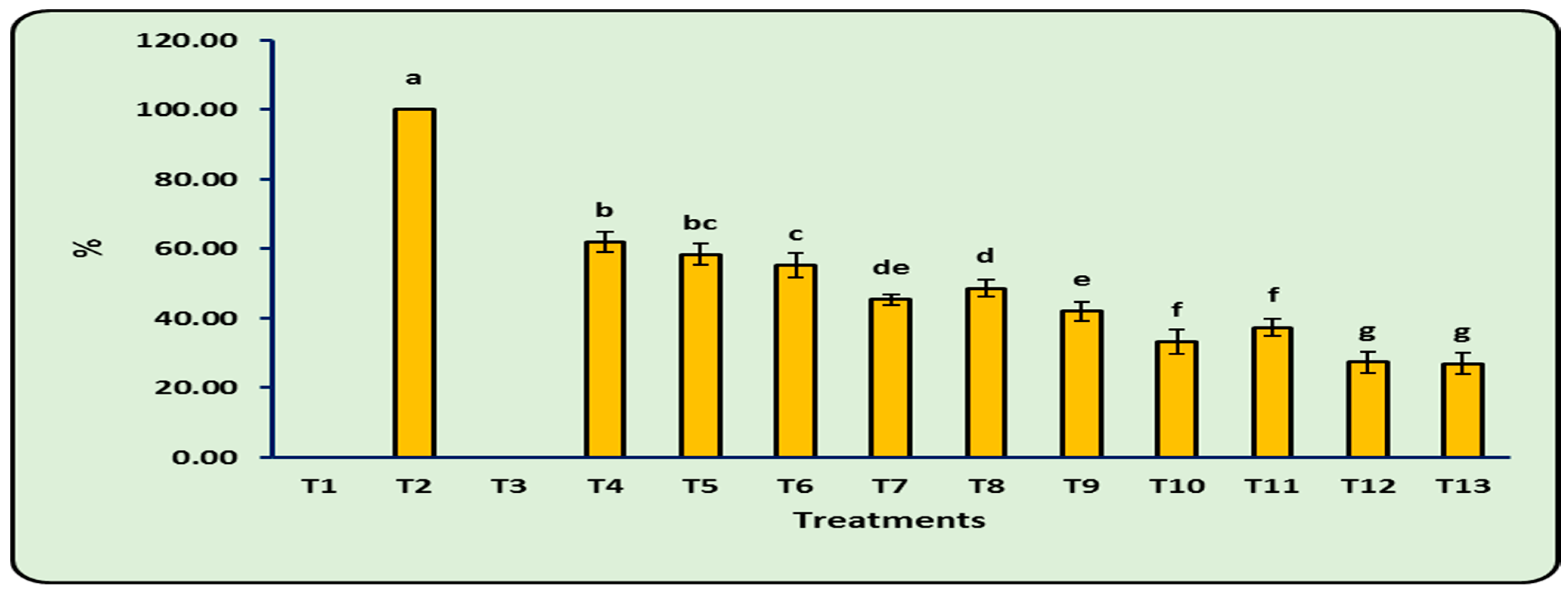
3.5. Disease Incidence (%)
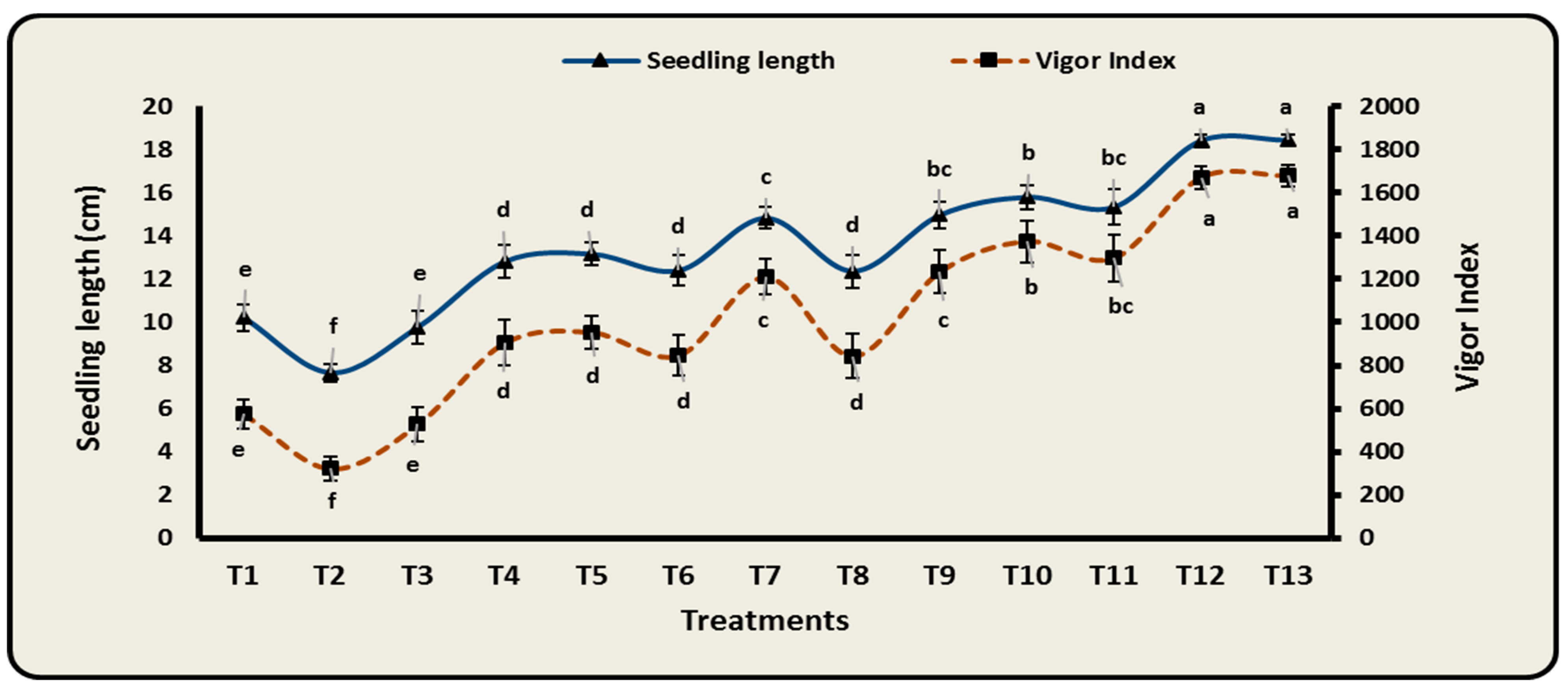
3.6. Effect of Different Bioformulations on Growth Characteristics of Cowpea Infected with F. oxysporum
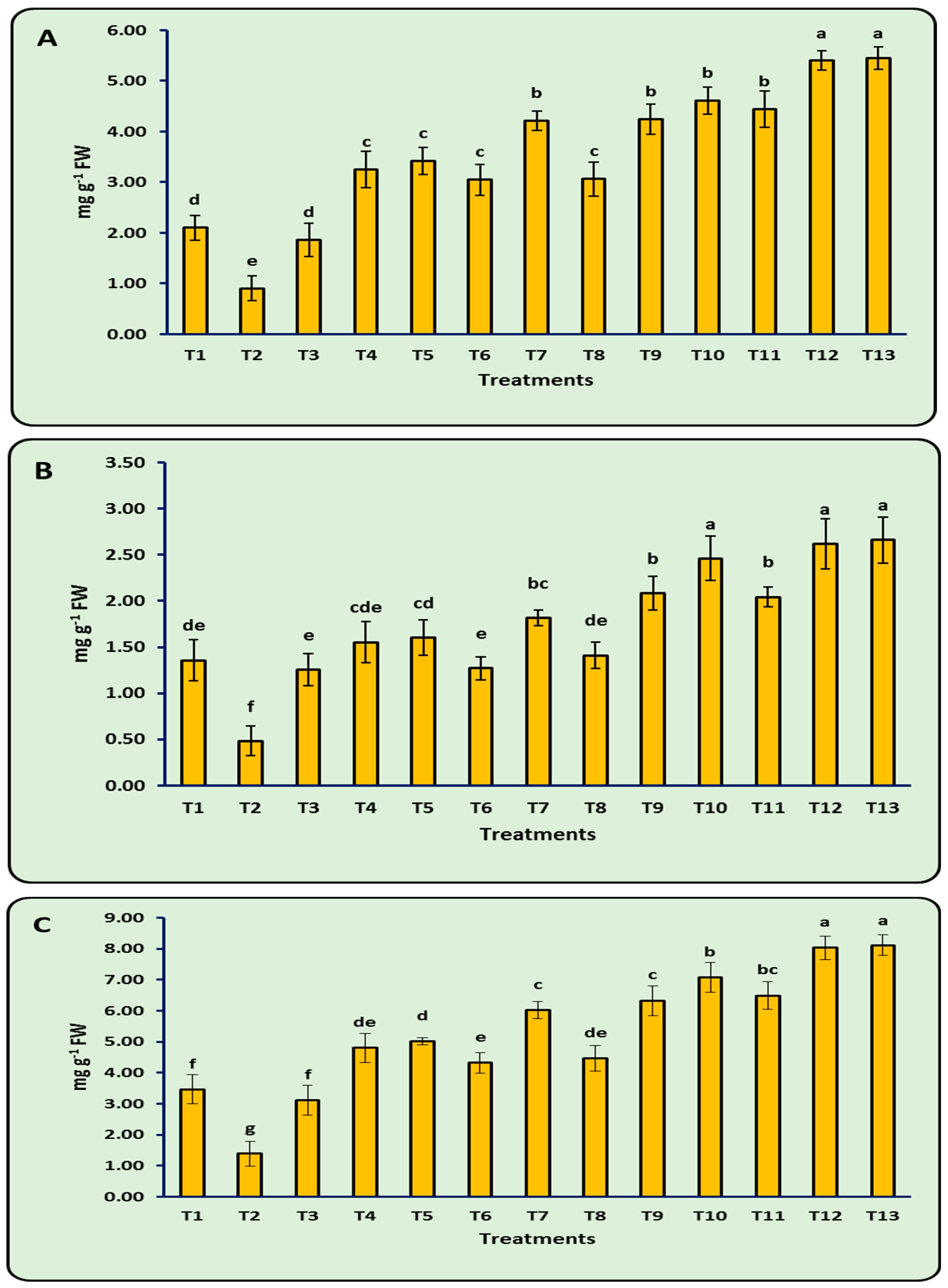
3.7. Photosynthetic Pigments
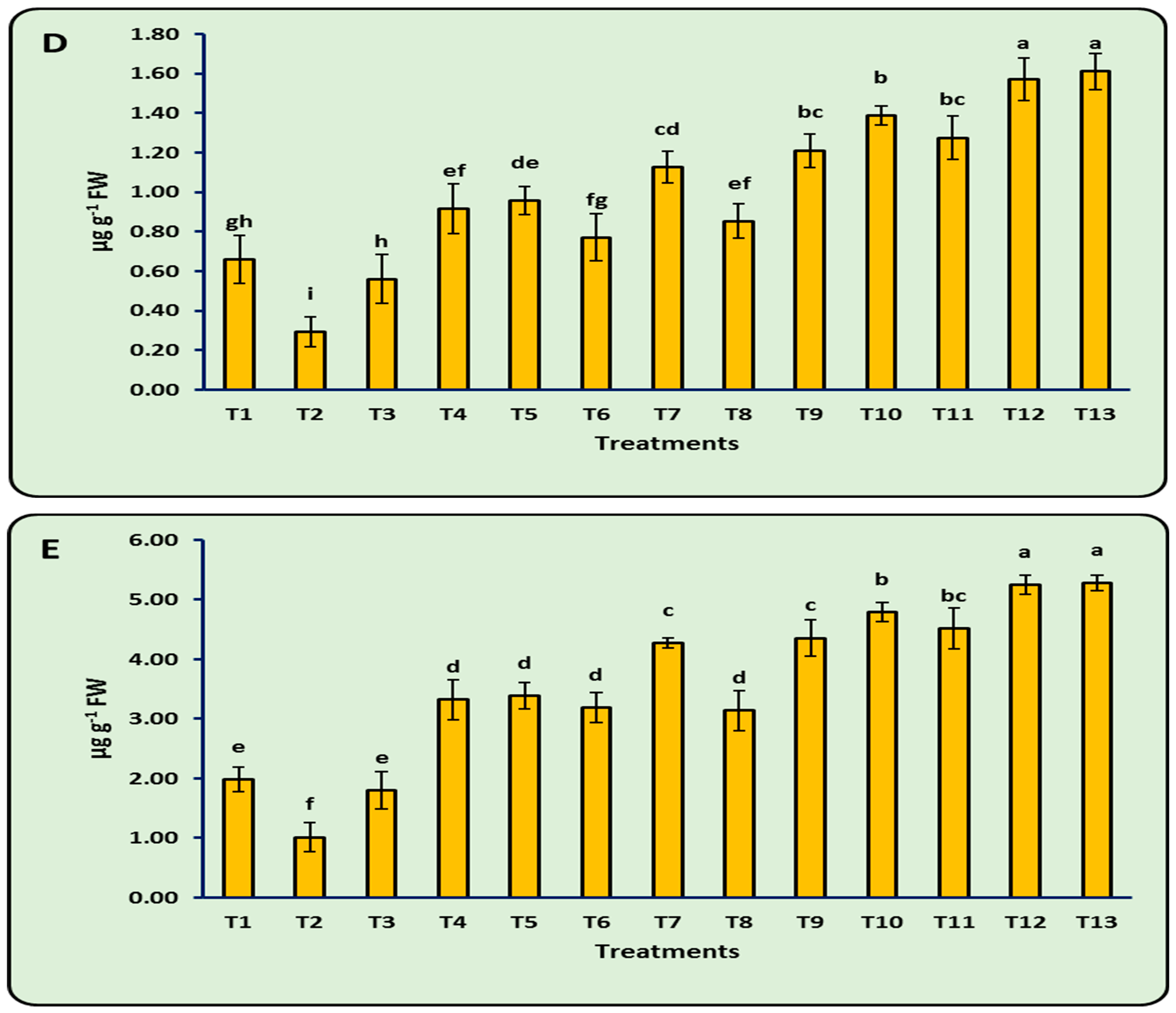
3.8. Antioxidant Enzymes
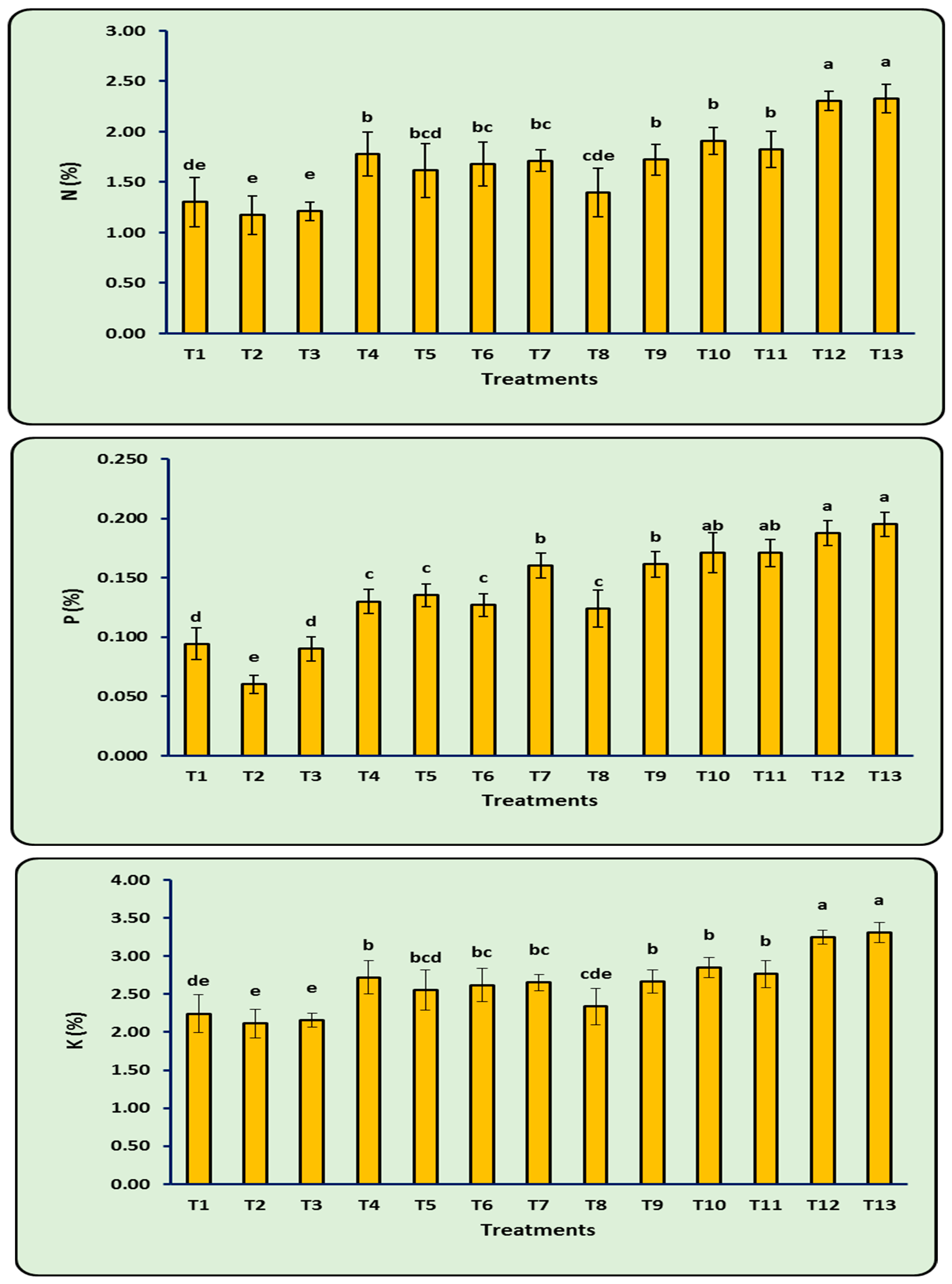
3.9. N, P, and K (%) of Leaves
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rajak, P.; Roy, S.; Ganguly, A.; Mandi, M.; Dutta, A.; Das, K.; Nanda, S.; Ghanty, S.; Biswas, G. Agricultural pesticides–Friends or foes to biosphere? J. Hazard. Mater. Adv. 2023, 10, 100264. [Google Scholar] [CrossRef]
- Ayilara, M.S.; Adeleke, B.S.; Akinola, S.A.; Fayose, C.A.; Adeyemi, U.T.; Gbadegesin, L.A.; Omole, R.K.; Johnson, R.M.; Uthman, Q.O.; Babalola, O.O. Biopesticides as a promising alternative to synthetic pesticides: A case for microbial pesticides, phytopesticides, and nanobiopesticides. Front. Microbiol. 2023, 14, 1040901. [Google Scholar] [CrossRef] [PubMed]
- Ngegba, P.M.; Cui, G.; Khalid, M.Z.; Zhong, G. Use of botanical pesticides in agriculture as an alternative to synthetic pesticides. Agriculture 2022, 12, 600. [Google Scholar] [CrossRef]
- El-Saadony, M.T.; Saad, A.M.; Soliman, S.M.; Salem, H.M.; Ahmed, A.I.; Mahmood, M.; El-Tahan, A.M.; Ebrahim, A.A.; El-Mageed, A.; Taia, A.; et al. Plant growth-promoting microorganisms as biocontrol agents of plant diseases: Mechanisms, challenges and future perspectives. Front. Plant Sci. 2022, 13, 923880. [Google Scholar] [CrossRef]
- Pathak, V.M.; Verma, V.K.; Rawat, B.S.; Kaur, B.; Babu, N.; Sharma, A.; Dewali, S.; Yadav, M.; Kumari, R.; Singh, S.; et al. Current status of pesticide effects on environment, human health and it’s eco-friendly management as bioremediation: A comprehensive review. Front. Microbiol. 2022, 13, 2833. [Google Scholar] [CrossRef] [PubMed]
- Naeimi, S.; Khosravi, V.; Varga, A.; Vágvölgyi, C.; Kredics, L. Screening of organic substrates for solid-state fermentation, viability and bioefficacy of Trichoderma harzianum AS12-2, a biocontrol Strain against Rice Sheath Blight Disease. J. Agron. 2020, 10, 1258. [Google Scholar] [CrossRef]
- Bonaterra, A.; Badosa, E.; Daranas, N.; Francés, J.; Roselló, G.; Montesinos, E. Bacteria as biological control agents of plant diseases. Microorganisms 2022, 10, 1759. [Google Scholar] [CrossRef]
- Vassileva, M.; Malusà, E.; Sas-Paszt, L.; Trzcinski, P.; Galvez, A.; Flor-Peregrin, E.; Shilev, S.; Canfora, L.; Mocali, S.; Vassilev, N. Fermentation strategies to improve soil bio-inoculant production and quality. Microorganisms 2021, 9, 1254. [Google Scholar] [CrossRef]
- Teixidó, N.; Usall, J.; Torres, R. Insight into a successful development of biocontrol agents: Production, formulation, packaging, and shelf life as key aspects. Horticulturae 2022, 8, 305. [Google Scholar] [CrossRef]
- Lewis, J.A.; Papavizas, G. Biocontrol of plant diseases: The approach for tomorrow. Crop. Prot. 1991, 10, 95–105. [Google Scholar] [CrossRef]
- Rayhane, H.; Josiane, M.; Gregoria, M.; Yiannis, K.; Nathalie, D.; Ahmed, M.; Sevastianos, R. From flasks to single used bioreactor: Scale-up of solid state fermentation process for metabolites and conidia production by Trichoderma asperellum. J. Environ. Manag. 2019, 252, 109496. [Google Scholar] [CrossRef] [PubMed]
- Sala, A.; Artola, A.; Sánchez, A.; Barrena, R. Rice husk as a source for fungal biopesticide production by solid-state fermentation using B. bassiana and T. harzianum. Bioresour. Technol. 2019, 296, 122322. [Google Scholar] [CrossRef] [PubMed]
- Mattedi, A.; Sabbi, E.; Farda, B.; Djebaili, R.; Mitra, D.; Ercole, C.; Cacchio, P.; Del Gallo, M.; Pellegrini, M. Solid-State Fermentation: Applications and Future Perspectives for Biostimulant and Biopesticides Production. Microorganisms 2023, 11, 1408. [Google Scholar] [CrossRef] [PubMed]
- Šelo, G.; Planinić, M.; Tišma, M.; Tomas, S.; Koceva Komlenić, D.; Bucić-Kojić, A. A comprehensive review on valorization of agro-food industrial residues by solid-state fermentation. Foods 2021, 10, 927. [Google Scholar] [CrossRef]
- López-Trujillo, J.; Mellado-Bosque, M.; Ascacio-Valdés, J.A.; Prado-Barragán, L.A.; Hernández-Herrera, J.A.; Aguilera-Carbó, A.F. Temperature and pH Optimization for Protease Production Fermented by Yarrowia lipolytica from Agro-Industrial Waste. Fermentation 2023, 9, 819. [Google Scholar] [CrossRef]
