Abstract
Meat contamination by microorganisms could occur during numerous processes linked to game meat animal slaughter. These contaminants could pose a risk to product quality and consumer health. Contamination often occurs around the wound caused by shooting. Animal slaughter plants are given a responsibility to identify, evaluate and control the occurrence of hazards in their processing plant. To improve this control plan, the effectiveness of lactic (LA) and acetic acids (AA) for reducing the microbiological load directly around the wound was investigated. After killing by means of an aerial (helicopter) shotgun (n = 12) firing lead pellets and land-based rifle bullet shots (n = 36), samples of the flesh directly around the wounds of impala (Aepyceros melampus) were taken immediately after dressing (AD) before any treatment was conducted. Thereafter, at the step where carcasses are typically washed with potable water, the flesh directly around the wound was subjected to a wash with either ≈5 mL potable water (T1), 5% LA solution (T2) or 5% AA solution (T3) and then chilled overnight. Samples of the flesh directly around the wounds were also taken after chilling (AC). The aim of the study was to determine the effectiveness of each organic acid in reducing the microbiological load (total plate count; E. coli; coliforms and Salmonella) present in the flesh directly around the wounds of impala carcasses. The study found that shotgun pellets caused less body damage with fewer microorganisms recorded compared to samples from rifle-killed carcasses. LA reduced the occurrence of Salmonella during slaughter. The results of the other microorganisms revealed inconclusive outcomes on whether the application of water, 5% LA or 5% AA was effective in the reduction of the microbial organisms on the flesh directly around the wounds.
1. Introduction
Generally, the slaughter of wildlife/game meat animals presents eminent risks of the contamination of carcasses during in-field killing, evisceration and further dressing processes [1,2]. Species of impala (Aepyceros melampus) are predominantly hunted because of their abundance in most game meat producing countries such as South Africa, Namibia, Zimbabwe and other game meat producing countries in Africa. Researchers, such as Taylor et al., reported that out of all small game animals hunted for meat production between 2013 and 2015, approximately 41% were impala [3]. This species of game is listed by the International Union for Conservation of Nature (IUCN) as of least concern and not endangered [4]. Different from domestic animal slaughter, the two most common methods employed to kill or harvest game include killing with a single projectile such as a 30.06 rifle (150 g Nosler Accubond Points) or killing with a shotgun utilizing numerous pellet sizes (such as 12-gauge that utilizes 35-g cartridges with a diameter of 5.2 mm per pellet, firing 44 pellets per round) [5,6]. Coupled with open wounds and related bruising caused by bullet entry and exit, these killing processes could potentially cause the microbial contamination of game carcasses that is further exasperated during exsanguination (neck slitting and/or thoracic sticking) for bleeding purposes, evisceration and final dressing of the carcass, which may or may not be performed in a registered abattoir. Other factors contributing to microbial contamination include carcass-to-carcass contact, contamination from faecal material, the paunch and hide, processing tools and equipment. Facility structure, human contact and the abattoir environment are also key points of potential contamination [7,8]. As a result, many researchers have highlighted the need for microbiological decontamination strategies [9,10,11]. The benefits of an effective decontamination plan include meat safety, and improved meat quality and shelf life. Available decontamination methods, single or in combination, include chlorinated/potable water (±50 ppm), steam (82–99 °C), tri-sodium phosphate (8–12% concentrations), pulsatile light exposure, pulsed electric fields or ionizing radiation and organic acids (OAs) such as LA (2–5%), AA (2–5%) and citric acid (2–5%) [12,13,14,15,16]. However, in the context of killing game in the field, it is not viable to use complex methods that require expensive equipment, skills and knowledge. The use of OAs as an added step in the slaughter processes to decontaminate carcasses appears to be more viable [17,18].
