Semi-VOCs of Wood Vinegar Display Strong Antifungal Activities against Oomycete Species Globisporangium ultimum and Pythium aphanidermatum
Abstract
1. Introduction
2. Materials and Methods
2.1. Pathogen Recovery
2.2. DNA Extraction and PCR Amplification
2.3. DNA Sequencing and Phylogeny
2.4. Extraction of Pyroligneous Acid (Wood Vinegar)
2.5. GC-FID Analysis of Pyroligneous Acid (Wood Vinegar)
2.6. In Vitro Inhibition of Semi-VOCs of Wood Vinegar against Globisporangium Ultimum and Pythium Aphanidermatum Mycelial Growth
2.7. Statistical Analysis
3. Results
3.1. Molecular Identification of Globisporangium and Pythium Isolates
3.2. Semi-VOCs Concentrations Activity against Globisporangium Ultimum Mycelial Growth
3.3. Efficacy of Semi-VOCs Concentrations against Pythium Aphanidermatum Mycelial Growth
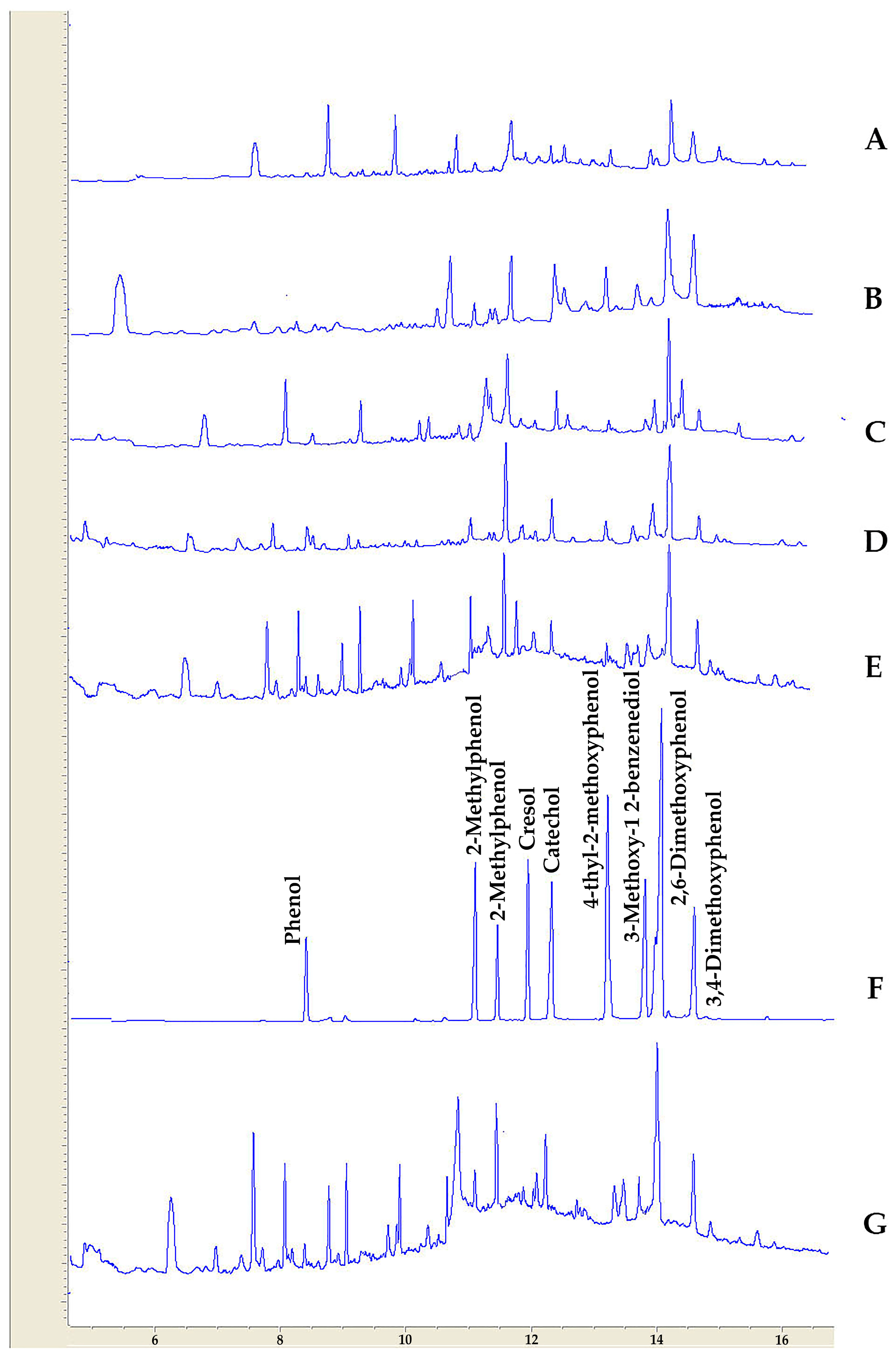
3.4. GC-FID Analysis of Wood Vinegar Samples
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Belbahri, L.; Chenari Bouket, A.; Rekik, I.; Alenezi, F.N.; Vallat, A.; Luptakova, L.; Petrovova, E.; Oszako, T.; Cherrad, S.; Vacher, S.; et al. Comparative Genomics of Bacillus amyloliquefaciens Strains Reveals a Core Genome with Traits for Habitat Adaptation and a Secondary Metabolites Rich Accessory Genome. Front. Microbiol. 2017, 8, 1438. [Google Scholar] [CrossRef]
- Ristaino, J.B.; Anderson, P.; Brauman, K.A.; Cunniffe, N.J.; Federoff, N.; Garrett, K.A.; Gilligan, C.; Holmes, T.; Martin, M.; MacDonald, G.K.; et al. The Persistent Threat of Emerging Plant Diseases to Global Food Security. Proc. Nat. Acad. Sci. USA 2021, 118, e2022239118. [Google Scholar] [CrossRef]
- Malhi, G.S.; Kaur, M.; Kaushik, P. Impact of Climate Change on Agriculture and Its Mitigation Strategies: A Review. Sustainability 2021, 13, 1318. [Google Scholar] [CrossRef]
- Desaint, H.; Aoun, N.; Deslandes, L.; Vailleau, F.; Roux, F.; Berthome, R. Fight hard or die trying: When plants face pathogens under heat stress. New Phytol. 2021, 229, 712–734. [Google Scholar] [CrossRef]
- Balla, A.; Silini, A.; Cherif-Silini, H.; Chenari Bouket, A.; Moser, W.K.; Nowakowska, J.A.; Oszako, T.; Benia, F.; Belbahri, L. The Threat of Pests and Pathogens and the Potential for Biological Control in Forest Ecosystems. Forests 2021, 12, 1579. [Google Scholar] [CrossRef]
- Prospero, S.; Vercauteren, A.; Heungens, K.; Belbahri, L.; Rigling, D. Phytophthora diversity and the population structure of Phytophthora ramorum in Swiss ornamental nurseries. Plant Pathol. 2013, 62, 1063–1071. [Google Scholar] [CrossRef]
- Corredor-Moreno, P.; Saunders, D.G.O. Expecting the unexpected: Factors influencing the emergence of fungal and oomycete plant pathogens. New Phytol. 2020, 225, 118–125. [Google Scholar] [CrossRef]
- Shahzad, A.; Ullah, S.; Dar, A.A.; Sardar, M.F.; Mehmood, T.; Tufail, M.A.; Shakoor, A.; Haris, M. Nexus on climate change: Agriculture and possible solution to cope future climate change stresses. Environ. Sci. Pollut. Res. Int. 2021, 28, 14211–14232. [Google Scholar] [CrossRef]
- FAO. FAO Database. Food and Agriculture Organization, United Nations. 2016. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 1 February 2023).
- Du, Y.D.; Cao, H.X.; Liu, S.Q.; Gu, X.B.; Cao, Y.X. Response of yield, quality, water and nitrogen use efficacy of tomato to different levels of water and nitrogen under drip irrigation in Northwestern China. J. Integr. Agric. 2017, 16, 1153–1161. [Google Scholar] [CrossRef]