- Sadh, P.K.; Duhan, S.; Duhan, J.S. Agro-industrial wastes and their utilization using solid state fermentation: A review. Bioresour. Bioprocess. 2018, 5, 1–5. [Google Scholar] [CrossRef]
- Steudler, S.; Werner, A.; Walther, T. It Is the Mix that Matters: Substrate-Specific Enzyme Production from Filamentous Fungi and Bacteria through Solid-State Fermentation. In Solid State Fermentation, Research and Industrial Applications; Werner, A., Steudler, S., Cheng, J.J., Eds.; Springer Nature: Cham, Switzerland, 2019; pp. 51–81. [Google Scholar]
- Zin, N.A.; Badaluddin, N.A. Biological functions of Trichoderma spp. for agriculture applications. Ann. Agric. Sci. 2020, 65, 168–178. [Google Scholar] [CrossRef]
- Collinge, D.B.; Jensen, D.F.; Rabiey, M.; Sarrocco, S.; Shaw, M.W.; Shaw, R.H. Biological control of plant diseases—What has been achieved and what is the direction? Plant Pathol. 2022, 71, 1024–1047. [Google Scholar] [CrossRef]
- Ayaz, M.; Li, C.H.; Ali, Q.; Zhao, W.; Chi, Y.K.; Shafiq, M.; Ali, F.; Yu, X.Y.; Yu, Q.; Zhao, J.T.; et al. Bacterial and Fungal Biocontrol Agents for Plant Disease Protection: Journey from Lab to Field, Current Status, Challenges, and Global Perspectives. Molecules 2023, 28, 6735. [Google Scholar] [CrossRef]
- Mendoza-Mendoza, A.; Clouston, A.; Li, J.-H.; Nieto-Jacobo, M.F.; Cummings, N.; Steyaert, J.; Hill, R. Isolation and Mass Production of Trichoderma. In Microbial-Based Biopesticides, Methods in Molecular Biology; Glare, T.R., Moran-Diez, M.E., Eds.; Humana Press: New York, NY, USA, 2016; Volume 1477, pp. 13–20. [Google Scholar]
- Abbey, L.; Abbey, J.; Leke-Aladekoba, A.; Iheshiulo, E.M.; Ijenyo, M. Biopesticides and Biofertilizers: Types, Production, Benefits, and Utilization. In Byproducts from Agriculture and Fisheries: Adding Value for Food, Feed, Pharma, and Fuels; Simpson, B.K., Aryee, A.N.A., Toldrá, F., Eds.; John Wiley & Sons Ltd.: New York, NY, USA, 2020; pp. 479–500. [Google Scholar]
- Mekonnen, T.W.; Gerrano, A.S.; Mbuma, N.W.; Labuschagne, M.T. Breeding of vegetable cowpea for nutrition and climate resilience in Sub-Saharan Africa: Progress, opportunities, and challenges. Plants 2022, 11, 1583. [Google Scholar] [CrossRef]
- Adekunle, A.T.; Cardwell, K.F.; Florini, D.A.; Ikotun, T. Seed treatment with Trichoderma species for control of damping-off of cowpea caused by Macrophomina phaseolina. Biocontrol. Sci. Technol. 2001, 11, 449–457. [Google Scholar] [CrossRef]
- Lewis, J.A.; Lumsden, R.D. Biocontrol of damping-off of greenhouse-grown crops caused by Rhizoctonia solani with a formulation of Trichoderma spp. Crop Prot. 2001, 20, 49–56. [Google Scholar] [CrossRef]
- Hamed, E.R.; Awad, H.M.; Ghazi, E.A.; El-Gamal, N.G.; Shehata, H.S. Trichoderma asperellum isolated from salinity soil using rice straw waste as biocontrol agent for cowpea plant pathogens. J. Appl. Pharm. Sci. 2015, 5, 91–98. [Google Scholar] [CrossRef]
- Naseri, B.; Younesi, H. Beneficial microbes in biocontrol of root rots in bean crops: A meta-analysis (1990–2020). Physiol. Mol. Plant Pathol. 2021, 116, 101712. [Google Scholar] [CrossRef]
- Fraceto, L.F.; Maruyama, C.R.; Guilger, M.; Mishra, S.; Keswani, C.; Singh, H.B.; de Lima, R. Trichoderma harzianum-based novel formulations: Potential applications for management of next-gen agricultural challenges. J. Chem.Technol. Biotechnol. 2018, 93, 2056–2063. [Google Scholar] [CrossRef]
- Box, G.E.P.; Wilson, K.B. On the experimental attainment of optimum conditions. J. Roy Stat. Soc. B 1951, 13, 27. [Google Scholar] [CrossRef]
- Box, G.E.P.; Hunter, J.S. Multifactor experimental design for exploring the response surfaces. Ann. Math. Stat. 1957, 28, 195–242. [Google Scholar] [CrossRef]
- Plackett, R.L.; Burman, J.P. The design of optimum multifactorial experiments. Biometrica 1946, 33, 305–325. [Google Scholar] [CrossRef]
- Mishra, P.K.; Khan, F.N. Effect of different growth media and physical factors on biomass production of Trichoderma viride. People’s J. Sci. Res. 2015, 8, 11–17. [Google Scholar]
- Carboué, Q.; Claeys-Bruno, M.; Bombarda, I.; Sergent, M.; Jolain, J.; Roussos, S. Experimental design and solid state fermentation: A holistic approach to improve cultural medium for the production of fungal secondary metabolites. Chemom. Intell. Lab. Syst. 2018, 176, 101–107. [Google Scholar] [CrossRef]
- El-Shabrawy, E.; Heba, S. Controlling maize late-wilt and enhancing plant salinity tolerance by some rhizobacterial strains. Egypt. J. Phytopathol. 2018, 46, 235–255. [Google Scholar] [CrossRef]