In most developed countries, the use and efficiency of OA on meat have been investigated in the past [9,19,20]. It is noted that most of these studies were conducted on processed meat products and poultry, and a few on raw domestic red meat carcasses [21]. The usage of OAs on fresh carcasses, including game carcasses during slaughter still needs to be investigated as it presents an added benefit of meat safety assurance [22,23]. Few developed and developing countries endorse the use of decontamination. For example, the United States of America (USA) allows for carcass decontamination plans, while some European countries do not fully approve the use of decontamination as a form of improving the safety of meat products with an exception for LA and chlorinated water [20,24]. In South Africa, no method of the decontamination of fresh red meat is allowed, except for the use of potable water for the rinsing of fresh carcasses after slaughter before chilling, except with the approval of a provincial executive officer of Veterinary Services [25].
South Africa is an important meat producer for the local and export markets. Meat that is exported is mostly frozen after deboning, and in the countries of destination it is normally defrosted and sold as “fresh” meat [26,27,28]. It is therefore in the interest of South Africa and other receiving countries to obtain more scientific information that will inform the current reservations among policy makers and the game meat industry. While there are many OA used for carcass decontamination interventions, LA and AA have predominantly been adopted in meat products and carcasses in developed countries; mainly because of their availability in many regions of the world [6,24]. This paper, therefore, investigated the decontamination effect and efficiency of potable water typically used for washing carcasses in comparison to the use of LA and AA as decontaminants of wound areas caused during the killing of impala at a commercial game abattoir in South Africa.
2. Materials and Methods
During a commercial game harvesting process that formed part of their game management plan, 48 impala (Aepyceros melampus) were shot at a game farm located in Mokopane, South Africa. Shooting was from a hunting vehicle by a proficient hunter with a 30.06 rifle (150 g Nosler Accubond Points) targeting the thorax (n = 36), and aerial (helicopter) shotgun (12-gauge, 35-g cartridges with a diameter of 5.2 mm per pellet, firing 44 pellets per round) targeting the head and neck (n = 12). The approximate distances for land-based rifle bullet shots ranged between 40–100 m and shotgun shots ranged between 15–20 m high. Immediately after in-field exsanguination, the temperature and pH were measured in the hind leg. A Mettler Toledo pH meter was calibrated using buffers pH 7 and pH 4. In addition, using a Testo 106-T1/T2 thermometer, the temperatures were also measured in the hind leg after overnight chilling. The shot and bled animals were transported on an open pickup (traditional method) to a registered game abattoir (registration number 2/4G) located on the same farm from where the animals were harvested, where slaughter (evisceration, dressing, meat inspection, weighing, chilling) was conducted.
2.1. Sampling in Relation to Treatment
The 36 thoracic shot carcasses rendered 67 bullet wounds, consisting of 36 entry and 31 exit wounds, while the shotgun pellets rendered multiple small entry points around the head and neck. Because of the small size of the aerial (helicopter) shotgun wounds, samples were pooled from 3 pellet entry points. From the land-based rifle bullet shots (n = 36) and aerial (helicopter) shotgun-killed animals (n = 12), the carcasses were randomly divided into three groups of 12 and 4, respectively. In each group, using sterile scalpels, samples of the flesh directly around the wound were taken immediately after dressing (AD) before any treatment was applied and placed into prelabelled sterile Whirl-Pack (Merck Pty Ltd., Modderfontein, South Africa) bags. Thereafter, at the step where washing of the carcasses with potable water typically occurs, the flesh directly around the wounds was subjected to five squirts (≈5 mL) from one of three different hand spray bottles containing, at an ambient temperature, potable water (±50 ppm chlorine) from the abattoir (T1), 5% LA solution (T2) or 5% AA solution (T3), whereafter the carcasses were chilled overnight. The solutions were made up by mixing LA and AA with commercially bought distilled water. The pH of the two acid solutions ranged between pH 3.3–3.5 at a temperature of ±21 °C. After chilling (AC), samples of the flesh directly around the wound were again taken. Samples were frozen at −18 °C and transported frozen to the laboratory where they were maintained in the same state until analysis [29]. The total number of wound samples from rifle thorax shots was 134, and 24 from aerial (helicopter) shots.