- Gadekallu, T.R.; Rajput, D.S.; Reddy, M.P.K.; Lakshmanna, K.; Bhattacharya, S.; Singh, S.; Jolfaei, A.; Alazab, M. A novel PCA–whale optimization-based deep neural network model for classification of tomato plant diseases using GPU. J. Real-Time Image Process. 2021, 18, 1383–1396. [Google Scholar] [CrossRef]
- Nankishore, A.; Farrell, A.D. The response of contrasting tomato genotypes to combined heat and drought stress. J. Plant Physiol. 2016, 202, 75–82. [Google Scholar] [CrossRef]
- Umetsu, N.; Shirai, Y. Development of novel pesticides in the 21st century. J. Pest. Sci. 2020, 45, 54–74. [Google Scholar] [CrossRef]
- Cherrad, S.; Charnay, A.; Hernandez, C.; Steva, H.; Belbahri, L.; Vacher, S. Emergence of boscalid-resistant strains of Erysiphe necator in French vineyards. Microbiol. Res. 2018, 216, 79–84. [Google Scholar] [CrossRef]
- Zhu, Q.; Yang, Y.; Zhong, Y.; Lao, Z.; O’Neill, P.; Hong, D.; Zhang, K.; Zhao, S. Synthesis, insecticidal activity, resistance, photodegradation and toxicity of pyrethroids (A review). Chemosphere 2020, 254, 126779. [Google Scholar] [CrossRef]
- Solla, A.; Moreno, G.; Malewski, T.; Jung, T.; Klisz, M.; Tkaczyk, M.; Siebyla, M.; Pérez, A.; Cubera, E.; Hrynyk, H.; et al. Phosphite Spray for the Control of Oak Decline Induced by Phytophthora in Europe. For. Ecol. Manag. 2021, 485, 118938. [Google Scholar] [CrossRef]
- Tkaczyk, A.; Mitrowska, K.; Posyniak, A. Synthetic organic dyes as contaminants of the aquatic environment and their implications for ecosystems: A review. Sci. Total Environ. 2020, 717, 137222. [Google Scholar] [CrossRef]
- Naamala, J.; Smith, D.L. Relevance of Plant Growth Promoting Microorganisms and Their Derived Compounds, in the Face of Climate Change. Agronomy 2020, 10, 1179. [Google Scholar] [CrossRef]
- Raveau, R.; Fontaine, J.; Lounès-Hadj Sahraoui, A. Essential Oils as Potential Alternative Biocontrol Products against Plant Pathogens and Weeds: A Review. Foods. 2020, 9, 365. [Google Scholar] [CrossRef]
- Tilocca, B.; Cao, A.; Migheli, Q. Scent of a Killer: Microbial Volatilome and Its Role in the Biological Control of Plant Pathogens. Front. Microbiol. 2020, 11, 41. [Google Scholar] [CrossRef]
- Chenari Bouket, A.; Narmani, A.; Tavasolee, A.; Elyasi, G.; Abdi, A.; Naeimi, S.; Sharifi, K.; Oszako, T.; Alenezi, F.N.; Belbahri, L. In Vitro Evaluation of Wood Vinegar (Pyroligneous Acid) Semi-VOCs Inhibitory Effect against a Fungus-like Microorganism Ovatisporangium (Phytopythium) Isolate Recovered from Tomato Fields in Iran. Agronomy 2022, 12, 1609. [Google Scholar] [CrossRef]
- Arshad, U.; Naveed, M.; Javed, N.; Gogi, M.D.; Ali, M.A. Biochar Application from Different Feedstocks Enhances Plant Growth and Resistance against Meloidogyne incognita in Tomato. Int. J. Agric. Biol. 2020, 24, 961–968. [Google Scholar]
- Thines, M. Phylogeny and evolution of plant pathogenic oomycetes—A global overview. Eur. J. Plant Pathol. 2014, 138, 431–447. [Google Scholar] [CrossRef]
- Van West, P.; Beakes, G.W. Animal pathogenic Oomycetes. Fungal Biol. 2014, 118, 525–526. [Google Scholar] [CrossRef]
- Larousse, M.; Rancurel, C.; Syska, C.; Palero, F.; Etienne, C.; Industri, B.; Nesme, X.; Bardin, M.; Galiana, E. Tomato root microbiota and Phytophthora parasitica-associated disease. Microbiome 2017, 5, 56. [Google Scholar] [CrossRef]
- Tiilikkala, K.; Fagernas, L.; Tiilikkala, J. History and use of wood pyrolysis liquids as biocide and plant protection product. Open Agric. J. 2010, 4, 111–118. [Google Scholar] [CrossRef]
- Grewal, A.; Abbey, L.; Gunupuru, L.R. Production, Prospects and Potential Application of Pyroligneous Acid in Agriculture. J. Anal. Appl. Pyrolysis. 2018, 135, 152–159. [Google Scholar] [CrossRef]