- Belimov, A.A.; Hontzeas, N.; Safronova, V.I.; Demchinskaya, S.V.; Piluzza, G.; Bullitta, S.; Glick, B.R. Cadmium-tolerant plant growth-promoting bacteria associated with the roots of Indian mustard (Brassica juncea L. Czern.). Soil Biol. Biochem. 2005, 37, 241–250. [Google Scholar] [CrossRef]
- Luo, J.; Tang, S.Q.; Hu, P.S.; Aleman, L.; Jiao, G.A.; Tang, J. Analysis on factors affecting seedling establishment in rice. Rice Sci. 2007, 14, 27–32. [Google Scholar] [CrossRef]
- Crowe, F.J.; Debons, J.; Darnell, T.; McGrath, D.; Koepsell, P.; Laborde, J.; Redondo, J. Control of Allium white rot with DADS and related products. In Proceedings of the Fifth International Workshop on Allium White Rot, Cordoba, Spain, 1994; Entwistle, A.R., Melero-Vara, J.M., Eds.; HRI: Norwich, UK, 1994; pp. 15–19. [Google Scholar]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. In Methods in Enzymology; Academic Press: San Diego, CA, USA, 1987; Volume 148, pp. 350–382. [Google Scholar]
- Hendrix, D.L. Rapid extraction and analysis of nonstructural carbohydrates in plant tissues. Crop. Sci. 1993, 33, 1306–1311. [Google Scholar] [CrossRef]
- Srivastava, S.K. Peroxidase and polyphenol-oxidase in Brassica juncea plants infected with Macrophomina phaseolina (Tassi): Goid of and their implication in disease resistance. Phytopathology 1987, 120, 249–254. [Google Scholar] [CrossRef]
- Matta, A.I.; Dimond, A.F. Symptoms of Fusarium wilt in relation to quantity of fungus and enzyme activity in tomato stems. Phytopathology 1963, 53, 574–578. [Google Scholar]
- Zucker, M. Sequential induction of phenylalanine ammonia layse and lyase in inactivating system in potato tuber disks. Plant Physiol. 1968, 43, 365–374. [Google Scholar] [CrossRef]
- Zieslin, N.; Ben-Zaken, R. Peroxidase activity and presence of phenolic substances in peduncles of rose flowers. Plant Physiol Biochem. 1993, 31, 333–339. [Google Scholar]
- Page, A.L.; Miller, R.H.; Keeny, D.R. Methods of Soil Analysis, Part II, Agronomy Monographs ASA and SSSA, 2nd ed.; Madison Book Company: Madison, WI, USA, 1982. [Google Scholar]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- USDA. Soil Survey LaboratoryMethodsManual Soil Survey Investigation Report, No. 42, Version 4; USDA-NRCS: Lincoln, NE, USA, 2004.
- Duncan, D.B. Multiple range and multiple F tests. Biometrics 1955, 11, 1–42. [Google Scholar] [CrossRef]
- Hynes, R.K.; Boyetchko, S.M. Research initiatives in the art and science of biopesticide formulations. Soil Biol. Biochem. 2006, 38, 845–849. [Google Scholar] [CrossRef]
- Mulatu, A.; Alemu, T.; Megersa, N.; Vetukuri, R.R. Optimization of culture conditions and production of bio-fungicides from Trichoderma species under solid-state fermentation using mathematical modeling. Microorganisms 2021, 9, 1675. [Google Scholar] [CrossRef] [PubMed]
- Yahya, S.; Jahangir, S.; Shaukat, S.S.; Sohail, M.; Khan, S.A. Production optimization by using Plackett-Burman design and partial characterization of amylase from Aspergillus tubingensis SY 1. Pak. J. Bot. 2016, 48, 2557–2561. [Google Scholar]
- Zalila-Kolsi, I.; Kessentini, S.; Tounsi, S.; Jamoussi, K. Optimization of Bacillus amyloliquefaciens BLB369 culture medium by response surface methodology for low cost production of antifungal activity. Microorganisms 2022, 10, 830. [Google Scholar] [CrossRef] [PubMed]
- Limón, M.C.; Chacón, M.R.; Mejías, R.; Delgado-Jarana, J.; Rincón, A.M.; Codón, A.C.; Benítez, T. Increased antifungal and chitinase specific activities of Trichoderma harzianum CECT 2413 by addition of a cellulose binding domain. Appl. Microbiol. Biotechnol. 2004, 64, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Bitton, G.; Boylan, R.A. Effect of Acid Precipitation on Soil Microbial Activity: I. Soil Core Studies; American Society of Agronomy, Crop Science Society of America, and Soil Science Society of America: Madison, WI, USA, 1985; Volume 14, pp. 66–69. [Google Scholar]
- Jackson, A.; Whipps, J.; Lynch, J. Production, delivery systems, and survival in soil of four fungi with disease biocontrol potential. Enzym. Microb. Technol. 1991, 13, 636–642. [Google Scholar] [CrossRef]
- Singh, A.; Sarma, B.K.; Singh, H.B.; Upadhyay, R. Trichoderma: A silent worker of plant rhizosphere. In Biotechnology and Biology of Trichoderma; Elsevier: Amsterdam, The Netherlands, 2014; pp. 533–542. [Google Scholar]