2.2. Sample Preparation and Analysis
Samples were defrosted overnight in a fridge at 4–6 °C [30]. Samples taken AD and AC were analysed for the presence of total plate count (TPC), coliforms, Escherichia coli (E. coli) and Salmonella. To obtain a 1:10 dilution, ten grams of the samples was weighed aseptically and homogenised in 90 mL sterile buffered peptone water (Merck Pty Ltd., Modderfontein, South Africa) with a stomacher for 60 s (BagMixer 400CC, Interscience, Saint Nom la Brétèche, France). Series dilutions were then prepared in buffered peptone water, and 0.1 mL of each dilution was transferred to duplicate petri dishes for the enumeration of TPC (Plate Count Agar, Merck), and the simultaneous detection and enumeration of coliform bacteria and E. coli (Chromocult® Coliforms Agar Sigma-Aldrich, St. Louis, MI, USA). The inoculated plates were then incubated at 35–37 °C/24–48 h. For Salmonella, a tube with enrichment broth (Rappaport-Vassiliadis Oxoid, Hants, England) was inoculated (0.1 mL), and after incubation at 37 °C for ±24 h, 0.1 mL was transferred to duplicate plates of Xylose Lysine Deoxycholate Agar (Sigma-Aldrich). Control plates were poured to confirm sterility of media. After incubation, TPC, coliform and E. coli colonies on the plates were manually counted. Salmonella was regarded as presumptive positive when black colonies with a pink periphery were formed due to its ability to produce hydrogen sulphide (H2S) on the XLD agar.
2.3. Data Analysis
Colony counts for each dilution (including duplicates) of the two process positions (AD and AC) were captured on an Excel spreadsheet. Using Excel formula function, the averages between the actual sample and the duplicate were calculated to represent the number of microorganisms in a sample, whereafter the related logs were calculated for each sample. A t-test for comparison between means was performed to determine whether there was a difference between the means of treatment methods applied AD and AC for both killing methods using Excel data analysis tools [30]. Results of p < 0.05 (CL = 95%) were regarded as significant.
3. Results
3.1. Characterization of Wound Areas
The affected areas caused by the shotgun and rifle bullets varied in sizes and shape. Figure 1 depicts examples of damage caused on carcasses. Aerial (helicopter) shotgun pellets caused smaller but multiple entry points with minor damage and bruising (Figure 1a). The effect of entry and exit points caused by bullets ranged from small (Figure 1b) to extensive (Figure 1c,d).

Figure 1.
Wound images caused by (a) shotgun pellets (b,c) land-based rifle bullet shots entry (d) land-based rifle bullet shots exit.
The mutilation was irregular in shape; in some superficial and in others deeper in muscle tissues. With rifle shots, substantial contamination was often caused by contact of the open entry and exit wounds with the ground and pickup surfaces that the dead animal was lying on; punctured organs such as the intestine walls; during the killing and dressing of the carcasses (Figure 1d).
3.2. Microbiological Analysis
The results are presented for all targeted microorganisms AD and AC. Generally, the counts from aerial (helicopter) shotgun wounds were lower than those from land-based rifle bullet shot-inflicted wounds. As depicted in Table 1, 33.2% (helicopter) and 28.4% (rifle) of TPC samples, 20.9% (helicopter) and 44% (rifle) of coliform samples and 66.6% (helicopter) and 59% (rifle) of E. coli samples were excluded for being either too low (<10) or too high (>300) to count. This resulted in uneven p-values where, in other instances, bigger differences from the counts resulted in no statistical differences (depending on the average count), and irregular standard deviations (s.d.) where the number close to zero meant that majority of the data were clustered near the mean.

Table 1.
Summary of wound sample results counted (10–300) and those not counted (<10 and >300).