- Mathew, S.; Zakaria, Z.A. Pyroligneous acid—The smoky acidic liquid from plant biomass. Appl. Microbiol. Biotechnol. 2015, 99, 611–622. [Google Scholar] [CrossRef]
- Souza, J.B.G.; Ré-Poppi, N.; Raposo, J.L. Characterization of pyroligneous acid used in agriculture by gas chromatography-mass spectrometry. J. Braz. Chem. Soc. 2012, 23, 610–617. [Google Scholar] [CrossRef]
- Balat, M.; Balat, M.; Kırtay, E.; Balat, H. Main routes for the thermo-conversion of biomass into fuels and chemicals. Part 1: Pyrolysis systems. Energy Convers. Manag. 2009, 50, 3147–3157. [Google Scholar] [CrossRef]
- Mansur, D.; Yoshikawa, T.; Norinaga, K.; Hayashi, J.; Tago, T.; Masuda, T. Production of ketones from pyroligneous acid of woody biomass pyrolysis over an iron-oxide catalyst. Fuel 2013, 103, 130–134. [Google Scholar] [CrossRef]
- Araujo, E.S.; Pimenta, A.S.; Feiji, F.M.C.; Castro, R.V.O.; Fasciotti, M.; Monteiro, T.V.C.; Lima, K.M.G. Antibacterial and antifungal activities of pyroligneous acid from wood of Eucalyptus urograndis and Mimosa tenuiflora. J. Appl. Microbiol. 2017, 124, 85–96. [Google Scholar] [CrossRef]
- Fanfarillo, E.; Fedeli, R.; Fiaschi, T.; de Simone, L.; Vannini, A.; Angiolini, C.; Loppi, S.; Maccherini, S. Effects of Wood Distillate on Seedling Emergence and First-Stage Growth in Five Threatened Arable Plants. Diversity 2022, 14, 669. [Google Scholar] [CrossRef]
- Fedeli, R.; Vannini, A.; Celletti, S.; Maresca, V.; Munzi, S.; Cruz, C.; Alexandrov, D.; Guarnieri, M.; Loppi, S. Foliar application of wood distillate boosts plant yield and nutritional parameters of chickpea. Ann. Appl. Biol. 2023, 182, 57–64. [Google Scholar] [CrossRef]
- Fedeli, R.; Vannini, A.; Guarnieri, M.; Monaci, F.; Loppi, S. Bio-Based Solutions for Agriculture: Foliar Application of Wood Distillate Alone and in Combination with Other Plant-Derived Corroborants Results in Different Effects on Lettuce (Lactuca sativa L.). Biology 2022, 11, 404. [Google Scholar] [CrossRef]
- Liu, X.; Zhan, Y.; Li, X.; Li, Y.; Feng, X.; Baghavathiannan, M.; Zhang, C.; Qu, M.; Yu, J. The use of wood vinegar as a non-synthetic herbicide for control of broadleaf weeds. Ind. Crops Prod. 2021, 73, 114105. [Google Scholar] [CrossRef]
- Chu, L.; Liu, H.; Zhang, Z.; Zhan, Y.; Wang, K.; Yang, D.; Liu, Z.; Yu, J. Evaluation of Wood Vinegar as an Herbicide for Weed Control. Agronomy 2022, 12, 3120. [Google Scholar] [CrossRef]
- Wititsiri, S. Production of wood vinegars from coconut shells and additional materials for control of termite workers, Odontotermes sp. and striped mealy bugs, Ferrisia virgata. Songklanakarin J. Sci. Technol. 2011, 33, 349–354. [Google Scholar]
- Mohan, D.; Pittman, C.U.; Steele, P.H. Pyrolysis of wood/biomass for bio-oil: A critical review. Energy Fuels 2006, 20, 848–889. [Google Scholar] [CrossRef]
- Steiner, C.; Das, K.C.; Garcia, M.; Förster, B.; Zech, W. Charcoal and smoke extract stimulate the soil microbial community in a highly weathered xanthic Ferralsol. Pedobiologia 2008, 51, 359–366. [Google Scholar] [CrossRef]
- Wei, Q.; Ma, X.; Dong, J. Preparation, chemical constituents and antimicrobial activity of pyroligneous acids from walnut tree branches. J. Anal. Appl. Pyrolysis 2010, 87, 24–28. [Google Scholar] [CrossRef]
- Jung, K.-H. Growth inhibition effect of pyroligneous acid on pathogenic fungus, Alternaria mali, the agent of Alternaria blotch of apple. Biotechnol. Bioprocess Eng. 2007, 12, 318–322. [Google Scholar] [CrossRef]