- Mohiddin, F.; Bashir, I.; Padder, S.A.; Hamid, B. Evaluation of different substrates for mass multiplication of Trichoderma species. J. Pharmacogn. Phytochem. 2017, 6, 563–569. [Google Scholar]
- Pandey, A. Aspects of fermenter design for solid-state fermentations. Process Biochem. 1991, 26, 355–361. [Google Scholar] [CrossRef]
- Mosier, N.; Wyman, C.; Dale, B.; Elander, R.; Lee, Y.Y.; Holtzapple, M.; Ladisch, M. Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour. Technol. 2005, 96, 673–686. [Google Scholar] [CrossRef]
- Sharma, A.; Salwan, R.; Kaur, R.; Sharma, R.; Sharma, V. Characterization and evaluation of bioformulation from antagonistic and flower inducing Trichoderma asperellum isolate UCRD5. Biocatal. Agric. Biotechnol. 2022, 43, 102437. [Google Scholar] [CrossRef]
- Subramaniam, S.; Zainudin, N.A.I.M.; Aris, A.; Hasan, Z.A.E. Role of Trichoderma in plant growth promotion. In Advances in Trichoderma Biology for Agricultural Applications; Springer: Berlin/Heidelberg, Germany, 2022; pp. 257–280. [Google Scholar]
- Poveda, J.; Eugui, D. Combined use of Trichoderma and beneficial bacteria (mainly Bacillus and Pseudomonas): Development of microbial synergistic bio-inoculants in sustainable agriculture. Biol. Control 2022, 176, 105100. [Google Scholar] [CrossRef]
- Dutta, P.; Mahanta, M.; Singh, S.B.; Thakuria, D.; Deb, L.; Kumari, A.; Upamanya, G.K.; Boruah, S.; Dey, U.; Mishra, A.K.; et al. Molecular interaction between plants and Trichoderma species against soil-borne plant pathogens. Front. Plant Sci. 2023, 14, 1145715. [Google Scholar] [CrossRef]
- Mastouri, F.; Björkman, T.; Harman, G.E. Seed treatment with Trichoderma harzianum alleviates biotic, abiotic, and physiological stresses in germinating seeds and seedlings. Phytopathology 2010, 100, 1213–1221. [Google Scholar] [CrossRef]
- Adan, M.J.; Baque, M.A.; Rahman, M.M.; Islam, M.R.; Jahan, A. Formulation of Trichoderma based biopesticide for controlling damping off pathogen of eggplant seedling. Univers. J. Agric. Res. 2015, 3, 106–113. [Google Scholar] [CrossRef]
- Dabire, T.G.; Bonzi, S.; Somda, I.; Legreve, A. Evaluation of the potential of Trichoderma harzianum as a plant growth promoter and biocontrol agent against Fusarium damping-off in onion in Burkina Faso. Asian J. Plant Pathol. 2016, 10, 49–60. [Google Scholar] [CrossRef][Green Version]
- Mukherjee, P.K.; Mehetre, S.T.; Sherkhane, P.D.; Muthukathan, G.; Ghosh, A.; Kotasthane, A.S.; Khare, N.; Rathod, P.; Sharma, K.K.; Nath, R.; et al. A novel seed-dressing formulation based on an improved mutant strain of Trichoderma virens, and its field evaluation. Front. Microbiol. 2019, 10, 1910. [Google Scholar] [CrossRef] [PubMed]
- Agostini, R.B.; Ariel, F.; Rius, S.P.; Vargas, W.A.; Campos-Bermudez, V.A. Trichoderma root colonization in maize triggers epigenetic changes in genes related to the jasmonic and salicylic acid pathways that prime defenses against Colletotrichum graminicola leaf infection. J. Exp. Bot. 2023, 74, 2016–2028. [Google Scholar] [CrossRef]
- Meah, M.B.; Islam, M.R.; Islam, M.M. Development of an integrated approach for management of Phomopsis Blight and Fruit rot of Eggplant in Bamgladesh. Ann. Res. Reptr. Dept. Plant Pathol. BAU 2004, 62. [Google Scholar]
- Kakvan, N.; Heydari, A.; Zamanizadeh, H.R.; Rezaee, S.; Naraghi, L. Development of new bioformulations using Trichoderma and Talaromyces fungal antagonists for biological control of sugar beet damping-off disease. Crop Prot. 2013, 53, 80–84. [Google Scholar] [CrossRef]
- Chin, J.M.; Lim, Y.Y.; Ting, A.S.Y. Biopolymers for biopriming of Brassica rapa seeds: A study on coating efficacy, bioagent viability and seed germination. J. Saudi Soc. Agric. Sci. 2021, 20, 198–207. [Google Scholar] [CrossRef]
- Manzar, N.; Kashyap, A.S.; Goutam, R.S.; Rajawat, M.V.S.; Sharma, P.K.; Sharma, S.K.; Singh, H.V. Trichoderma: Advent of versatile biocontrol agent, its secrets and insights into mechanism of biocontrol potential. Sustainability 2022, 14, 12786. [Google Scholar] [CrossRef]
- Harman, G.E.; Howell, C.R.; Viterbo, A.; Chet, I.; Lorito, M. Trichoderma species opportunistic, avirulent plant symbionts. Nat. Rev. Microbiol. 2004, 2, 43–56. [Google Scholar] [CrossRef]
- Alfiky, A.; Weisskopf, L. Deciphering Trichoderma–Plant–Pathogen Interactions for Better Development of Biocontrol Applications. J. Fungi 2021, 7, 61. [Google Scholar] [CrossRef]