3.2.1. Total Plate Count
None of the helicopter wound samples had TPC > 300 colonies, while 20.2% (AD 11.2%; AC 9%) from rifle-inflicted wound samples had TPC > 300 and were thus not counted (Table 1). However, 33.2% (AD 12.5%; AC 20.7%) of the helicopter and 8.2% (AD 3%; AC 5.2%) of the thoracic shots had low TPC (<10) counts, indicating a large variation in potential carcass (wound) contamination found between the various killing methods. Out of the eight excluded samples, three were from AD and five from AC. Total plate counts presented contrasting results on the efficiency of potable water, LA and AA on the reduction of TPC on shotgun pellet- (Figure 2) and rifle bullet- (Figure 2) inflicted wounds from AD (no treatment) to AC. All treatments of wounds caused by shotgun pellets had a reduction in counts from AD to AC with the biggest reduction caused by the potable water (1.6 log), while wounds caused by rifle bullets showed little change between AD and AC (±s.d. = 0.7). Considering killing methods and treatments, the mean results obtained from AD and AC were within the VPN 15 limit of 5.7 logas prescribed by the South African Veterinary Procedure Notice (VPN 15) on microbial limits in game raw meat standards used for export purposes [31]. One sample AD from the helicopter was above 5.7 log(log 6.1) but reduced to log 4.4 AC. The rifle killing showed the same results, where water (AD) had one sample with log 6.4, which stayed the same AC; LA (AC) had one sample above 5.7; and AA (AD) had two samples above 5.7 of which AC, one was reduced to log 6.3 and the other to log 5.4. In none of the instances were the mean differences between AD and AC significant (p > 0.05) (Figure 2). Of note is that there was a decrease (Table 1) in the number of TPC > 300 colonies that reduced from 15 AD to 12 AC, and a horizontal movement and reduction in three samples from >300 to 10–300 to <10.
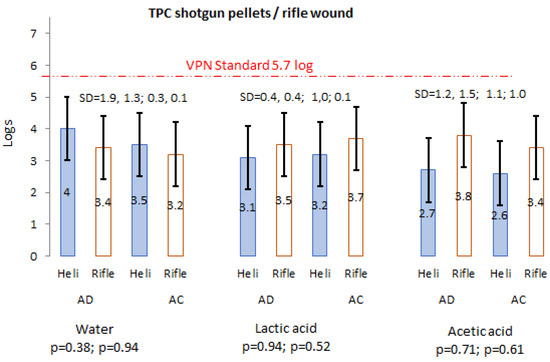
Figure 2.
Average log counts of TPC helicopter and rifle wounds before and after water, LA and AA treatment.
3.2.2. Coliforms
Similar to TPC, a large variation was caused by the differences between the number samples excluded from helicopter and rifle wounds. Of the helicopter AD samples, 23 (95.8%) were included and one (4.2%) was excluded from the results; and AC three (12.5%) were excluded (<10) and one (4.2%) was excluded based on the counts being >300. Conversely, rifle wound samples had larger numbers of <10 (AD 7.5%; AC 15%) and >300 (AD 13.4%; AC 8.1%). Similar to the TPC, mean coliforms counts (Figure 3) presented different results on the effect of water, LA and AA on the reduction of shotgun pellet- and rifle bullet-inflicted wounds from AD (no treatment) to AC after chilling. Similar to TPC, samples with >300 colonies were reduced from 18 AD to 11 AC; with an increase in samples with <10 from 10 AD to 20 AC (Figure 3). The South African Veterinary Procedure Notice (VPN 15) does not make provision for coliform limits.
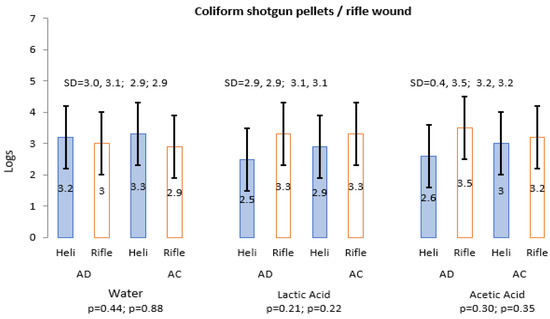
Figure 3.
Average log counts of coliform helicopter and rifle wound before and after water, LA and AA treatment.