- Chen, S.; Feng, Y.; Li, S.; Mu, J. Research on Inhibition Effect of MDF Pyrolysis Condensate Liquids against Two Kinds of Fungi. In Proceedings of the 55th International Convention of Society of Wood Science and Technology, Beijing, China, 27–31 August 2012; pp. 1–7. [Google Scholar]
- Mmojieje, J.; Hornung, A. The potential application of pyroligneous acid in the UK Agricultural Industry. J. Crop. Improv. 2015, 29, 228–246. [Google Scholar] [CrossRef]
- Chalermsan, Y.; Peerapan, S. Wood-vinegar: By-product from rural charcoal kiln and its roles in plant protection. Asian J. Food. Agro-Indust. 2009, 2, 189–195. [Google Scholar]
- Mahmud, K.N.; Yahayu, M.; Sarip, S.H.M.; Rizan, N.H.; Min, C.B.; Mustafa, N.F.; Ngadiran, S.; Ujang, S.; Zakaria, Z.A. Evaluation on Efficiency of Pyroligneous Acid from Palm Kernel Shell as Antifungal and Solid Pineapple Biomass as Antibacterial and Plant Growth Promoter. Sains Malays. 2016, 45, 1423–1434. [Google Scholar]
- Saberi, M.; Sarpeleh, A.; Askary, H. Management of damping-off and increasing of dome growth traits of cucumber in greenhouse culture using citrus wood vinegar. Appl. Res. Plant Protec. 2015, 4, 99–111. [Google Scholar]
- Chuaboon, W.; Ponghirantanachoke, N.; Athinuwat, D. Application of wood vinegar for fungal disease controls in paddy rice. Appl. Environ. Res. 2016, 38, 77–85. [Google Scholar] [CrossRef]
- Hwang, Y.H.; Matsushita, Y.I.; Sugamoto, K.; Matsui, T. Antimicrobial effect of the wood vinegar from Cryptomeria japonica sapwood on plant pathogenic microorganisms. J. Microbiol. Biotechnol. 2005, 15, 1106–1109. [Google Scholar]
- Gao, T.; Bian, R.; Joseph, S.; Taherymoosavi, S.; Mitchell, D.R.G.; Munroe, P.; Xu, J.; Shi, J. Wheat straw vinegar: A more cost-effective solution than chemical fungicides for sustainable wheat plant protection. Sci. Total Environ. 2020, 725, 138359. [Google Scholar] [CrossRef] [PubMed]
- Maliang, H.D.; Wang, P.W.; Chen, A.; Liu, H.; Lin, H.; Ma, J.Y. Bamboo Tar as a Novel Fungicide: Its Chemical Components, Laboratory Evaluation, and Field Efficacy against False Smut and Sheath Blight of Rice and Powdery Mildew and Fusarium Wilt of Cucumber. Plant Dis. 2021, 105, 331–338. [Google Scholar] [CrossRef]
- Bahoram, N.A.; Rahman, M.H.A.; Shahrun, M.S.; Suherman, F.H.S.; Masdar, S.N.H. Chemical composition and antimicrobial activities of wood vinegars fromcarambola, coconut shells and mango against selectedplant pathogenic microorganisms. Malaysian J. Microbiol. 2020, 16, 438–445. [Google Scholar] [CrossRef]
- Lara, E.; Belbahri, L. SSU rRNA reveals major trends in oomycete evolution. Fungal Divers. 2011, 49, 93. [Google Scholar] [CrossRef]
- Fisher, M.C.; Gurr, S.J.; Cuomo, C.A.; Blehert, D.S.; Jin, H.; Stukenbrock, E.H.; Stajich, J.E.; Kahmann, R.; Boone, C.; Denning, D.W.; et al. Threats posed by the fungal kingdom to humans, wildlife, and agriculture. mBio 2020, 11, e00449-20. [Google Scholar] [CrossRef]
- Chepsergon, J.; Motaung, T.E.; Bellieny-Rabelo, D.; Moleleki, L.N. Organize, Don’t Agonize: Strategic Success of Phytophthora Species. Microorganisms 2020, 8, 917. [Google Scholar] [CrossRef] [PubMed]
- Chenari Bouket, A.; Arzanlou, M.; Tojo, M.; Babai-Ahari, A. Pythium kandovanense sp. nov., a fungus-like eukaryotic microorganism (Stramenopila, Pythiales) isolated from snow-covered ryegrass leaves. Int. J. Syst. Evol. Microbiol. 2015, 65, 2500–2506. [Google Scholar] [CrossRef] [PubMed]
- Möller, E.M.; Bahnweg, G.; Sandermann, H.; Geiger, H.H. A simple and efficient protocol for isolation of high molecular weight DNA from filamentous fungi, fruit bodies, and infected plant tissues. Nucleic Acids Res. 1992, 20, 6115–6116. [Google Scholar] [CrossRef] [PubMed]