- Yedidia, I.; Srivastva, A.K.; Kapulnik, Y.; Chet, I. Effect of Trichoderma harzianum on microelement concentrations and increased growth of cucumber plants. Plant Soil 2001, 235, 235–242. [Google Scholar] [CrossRef]
- Aseel, D.G.; Soliman, S.A.; Al-Askar, A.A.; Elkelish, A.; Elbeaino, T.; Abdelkhalek, A. Trichoderma viride Isolate Tvd44 Enhances Potato Growth and Stimulates the Defense System against Potato Virus Y. Horticulturae 2023, 9, 716. [Google Scholar] [CrossRef]
- Yang, C.A.; Cheng, C.H.; Lo, C.T.; Liu, S.Y.; Lee, J.W.; Peng, K.H. A novel L-amino acid oxidase from Trichoderma harzianum EST 323 associated with antagonism of R. Solani. J. Agric. Food Chem. 2011, 59, 4519–4526. [Google Scholar] [CrossRef]
- Durairaj, K.; Velmurugan, P.; Park, J.H.; Chang, W.S.; Park, Y.J.; Senthilkumar, P. 2018. Characterization and assessment of two biocontrol bacteria against Pseudomonas syringae wilt in Solanum lycopersicum and its genetic responses. Microbiol. Res. 2018, 206, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Moharan, S.; Dutta, S. Spatial variability of chlorophyll and nitrogen content of rice from hyperspectral imagery. ISPRS J. Photogramm 2016, 122, 17–29. [Google Scholar] [CrossRef]
- Pérez-Bueno, M.L.; Pineda, M.; Barón, M. Phenotyping plant responses to biotic stress by chlorophyll fluorescence imaging. Front. Plant Sci. 2019, 10, 1135. [Google Scholar] [CrossRef]
- Inayati, A.; Sulistyowati, L.; Aini, L.Q.; Yusnawan, E. Trichoderma virens-Tv4 enhances growth promoter and plant defense-related enzymes of mungbean (Vigna radiata) against soil-borne pathogen Rhizoctonia solani. Biodiversitas J. Biol. Divers. 2020, 21. [Google Scholar] [CrossRef]
- Adhilakshmi, M.; Paranidharan, V.; Balachandar, D.; Ganesamurthy, K.; Velazhahan, R. Suppression of root rot of mung bean (Vigna radiata L.) by Streptomyces sp. is associated with induction of peroxidase and polyphenol oxidase. Arch Phytopathol Plant Prot. 2014, 47, 71–583. [Google Scholar] [CrossRef]
- Kavitha, R.; Umesha, S. Regulation of defense-related enzymes associated with bacterial spot resistance in tomato. Phytoparasitica 2008, 36, 144. [Google Scholar] [CrossRef]
- Constabel, C.P.; Barbehenn, R. Defensive roles of polyphenol oxidase in plants. In Induced Plant Resistance to Herbivory; Schaller, A., Ed.; Springer: Dordrecht, Germany, 2008. [Google Scholar]
- Perincherry, L.; Lalak-Kańczugowska, J.; Stępień, Ł. Fusarium-produced mycotoxins in plant-pathogen interactions. Toxins 2019, 11, 664. [Google Scholar] [CrossRef]
- Kubalt, K. The role of phenolic compounds in plant resistance. Biotechnol. Food Sci. 2016, 80, 97–108. [Google Scholar]
- Bhattacharya, A.; Sood, P.; Citovsky, V. The roles of plant phenolics in defense and communication during Agrobacterium and Rhizobium infection. J. Mol. Plant Pathol. 2010, 11, 705–719. [Google Scholar] [CrossRef] [PubMed]
- Løvdal, T.; Olsen, K.M.; Slimestad, R.; Verheul, M.; Lillo, C. Synergetic effects of nitrogen depletion, temperature, and light on the content of phenolic compounds and gene expression in leaves of tomato. Phytochemistry 2010, 71, 605–613. [Google Scholar] [CrossRef]
- Hesham, A.E.-L.; Upadhyay, R.S.; Sharma, G.D.; Manoharachary, C.; Gupta, V.K. Fungal Biotechnology and Bioengineering; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Li, R.; Cai, F.; Pang, G.; Shen, Q.; Li, R.; Chen, W. Solubilisation of phosphate and micronutrients by Trichoderma harzianum and its relationship with the promotion of tomato plant growth. PLoS ONE 2015, 10, e0130081. [Google Scholar] [CrossRef] [PubMed]
- Mallhi, Z.I.; Rizwan, M.; Mansha, A.; Ali, Q.; Asim, S.; Ali, S.; Hussain, A.; Alrokayan, S.H.; Khan, H.A.; Alam, P.; et al. Citric acid enhances plant growth, photosynthesis, and phytoextraction of lead by alleviating the oxidative stress in castor beans. Plan Theory 2019, 8, 525. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Arasu, V.S.; Kathiresan, K. Effect of Trichoderma on soil phosphate solubilization and growth improvement of Avicennia Mar. Aquat Bot. 2013, 104, 101–105. [Google Scholar] [CrossRef]
- Li, Y.; Hwang, S.; Huang, Y.; Huang, C. Effects of Trichoderma asperellum on nutrient uptake and Fusarium wilt of tomato. Crop Prot. 2018, 110, 275–282. [Google Scholar] [CrossRef]
- Fiorentino, N.; Ventorino, V.; Woo, S.L.; Pepe, O.; Rosa, A.D.; Gioia, L.; Romano, I.; Lombardi, N.; Napolitano, M.; Colla, G.; et al. Trichoderma -based biostimulants modulate rhizosphere microbial populations and improve n uptake efficiency, yield, and nutritional quality of leafy vegetables. Front. Plant Sci. 2018, 9, 743. [Google Scholar] [CrossRef] [PubMed]
| Parameters (%) | Faba Bean | Cowpea | Sweet Potato | Pumpkin | Cassava |
|---|---|---|---|---|---|
| OC | 42.0 | 41.39 | 39.99 | 44.29 | 42.00 |
| C/N ratio | 28.3 | 27.88 | 30.21 | 28.11 | 29.92 |
| Total N | 1.47 | 1.29 | 1.43 | 1.59 | 1.66 |
| Total P | 0.37 | 0.39 | 0.48 | 0.52 | 0.56 |
| Total K | 1.48 | 1.41 | 1.76 | 1.83 | 1.73 |
| Independent Variables | Units | Coded Levels | ||
|---|---|---|---|---|
| Low | High | |||
| W1 | Faba bean waste | g | 0 | 1 |
| W2 | Cowpea waste | g | 0 | 1 |
| W3 | Sweet potato waste | g | 0 | 1 |
| W4 | Pumpkin waste | g | 0 | 1 |
| W5 | Cassava waste | g | 0 | 1 |
| pH | pH | - | 4 | 6 |
| Moisture | Moisture | % | 65 | 75 |
| Temperature | Temperature | °C | 20 | 25 |
| Pre-treatment | Pre-treatment of substrate | - | Untreated | Treated with water |
| Run | W1 | W2 | W3 | W4 | W5 | Moisture | pH | Temperature | Pre-Treatment | C/N Ratio |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 0 | 1 | 1 | 0 | 75 | 6 | 25 | 0 | 36:1 |
| 2 | 1 | 1 | 1 | 0 | 0 | 65 | 6 | 20 | 1 | 38:1 |
| 3 | 0 | 0 | 1 | 0 | 1 | 75 | 4 | 25 | 1 | 22:1 |
| 4 | 0 | 1 | 1 | 1 | 0 | 65 | 4 | 25 | 0 | 34:1 |
| 5 | 1 | 0 | 1 | 1 | 1 | 65 | 4 | 20 | 1 | 48:1 |
| 6 | 0 | 1 | 1 | 0 | 1 | 75 | 6 | 20 | 0 | 32:1 |
| 7 | 0 | 0 | 0 | 1 | 0 | 75 | 6 | 20 | 1 | 20:1 |
| 8 | 1 | 0 | 0 | 0 | 1 | 65 | 6 | 25 | 0 | 28:1 |
| 9 | 1 | 1 | 0 | 0 | 0 | 75 | 4 | 25 | 1 | 21:1 |
| 10 | 1 | 1 | 0 | 1 | 1 | 75 | 4 | 20 | 0 | 46:1 |
| 11 | 0 | 0 | 0 | 0 | 0 | 65 | 4 | 20 | 0 | 0 |
| 12 | 0 | 1 | 0 | 1 | 1 | 65 | 6 | 25 | 1 | 35:1 |
| Independent Variables | Units | Coded Levels | |||
|---|---|---|---|---|---|
| Level 1 | Level 2 | Level 3 | |||
| W2 | Sweet potato waste | g | 2 | 3 | 4 |
| W5 | Cassava waste | g | 2 | 3 | 4 |
| pH | pH | 4 | 6 | 8 | |
| Temp | Temperature | °C | 20 | 25 | 30 |
| Pre-treatment | Pre-treatment of substrate | - | Untreated | Treated with water (0.5) | Treated with 0.5% NaOH (1) |
| Run | W2 | W5 | pH | Temperature | Pre-Treatment | C/N Ratio |
|---|---|---|---|---|---|---|
| 1 | 2 | 2 | 8 | 30 | −1 | 25:1 |
| 2 | 3 | 3 | 6 | 20 | 0 | 34:1 |
| 3 | 2 | 3 | 6 | 25 | 0 | 25:1 |
| 4 | 4 | 2 | 8 | 20 | 1 | 31:1 |
| 5 | 3 | 3 | 6 | 30 | 0 | 34:1 |
| 6 | 4 | 4 | 8 | 20 | 1 | 48:1 |
| 7 | 4 | 4 | 4 | 20 | −1 | 48:1 |
| 8 | 4 | 2 | 8 | 30 | 1 | 31:1 |
| 9 | 4 | 4 | 8 | 30 | 1 | 48:1 |
| 10 | 4 | 4 | 4 | 30 | 1 | 48:1 |
| 11 | 2 | 4 | 8 | 30 | 1 | 35:1 |
| 12 | 3 | 3 | 6 | 25 | 1 | 34:1 |
| 13 | 2 | 4 | 4 | 30 | −1 | 35:1 |
| 14 | 3 | 4 | 6 | 25 | 0 | 37:1 |
| 15 | 2 | 2 | 4 | 30 | −1 | 25:1 |
| 16 | 4 | 4 | 8 | 20 | −1 | 48:1 |
| 17 | 2 | 4 | 4 | 30 | 1 | 35:1 |
| 18 | 2 | 4 | 8 | 20 | 1 | 35:1 |
| 19 | 3 | 3 | 6 | 25 | 0 | 34:1 |
| 20 | 4 | 2 | 4 | 20 | −1 | 31:1 |
| 21 | 2 | 2 | 8 | 20 | 1 | 25:1 |
| 22 | 3 | 3 | 8 | 25 | 0 | 34:1 |
| 23 | 3 | 3 | 6 | 25 | −1 | 34:1 |
| 24 | 2 | 2 | 4 | 20 | 1 | 25:1 |
| 25 | 4 | 2 | 4 | 30 | −1 | 31:1 |
| 26 | 4 | 4 | 4 | 30 | −1 | 48:1 |
| 27 | 3 | 2 | 6 | 25 | 0 | 27:1 |
| 28 | 4 | 2 | 8 | 30 | −1 | 31:1 |
| 29 | 4 | 2 | 8 | 20 | −1 | 31:1 |
| 30 | 4 | 4 | 8 | 30 | −1 | 48:1 |
| 31 | 2 | 2 | 8 | 30 | 1 | 25:1 |
| 32 | 4 | 4 | 4 | 20 | 1 | 48:1 |
| 33 | 2 | 4 | 4 | 20 | −1 | 35:1 |
| 34 | 2 | 2 | 4 | 20 | −1 | 35:1 |
| 35 | 3 | 3 | 4 | 25 | 0 | 25:1 |
| 36 | 2 | 4 | 8 | 30 | −1 | 35:1 |
| 37 | 4 | 2 | 4 | 30 | 1 | 31:1 |
| 38 | 4 | 2 | 4 | 20 | 1 | 31:1 |
| 39 | 2 | 2 | 4 | 30 | 1 | 25:1 |
| 40 | 4 | 3 | 6 | 25 | 0 | 44:1 |
| 41 | 2 | 2 | 8 | 20 | −1 | 25:1 |
| 42 | 2 | 4 | 8 | 20 | −1 | 35:1 |
| 43 | 2 | 4 | 4 | 20 | 1 | 35:1 |
| Mechanical Analysis (%) | Texture | pH | EC | OM (g kg−1) | Available Elements (mg kg−1) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Sand | Silt | Clay | N | P | K | ||||
| 20.91 | 24.87 | 54.22 | Clay | 7.63 | 2.92 | 15.56 | 9.77 | 8.90 | 343.37 |
| Symbol | Description |
|---|---|
| T1 | Control (neither bioformulation nor infected stress) |
| T2 | Control (soil infected with F. oxysporum) |
| T3 | Control (soil infected with F. oxysporum + seeds treated with Vitavax fungicide (Carboxin 37.5% + Thiram 37.5% DS, 1 g kg−1, Dhanuka Agritech Ltd. India) |
| T4 | Seeds treated with T. lixii by a carrier of sweet potato waste (2 g) + Cassava waste (2 g) |
| T5 | Seeds treated with T. lixii by a carrier of sweet potato waste (4 g) + Cassava waste (4 g) |
| T6 | Seeds treated with T. lixii by a carrier of sweet potato waste (3 g) + Cassava waste (4 g) |