- Belbahri, L.; Calmin, G.; Sanchez-Hernandez, E.; Oszako, T.; Lefort, F. Pythium sterilum sp. nov. isolated from Poland, Spain and France: Its morphology and molecular phylogenetic position. FEMS Microbiol. Lett. 2006, 255, 209–214. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. Mega6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for re-constructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar]
- Kimura, M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols, a Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar]
- Bridgewater, A.V. Renewable fuels and chemicals by thermal processing of biomass. Chem. Eng. J. 2003, 91, 87–102. [Google Scholar] [CrossRef]
- Bridgewater, A.V. Biomass fast pyrolysis. Thermal Sci. 2004, 8, 21–50. [Google Scholar] [CrossRef]
- Theapparat, Y.; Chandumpai, A.; Leelasuphakul, W.; Laemask, N.; Ponglimanont, C. Physicochemical Characteristics of Wood Vinegars from Carbonization of Leucaena leucocephala, Azadirachta indica, Eucalyptus camaldulensis, Hevea brasiliensis and Dendrocalamus asper. Kasetsart. J. (Nat. Sci.) 2014, 48, 916–928. [Google Scholar]
- Pimenta, A.; Fasciotti, M.; Monteiro, T.V.C.; Lima, K.M.G. Chemical Composition of Pyroligneous Acid Obtained from Eucalyptus GG100 Clone. Molecules 2018, 23, 426. [Google Scholar] [CrossRef]
- Miyardan, F.N.; Mogaddam, M.R.A.; Farajzadeh, M.A.; Nemati, M. Combining modified graphene oxide-based dispersive micro solid phase extraction with dispersive liquid–liquid microextraction in the extraction of some pesticides from zucchini samples. Microchem. J. 2022, 182, 107884. [Google Scholar] [CrossRef]
- Alves, M.J.; Ferrerira, I.C.F.R.; Forufe, H.J.C.; Abreu, R.M.V.; Martins, A.; Pintado, M. Antimicrobial activity of phenolic compounds identified in wild mushrooms, SAR analysis and docking studies. J. Appl. Microbiol. 2013, 115, 346–357. [Google Scholar] [CrossRef]
- Fujisawa, S.; Ishihara, M.; Murakami, Y.; Atsumi, T.; Kadoma, Y.; Yokoe, I. Predicting the Biological Activities of 2-Methoxyphenol Antioxidants: Effects of Dimers. In Vivo 2007, 21, 181–188. [Google Scholar]
- Zaborowska, M.; Kucharski, J.; Wyszkowska, J. Biochemical and microbiological activity of soil contaminated with o-cresol and biostimulated with Perna canaliculus mussel meal. Environ. Monit. Assess. 2018, 190, 602. [Google Scholar] [CrossRef]
- Kocaçalışkan, I.; Talan, I.; Terzi, I. Antimicrobial Activity of Catechol and Pyrogallol as Allelochemicals. Z. Naturforsch. 2006, 61, 639–642. [Google Scholar] [CrossRef]
- Gao, T.; Zhang, Y.; Shi, J.; Mohamed, S.R.; Xu, J.; Liu, X. The Antioxidant Guaiacol Exerts Fungicidal Activity Against Fungal Growth and Deoxynivalenol Production in Fusarium graminearum. Front. Microbiol. 2021, 12, 762844. [Google Scholar] [CrossRef]
- Kim, M.-G.; Lee, H.-S. 1,2-Benzendiol Isolated from Persimmon Roots and Its Structural Analogues Show Antimicrobial Activities against Food-borne Bacteria. J. Korean Soc. Appl. Biol. Chem. 2014, 57, 429–433. [Google Scholar] [CrossRef]
- Oka, S.; Kuniba, R.; Tsuboi, N.; Tsuchida, S.; Ushida, K.; Tomoshige, S.; Kuramochi, K. Isolation, synthesis, and biological activities of a bibenzyl from Empetrum nigrum var. japonicum. Biosci. Biotechnol. Biochem. 2020, 84, 31–36. [Google Scholar] [CrossRef]
- Paul, B.; Bala, K.; Lassaad, B.; Calmin, G.; Sanchez-Hernandez, E.; Lefort, F. A new species of Pythium with ornamented oogonia: Morphology, taxonomy, internal transcribed spacer region of its ribosomal RNA, and its comparison with related species. FEMS Microbiol. Lett. 2006, 254, 317–323. [Google Scholar] [CrossRef]