| T7 | Seeds treated with T. lixii by a carrier of sweet potato waste (2 g) + Cassava waste (2 g) |
| T8 | Seeds treated with T. lixii by a carrier of sweet potato waste (2 g) + Cassava waste (4 g) |
| T9 | Seeds treated with T. lixii by a carrier of sweet potato waste (4 g) + Cassava waste (3 g) |
| T10 | Seeds treated with T. lixii by a carrier of sweet potato waste (3 g) + Cassava waste (2 g) |
| T11 | Seeds treated with T. lixii by a carrier of sweet potato waste (3 g) + Cassava waste (3 g) |
| T12 | Seeds treated with T. lixii by a carrier of sweet potato waste (4 g) + Cassava waste (2 g) |
| T13 | Seeds treated with T. lixii by a carrier of talc (reference treatment) |
| Run | W1 | W2 | W3 | W4 | W5 | Moisture | pH | Temperature | Pre-Treatment | log10(Y)-Conidia/g Dry Substrate | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Experimental | Predicted | ||||||||||
| 1 | 1 | 0 | 1 | 1 | 0 | 75 | 6 | 25 | 0 | 6.95 ± 0.15 | 6.98 |
| 2 | 1 | 1 | 1 | 0 | 0 | 65 | 6 | 20 | 1 | 6.67 ± 0.14 | 6.63 |
| 3 | 0 | 0 | 1 | 0 | 1 | 75 | 4 | 25 | 1 | 7.52 ± 0.23 | 7.34 |
| 4 | 0 | 1 | 1 | 1 | 0 | 65 | 4 | 25 | 0 | 6.86 ± 0.16 | 6.54 |
| 5 | 1 | 0 | 1 | 1 | 1 | 65 | 4 | 20 | 1 | 8.67 ± 0.72 | 8.35 |
| 6 | 0 | 1 | 1 | 0 | 1 | 75 | 6 | 20 | 0 | 7.4 ± 0.63 | 7.57 |
| 7 | 0 | 0 | 0 | 1 | 0 | 75 | 6 | 20 | 1 | 0 | 0.17 |
| 8 | 1 | 0 | 0 | 0 | 1 | 65 | 6 | 25 | 0 | 7.36 ± 0.47 | 7.67 |
| 9 | 1 | 1 | 0 | 0 | 0 | 75 | 4 | 25 | 1 | 6.57 ± 0.47 | 6.88 |
| 10 | 1 | 1 | 0 | 1 | 1 | 75 | 4 | 20 | 0 | 8.05 ± 0.11 | 8.23 |
| 11 | 0 | 0 | 0 | 0 | 0 | 65 | 4 | 20 | 0 | 0 | 0.000 |
| 12 | 0 | 1 | 0 | 1 | 1 | 65 | 6 | 25 | 1 | 7.12 ± 0.12 | 7.29 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 92.98 | 9 | 10.33 | 679.29 | 0.0015 * |
| A—W1 | 19.69 | 1 | 19.69 | 1294.45 | 0.0008 |
| B—W2 | 12.34 | 1 | 12.34 | 811.56 | 0.0012 |
| C—W3 | 18.68 | 1 | 18.68 | 1227.95 | 0.0008 |
| D—W4 | 0.3781 | 1 | 0.3781 | 24.86 | 0.0380 |
| E—W5 | 30.31 | 1 | 30.31 | 1992.68 | 0.0005 |
| F—Moisture | 0.0030 | 1 | 0.0030 | 0.1978 | 0.7000 |
| G—pH | 0.3924 | 1 | 0.3924 | 25.80 | 0.0366 |
| H—Temperature | 11.19 | 1 | 11.19 | 736.04 | 0.0014 |
| J—Pre-Treatment | 0.0004 | 1 | 0.0004 | 0.0268 | 0.8849 |
| Residual | 0.0400 | 2 | 0.0152 | ||
| Lack of Fit | 0.01 | 1 | 0.01 | 0.53 | 0.54 |
| Pure Error | 0.03 | 6 | 0.02 | ||
| Cor Total | 92.17 | 11 |
| Run | W2 | W5 | pH | Temperature | Pre-Treatment | log10(Y)-Conidia/g Dry Substrate | |
|---|---|---|---|---|---|---|---|
| Experimental | Predicted | ||||||
| 1 | 2 | 2 | 8 | 30 | −1 | 0 | 0.16 |
| 2 | 3 | 3 | 6 | 20 | 0 | 8.71 ± 0.19 | 7.47 |
| 3 | 2 | 3 | 6 | 25 | 0 | 7.58 ± 0.54 | 7.51 |
| 4 | 4 | 2 | 8 | 20 | 1 | 7.95 ± 0.66 | 7.74 |
| 5 | 3 | 3 | 6 | 30 | 0 | 0 | 0.95 |
| 6 | 4 | 4 | 8 | 20 | 1 | 7.45 ± 0.27 | 7.09 |
| 7 | 4 | 4 | 4 | 20 | −1 | 7.56 ± 0.45 | 7.11 |
| 8 | 4 | 2 | 8 | 30 | 1 | 0 | −0.05 |
| 9 | 4 | 4 | 8 | 30 | 1 | 0 | 0.24 |
| 10 | 4 | 4 | 4 | 30 | 1 | 0 | 0.16 |
| 11 | 2 | 4 | 8 | 30 | 1 | 0 | −0.28 |
| 12 | 3 | 3 | 6 | 25 | 1 | 7.58 ± 0.37 | 7.05 |
| 13 | 2 | 4 | 4 | 30 | −1 | 0 | −0.17 |
| 14 | 3 | 4 | 6 | 25 | 0 | 5.89 ± 0.27 | 6.93 |
| 15 | 2 | 2 | 4 | 30 | −1 | 0 | 0.42 |
| 16 | 4 | 4 | 8 | 20 | −1 | 4.23 ± 0.17 | 4.82 |
| 17 | 2 | 4 | 4 | 30 | 1 | 0 | −0.73 |
| 18 | 2 | 4 | 8 | 20 | 1 | 4.65 ± 0.45 | 4.66 |
| 19 | 3 | 3 | 6 | 25 | 0 | 6.27 ± 0.36 | 7.41 |
| 20 | 4 | 2 | 4 | 20 | −1 | 7.67 ± 0.37 | 8.01 |
| 21 | 2 | 2 | 8 | 20 | 1 | 5.46 ± 0.31 | 5.95 |
| 22 | 3 | 3 | 8 | 25 | 0 | 5.68 ± 0.29 | 6.24 |
| 23 | 3 | 3 | 6 | 25 | −1 | 5.88 ± 0.31 | 6.12 |
| 24 | 2 | 2 | 4 | 20 | 1 | 8.34 ± 0.31 | 7.98 |
| 25 | 4 | 2 | 4 | 30 | −1 | 0 | −0.08 |
| 26 | 4 | 4 | 4 | 30 | −1 | 0 | −0.04 |
| 27 | 3 | 2 | 6 | 25 | 0 | 8.87 ± 0.30 | 7.55 |
| 28 | 4 | 2 | 8 | 30 | −1 | 0 | −0.71 |
| 29 | 4 | 2 | 8 | 20 | −1 | 4.8 ± 0.82 | 5.31 |
| 30 | 4 | 4 | 8 | 30 | −1 | 0 | −0.25 |
| 31 | 2 | 2 | 8 | 30 | 1 | 0 | 0.06 |
| 32 | 4 | 4 | 4 | 20 | 1 | 9.45 ± 0.98 | 9.07 |
| 33 | 2 | 4 | 4 | 20 | −1 | 4.65 ± 0.20 | 5.08 |
| 34 | 2 | 2 | 4 | 20 | −1 | 6.85 ± 1.07 | 6.62 |
| 35 | 3 | 3 | 4 | 25 | 0 | 8.21 ± 1.04 | 7.36 |
| 36 | 2 | 4 | 8 | 30 | −1 | 0 | −0.01 |
| 37 | 4 | 2 | 4 | 30 | 1 | 0 | 0.28 |
| 38 | 4 | 2 | 4 | 20 | 1 | 9.53 ± 0.27 | 10.14 |
| 39 | 2 | 2 | 4 | 30 | 1 | 0 | 0.02 |
| 40 | 4 | 3 | 6 | 25 | 0 | 8.68 ± 0.40 | 8.47 |
| 41 | 2 | 2 | 8 | 20 | −1 | 4.23 ± 0.41 | 4.28 |
| 42 | 2 | 4 | 8 | 20 | −1 | 3.98 ± 0.31 | 3.16 |
| 43 | 2 | 4 | 4 | 20 | 1 | 5.28 ± 1.02 | 6.28 |
| Treatments | Storage Duration in Months (log10 CFU g−1) | |||||
|---|---|---|---|---|---|---|
| Initial | 3 | 5 | 7 | 9 | 12 | |
| C | 8.89 ± 0.34 | 8.20 ± 0.21 | 7.76 ± 0.22 | 6.72 ± 0.17 | 5.54 ± 0.11 | 5.20 ± 0.19 |
| T4 | 8.21 ± 0.12 | 7.90 ± 0.18 | 7.69 ± 0.19 | 6.95 ± 0.16 | 5.86 ± 0.18 | 5.63 ± 0.15 |
| T5 | 8.34 ± 0.11 | 8.04 ± 0.19 | 7.71 ± 0.16 | 7.06 ± 0.22 | 5.98 ± 0.12 | 5.81 ± 0.21 |
| T6 | 8.18 ± 0.27 | 7.48 ± 0.27 | 7.60 ± 0.27 | 6.80 ± 0.27 | 5.65 ± 0.17 | 5.45 ± 0.27 |
| T7 | 8.68 ± 0.33 | 8.15 ± 0.31 | 7.73 ± 0.32 | 7.13 ± 0.32 | 6.07 ± 0.19 | 5.94 ± 0.17 |
| T8 | 7.58 ± 0.26 | 7.70 ± 0.38 | 7.68 ± 0.31 | 6.92 ± 0.31 | 5.81 ± 0.22 | 5.62 ± 0.14 |
| T9 | 8.71 ± 0.17 | 8.26 ± 0.27 | 7.77 ± 0.32 | 7.17 ± 0.36 | 6.11 ± 0.28 | 5.95 ± 0.19 |
| T10 | 9.45 ± 0.19 | 8.30 ± 0.24 | 7.83 ± 0.33 | 7.40 ± 0.27 | 6.37 ± 0.19 | 6.27 ± 0.22 |
| T11 | 8.87 ± 0.27 | 8.28 ± 0.30 | 7.70 ± 0.31 | 7.33 ± 0.36 | 6.29 ± 0.25 | 6.18 ± 0.27 |
| T12 | 9.53 ± 0.29 | 9.38 ± 0.35 | 8.83 ± 0.26 | 7.78 ± 0.28 | 6.63 ± 0.26 | 6.36 ± 0.21 |
| Treatments | Fresh Mass (g Plant−1) | Dry Mass (g Plant−1) | Shoot Length (cm Plant−1) | Root Length (cm Plant−1) |
|---|---|---|---|---|
| T1 | 39.73 ± 7.42 e | 7.54 ± 0.95 d | 17.39 ± 1.07 e | 8.13 ± 0.50 e |
| T2 | 16.73 ± 5.02 f | 3.21 ± 0.90 e | 12.98 ± 0.73 f | 6.15 ± 0.35 f |
| T3 | 36.21 ± 5.13 e | 6.82 ± 1.23 d | 16.56 ± 1.27 e | 7.87 ± 0.63 e |
| T4 | 62.93 ± 6.51 cd | 11.82 ± 1.23 c | 21.69 ± 1.31 d | 10.35 ± 0.64 d |
| T5 | 68.29 ± 5.30 c | 12.45 ± 1.00 c | 22.28 ± 0.89 d | 10.62 ± 0.44 d |
| T6 | 59.59 ± 8.21 d | 11.18 ± 1.12 c | 21.02 ± 1.17 d | 9.99 ± 0.53 d |
| T7 | 83.90 ± 4.05 b | 15.53 ± 0.75 b | 25.16 ± 0.87 c | 11.91 ± 0.47 c |
| T8 | 59.21 ± 3.91 d | 11.30 ± 1.27 c | 21.00 ± 1.17 d | 9.92 ± 0.73 d |
| T9 | 84.93 ± 6.05 b | 15.63 ± 1.12 b | 25.33 ± 0.99 bc | 12.11 ± 0.53 bc |
| T10 | 89.29 ± 2.35 b | 17.06 ± 0.98 b | 26.79 ± 0.98 b | 12.72 ± 0.42 b |
| T11 | 88.93 ± 7.24 b | 16.47 ± 1.34 b | 26.04 ± 1.57 bc | 12.33 ± 0.54 bc |
| T12 | 103.56 ± 2.72 a | 19.81 ± 0.81 a | 29.54 ± 0.56 a | 14.03 ± 0.11 a |
| T13 | 105.16 ± 1.82 a | 19.97 ± 0.71 a | 29.79 ± 0.51 a | 14.18 ± 0.09 a |
| LSD 0.05 | 7.84 | 1.63 | 1.57 | 0.80 |
| Treatments | PO (μM H2O2 g−1 FW min−1) | PPO (μM Tetra–Guaiacol g−1 min−1 FW) | PAL (μmol min−1 g−1 FW) | TPC (mg GAE g−1 FW) |
|---|---|---|---|---|
| T1 | 0.71 ± 0.25 d | 0.14 ± 0.03 d | 2.24 ± 0.20 e | 13.15 ± 1.16 d |
| T2 | 0.25 ± 0.06 e | 0.07 ± 0.02 e | 1.10 ± 0.24 f | 9.38 ± 0.91 e |
| T3 | 0.51 ± 0.30 de | 0.13 ± 0.02 d | 2.01 ± 0.23 e | 11.92 ± 1.10 d |
| T4 | 1.90 ± 0.39 c | 0.21 ± 0.02 c | 3.37 ± 0.33 d | 17.72 ± 1.20 c |
| T5 | 2.08 ± 0.27 c | 0.22 ± 0.02 c | 3.47 ± 0.37 d | 18.01 ± 0.69 c |
| T6 | 1.70 ± 0.30 c | 0.20 ± 0.02 c | 3.27 ± 0.30 d | 17.31 ± 1.12 c |
| T7 | 2.81 ± 0.26 b | 0.27 ± 0.02 b | 4.41 ± 0.22 c | 21.29 ± 1.16 b |
| T8 | 1.73 ± 0.33 c | 0.20 ± 0.03 c | 3.11 ± 0.16 d | 17.03 ± 1.74 c |
| T9 | 2.82 ± 0.26 b | 0.27 ± 0.02 b | 4.39 ± 0.33 c | 21.76 ± 1.12 b |
| T10 | 3.22 ± 0.19 b | 0.29 ± 0.03 ab | 4.81 ± 0.24 b | 22.79 ± 1.53 b |
| T11 | 3.05 ± 0.31 b | 0.29 ± 0.02 ab | 4.60 ± 0.33 bc | 21.83 ± 1.33 b |
| T12 | 4.05 ± 0.13 a | 0.33 ± 0.02 a | 5.43 ± 0.21 a | 25.07 ± 1.04 a |
| T13 | 4.12 ± 0.11 a | 0.37 ± 0.03 a | 5.45 ± 0.17 a | 25.11 ± 1.08 a |
| LSD 0.05 | 0.42 | 0.03 | 0.36 | 1.86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omara, A.E.-D.; El-maghraby, F.M. Novel Bioformulations with Trichoderma lixii to Improve the Growth Dynamics and Biocontrol of the Cowpea Damping-Off Disease. Microbiol. Res. 2023, 14, 2041-2066. https://doi.org/10.3390/microbiolres14040138
Omara AE-D, El-maghraby FM. Novel Bioformulations with Trichoderma lixii to Improve the Growth Dynamics and Biocontrol of the Cowpea Damping-Off Disease. Microbiology Research. 2023; 14(4):2041-2066. https://doi.org/10.3390/microbiolres14040138
Chicago/Turabian StyleOmara, Alaa El-Dein, and Fatma M. El-maghraby. 2023. "Novel Bioformulations with Trichoderma lixii to Improve the Growth Dynamics and Biocontrol of the Cowpea Damping-Off Disease" Microbiology Research 14, no. 4: 2041-2066. https://doi.org/10.3390/microbiolres14040138
APA StyleOmara, A. E.-D., & El-maghraby, F. M. (2023). Novel Bioformulations with Trichoderma lixii to Improve the Growth Dynamics and Biocontrol of the Cowpea Damping-Off Disease. Microbiology Research, 14(4), 2041-2066. https://doi.org/10.3390/microbiolres14040138