- Moralejo, E.; Clemente, A.; Descals, E.; Belbahri, L.; Calmin, G.; Lefort, F.; Spies, C.F.; McLeod, A. Pythium recalcitrans sp. nov. revealed by multigene phylogenetic analysis. Mycologia 2008, 100, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Meftaul, I.M.; Venkateswarlu, K.; Dharmarjan, R.; Annamali, P.; Megharaj, M. Pesticides in the urban environment: A potential threat that knocks at the door. Sci. Total Environ. 2020, 711, 134612. [Google Scholar] [CrossRef]
- Dong, W.W.; Zhang, Y.; Quan, X. Health risk assessment of heavy metals and pesticides: A case study in the main drinking water source in Dalian, China. Chemosphere 2020, 242, 125113. [Google Scholar] [CrossRef]
- Maggi, F.; la Cecilia, D.; Tang, F.H.M.; McBratney, A. The global environmental hazard of glyphosate use. Sci. Total Environ. 2020, 717, 137167. [Google Scholar] [CrossRef]
- Cheffi, M.; Chenari Bouket, A.; Alenezi, F.N.; Luptakova, L.; Belka, M.; Vallat, A.; Rateb, M.E.; Tounsi, S.; Triki, M.A.; Belbahri, L. Olea europaea L. Root Endophyte Bacillus velezensis OEE1 Counteracts Oomycete and Fungal Harmful Pathogens and Harbours a Large Repertoire of Secreted and Volatile Metabolites and Beneficial Functional Genes. Microorganisms 2019, 7, 314. [Google Scholar] [CrossRef]
- Alenezi, F.N.; Slama, H.B.; Bouket, A.C.; Cherif-Silini, H.; Silini, A.; Luptakova, L.; Nowakowska, J.A.; Oszako, T.; Belbahri, L. Bacillus velezensis: A Treasure House of Bioactive Compounds of Medicinal, Biocontrol and Environmental Importance. Forests 2021, 12, 1714. [Google Scholar] [CrossRef]
- Yang, J.F.; Yang, C.H.; Liang, M.T.; Gao, Z.J.; Wu, Y.W.; Chuang, L.Y. Chemical composition, antioxidant, and antibacterial activity of wood vinegar from Litchi chinensis. Molecules 2016, 21, 1150. [Google Scholar] [CrossRef]
- Velmurugan, N.; Han, S.S.; Lee, Y.S. Antifungal activity of neutralized wood vinegar with water extracts of Pinus densiflora and Quercus serrata saw dusts. Int. J. Environ. Res. 2009, 3, 167–176. [Google Scholar]
- Baimark, Y.; Niamsa, N. Study on wood vinegars for use as coagulating and antifungal agents on the production of natural rubber sheets. Biomass Bioenergy 2009, 33, 994–998. [Google Scholar] [CrossRef]
- Ma, X.H.; Wei, Q.; Zhang, S.S.; Shi, L.; Zhao, Z. Isolation and bioactivities of organic acids and phenols from walnut shell pyroligneous acid. J. Anal. Appl. Pyrolysis 2011, 91, 338–343. [Google Scholar] [CrossRef]
- Amen-Chen, C.; Pakdel, H.; Roy, C. Production of monomeric phenols by thermochemical conversion of biomass: A review. Bioresour. Technol. 2001, 79, 277–299. [Google Scholar] [CrossRef]
- Aguirre, J.L.; Baena, J.; Martín, M.T.; Nozal, L.; González, S.; Manjón, J.L.; Peinado, M. Composition, Ageing and Herbicidal Properties of Wood Vinegar Obtained through Fast Biomass Pyrolysis. Energies 2020, 13, 2418. [Google Scholar] [CrossRef]
- Baimark, Y.; Threeprom, J.; Dumrongchai, N. Utilization of wood vinegars as sustainable coagulating and antifungal agents in the production of natural rubber sheets. J. Environ. Sci. Technol. 2008, 1, 157–163. [Google Scholar] [CrossRef]
- Kadota, M.; Niimi, Y. Effects of charcoal with pyroligneous acid and barnyard manure on bedding plants. Sci. Hort. 2004, 101, 327–332. [Google Scholar] [CrossRef]
- Orlo, E.; Russo, C.; Nugnes, R.; Lavorgna, M.; Isidori, M. Natural Methoxyphenol Compounds: Antimicrobial Activity against Foodborne Pathogens and Food Spoilage Bacteria, and Role in Antioxidant Processes. Foods 2021, 10, 1807. [Google Scholar] [CrossRef]
- Li, N.; Jing, S.; Wang, H.; Cavaco-Paulo, A. Production of antimicrobial powders of guaiacol oligomers by a laccase-catalyzed synthesis reaction. Process. Biochem. 2021, 111, 213–220. [Google Scholar] [CrossRef]
- Congguang, Z.; Shihai, L.; Fuqing, X.; Quan, B.; Ling, Q. Chemical Characteristics and Antimicrobial Performance of Wood Vinegar Produced from Pyrolysis of Polyploidy Mulberry Branches. J. Biobased Mater. Bioenergy 2019, 13, 812–819. [Google Scholar]
- Mederios, L.C.D.; Gasparotto, L.H.S. Pyroligneous acid and antibacterial activity: Criticism of a paper by Araújo et al. (2018). J. Appl. Microbiol. 2022, 132, 1768–1770. [Google Scholar] [CrossRef]
- Hoitink, H.A.J.; Powell, C.C. Fighting Phytophthora—A guide to combating Phytophthora root rot and dieback in ericaceous crops. Am. Nurserym. 1990, 171, 67–73. [Google Scholar]
- Hoitink, H.A.J.; Inbar, Y.; Boehm, M.J. Status of compost-amended potting mixes naturally suppressive to soilborne diseases of floricultural crops. Plant Dis. 1991, 75, 869–873. [Google Scholar] [CrossRef]




| Treatment-Concentration | Mycelial Growth (mm) | Tukey Multi-Comparison Analysis |
|---|---|---|
| Almond wood vinegar + C 100% | 0 | a |
| Cypress wood vinegar + C 100% | 1.5 ± 0.5 | ab |
| Pine wood vinegar + C 100% | 6.5 ± 0.5 | c |
| Pistachio wood vinegar + C 100% | 14.5 ± 0.5 | e |
| Pomegranate wood vinegar + C 100% | 23 ± 0.0 | h |
| Walnut wood vinegar + C 100% | 1.5 ± 0.5 | ab |
| Almond wood vinegar + C 50% | 1.5 ± 0.5 | ab |
| Cypress wood vinegar + C 50% | 10.5 ± 0.5 | d |
| Pine wood vinegar + C 50% | 17.5 ± 0.5 | f |
| Pistachio wood vinegar + C 50% | 26.5 ± 0.5 | i |
| Pomegranate wood vinegar + C 50% | 2.5 ± 0.5 | b |
| Walnut wood vinegar + C 50% | 11.5 ± 0.5 | d |
| Almond wood vinegar + C 25% | 20.5 ± 0.5 | g |
| Cypress wood vinegar + C 25% | 29.5 ± 0.5 | jk |
| Pine wood vinegar + C 25% | 30 ± 0.0 | k |
| Pistachio wood vinegar + C 25% | 0 | a |
| Pomegranate wood vinegar + C 25% | 0 | a |
| Walnut wood vinegar + C 25% | 3.5 ± 0.5 | b |
| Almond wood vinegar + C 12.5% | 11.5 ± 0.5 | d |
| Cypress wood vinegar + C 12.5% | 18 ± 0.0 | f |
| Pine wood vinegar + C 12.5% | 1.5 ± 0.5 | ab |
| Pistachio wood vinegar + C 12.5% | 3 ± 0.0 | b |
| Pomegranate wood vinegar + C 12.5% | 10.5 ± 0.5 | d |
| Walnut wood vinegar + C 12.5% | 14.5 ± 0.5 | e |
| Almond wood vinegar + C 6.25% | 27.5 ± 0.5 | ij |
| Cypress wood vinegar + C 6.25% | 0 | a |
| Pine wood vinegar + C 6.25% | 6.5 ± 0.5 | c |
| Pistachio wood vinegar + C 6.25% | 14.5 ± 0.5 | e |
| Pomegranate wood vinegar + C 6.25% | 20.5 ± 0.5 | g |
| Walnut wood vinegar + C 6.25% | 29 ± 0.0 | jk |
| Treatment-Concentration | Mycelial Growth (mm) | Tukey Multi-Comparison Analysis |
|---|---|---|
| Almond wood vinegar + C 100% | 0 | a |
| Cypress wood vinegar + C 100% | 3.5 ± 0.5 | bc |
| Pine wood vinegar + C 100% | 7.5 ± 0.5 | de |
| Pistachio wood vinegar + C 100% | 0 | a |
| Pomegranate wood vinegar + C 100% | 11 ± 0.0 | fg |
| Walnut wood vinegar + C 100% | 2.5 ± 0.5 | b |
| Almond wood vinegar + C 50% | 0 | a |
| Cypress wood vinegar + C 50% | 7.5 ± 0.5 | de |
| Pine wood vinegar + C 50% | 11.5 ± 0.5 | fg |
| Pistachio wood vinegar + C 50% | 2.5 ± 0.5 | b |
| Pomegranate wood vinegar + C 50% | 16.5 ± 0.5 | hi |
| Walnut wood vinegar + C 50% | 5.5 ± 0.5 | cd |
| Almond wood vinegar + C 25% | 0 | a |
| Cypress wood vinegar + C 25% | 11 ± 0.5 | fg |
| Pine wood vinegar + C 25% | 17.5 ± 0.5 | i |
| Pistachio wood vinegar + C 25% | 12.5 ± 0.5 | g |
| Pomegranate wood vinegar + C 25% | 21.5 ± 0.5 | jk |
| Walnut wood vinegar + C 25% | 9.5 ± 0.5 | ef |
| Almond wood vinegar + C 12.5% | 11.5 ± 0.5 | fg |
| Cypress wood vinegar + C 12.5% | 17.5 ± 0.5 | i |
| Pine wood vinegar + C 12.5% | 23 ± 0.0 | k |
| Pistachio wood vinegar + C 12.5% | 16 ± 0.0 | hi |
| Pomegranate wood vinegar + C 12.5% | 30 ± 0.0 | m |
| Walnut wood vinegar + C 12.5% | 15 ± 0.0 | h |
| Almond wood vinegar + C 6.25% | 17.5 ± 0.5 | i |
| Cypress wood vinegar + C 6.25% | 22.5 ± 0.5 | jk |
| Pine wood vinegar + C 6.25% | 27.5 ± 0.5 | l |
| Pistachio wood vinegar + C 6.25% | 23.5 ± 0.5 | k |
| Pomegranate wood vinegar + C 6.25% | 30 ± 0.0 | m |
| Walnut wood vinegar + C 6.25% | 20.5 ± 0.5 | j |
| Compound Name | Almond | Walnut | Pinus | Pomegranate | Pistachio | Cypress | Biological Activity | References |
|---|---|---|---|---|---|---|---|---|
| Phenol | 1.9 | 1.3 | 0.6 | 1.2 | 0.9 | 0.6 | Antimicrobial activity | [68] |
| 2-methylphenol | 2.9 | 2.7 | 2.4 | 2.2 | 2.3 | 1.9 | Cyclooxygenase (COX)-2 inhibitor | [69] |
| 2-methoxyphenol | 10.2 | 11.1 | 19.3 | 11.4 | 17.2 | 17.3 | Antibacterial activity | [27] |
| Cresol | 1.6 | 2.3 | 0.97 | 0.21 | 0.21 | 0.13 | Inhibition of biofilm formation and neutralization of bacterial toxins | [70] |
| Catechol | 3.2 | 4.6 | 4.2 | 5.9 | 8.3 | 3.2 | Antimicrobial activities on three bacteria (Pseudomonas putida, Pseudomonas pyocyanea, Corynebacterium xerosis) and two fungi (Fusarium oxysporum, Penicillium italicum) | [71] |
| 4-ethyl-2-metoxyphenol | 2.6 | 2.9 | 3.9 | 3.6 | 5.6 | 2.3 | Inhibitory effects against mycelial growth, conidial formation, and germination, and deoxynivalenol (DON) biosynthesis in Fusarium graminearum | [72] |
| 4-methoxy-1,2-benzenediole | 4.9 | 4.9 | 5.6 | 4.9 | 4.8 | 4.1 | antimicrobial activities against food-borne bacteria | [73] |
| 2,6-dimethoxy phenol | 26.4 | 27.6 | 32.3 | 21.4 | 22.1 | 25.9 | Inhibitory effect against Escherichia coli (NBRC 3301) and Bacillus subtilis (NBRC 3134). | [74] |
| 3,4-dimethoxyphenol | 2.6 | 4.9 | 2.4 | 5.3 | 3.4 | 3.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chenari Bouket, A.; Narmani, A.; Sharifi, K.; Naeimi, S.; Afshar Mogaddam, M.R.; Hamidi, A.A.; Luptakova, L.; Alenezi, F.N.; Belbahri, L. Semi-VOCs of Wood Vinegar Display Strong Antifungal Activities against Oomycete Species Globisporangium ultimum and Pythium aphanidermatum. Microbiol. Res. 2023, 14, 371-389. https://doi.org/10.3390/microbiolres14010029
Chenari Bouket A, Narmani A, Sharifi K, Naeimi S, Afshar Mogaddam MR, Hamidi AA, Luptakova L, Alenezi FN, Belbahri L. Semi-VOCs of Wood Vinegar Display Strong Antifungal Activities against Oomycete Species Globisporangium ultimum and Pythium aphanidermatum. Microbiology Research. 2023; 14(1):371-389. https://doi.org/10.3390/microbiolres14010029
Chicago/Turabian StyleChenari Bouket, Ali, Abolfazl Narmani, Kasra Sharifi, Shahram Naeimi, Mohammad Reza Afshar Mogaddam, Ali Asghar Hamidi, Lenka Luptakova, Faizah N. Alenezi, and Lassaad Belbahri. 2023. "Semi-VOCs of Wood Vinegar Display Strong Antifungal Activities against Oomycete Species Globisporangium ultimum and Pythium aphanidermatum" Microbiology Research 14, no. 1: 371-389. https://doi.org/10.3390/microbiolres14010029
APA StyleChenari Bouket, A., Narmani, A., Sharifi, K., Naeimi, S., Afshar Mogaddam, M. R., Hamidi, A. A., Luptakova, L., Alenezi, F. N., & Belbahri, L. (2023). Semi-VOCs of Wood Vinegar Display Strong Antifungal Activities against Oomycete Species Globisporangium ultimum and Pythium aphanidermatum. Microbiology Research, 14(1), 371-389. https://doi.org/10.3390/microbiolres14010029