3.2.3. Escherichia coli
Of the helicopter AD and AC samples, respectively, 16 (66.6%) were included and eight (33.3%) were excluded from the results (<10). None of the samples were >300. Contrariwise, rifle wound samples had larger numbers of <10 (AD 20.9%; AC 27.6%) and >300 (AD 7.5%; AC 3%). The results show lower occurrences of E. coli on helicopter wounds and rifle bullet-inflicted wounds as compared to TPC and coliforms. Samples from helicopter wounds treated with LA showed a reduction of 0.5 logs and 1.7 logs from AA, while for wounds caused by rifle bullets, a reduction in counts was observed from AD to AC (0.5 logs from LA and 1.1 logs from AA) (Figure 4). The LA treatment of wounds related to both killing methods differed significantly (p < 0.05). Six (25%) of the twenty-four helicopter samples had counts above the VPN limit of log 2.7 log, of which four (16.7%) were from AD samples and two (8.3%) were from AC, representing a 50% reduction. Of the included rifle bullet-inflicted wound samples, 35 (26.1%) samples were above the VPN limit of 2.7 log of which 21 (60%) samples were from AD and 14 (40%) were from AC. Similar to TPC and coliform, there was a decrease (Table 1) in the number of TPC > 300 colonies that reduced from 10 AD to 4 AC, signifying a 40% reduction as these 4 carcasses were from the same 10 that had too many to count AD. In general, a horizontal movement and reduction in samples from >300 to 10–300 to <10 was observed.
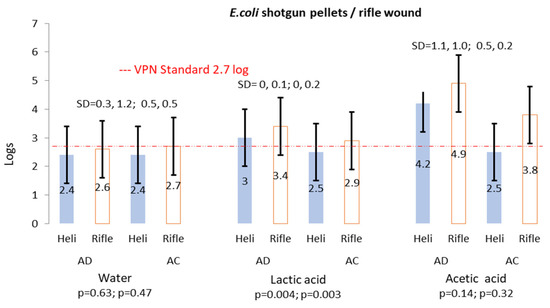
Figure 4.
Average log counts of E. coli helicopter and rifle wounds before and after water, LA and AA treatment.
3.2.4. Salmonella
With respect to the aerial (helicopter) shotgun pellet- and land-based rifle bullet shot-inflicted wounds, presumptive Salmonella was recorded in only 4 of the 24 (11.1%) and 20 of 134 (10.7%) samples, respectively (Table 2).

Table 2.
Salmonella positive samples, position and treatment.
3.3. Meat Traits, Carcasses’ pH and Temperature
An initial darkening of LA-treated areas was observed while areas exposed to AA had a pungent vinegar smell after treatment. This darkening and strong smell disappeared after overnight chilling. The initial pH and temperature of aerial (helicopter) shotgun-killed carcasses (measured immediately post exsanguination) ranged between pH 6.19 to 6.75 (average pH 6.6) and 32.7 °C to 36.4 °C (average 35 °C), respectively. After overnight chilling the pH dropped to between pH 5.65 and 5.85 (average pH 5.7) and the temperature dropped to between 4.5 °C and 5.3 °C (average 4.8 °C). The same pattern was observed with land-based rifle bullet shot-killed carcasses, where post exsanguination, the pH ranged between pH 6.2 and 7.52 (average pH 6.55) after killing and between 5.55 and 6.89 (average pH 5.7) after chilling, while the carcass temperature dropped from between 25.6 °C and 37.9 °C (average 32.3 °C) after killing to between 4.5 °C and 7.9 °C (average 5.4 °C) after chilling. The initial pH of all the aerial shotgun carcasses measured >6, of which all dropped to <6 after chilling, while the land-based rifle bullet shot-killed carcasses all had an initial pH > 6, but with the exception of two carcasses (6.08 and 6.89), all others also dropped to <6.
4. Discussion
The appearances of the mutilations around the bullet wounds or points of impact were consistent with those described where different animals were hunted for human consumption [31]. The type and severity of mutilation determines the distribution and the number of microorganisms at that particular site [32,33]. At 5%, OA mixtures are still acceptable in the food industry; hence, this concentration was selected for this experiment [6,34,35,36]. The use of indicator microorganisms, such as total aerobic plate count (APC), coliform count and Escherichia coli count could be used for raw meat safety and quality monitoring [37].
4.1. Effect of Treatment Methods on Microbiological Counts
Carcass contamination could occur during uncontrolled slaughter [8,38]. In general, wound samples derived from aerial helicopter shotgun killing presented lower microbiological growth than that of samples obtained from land-based rifle bullet shot-inflicted wounds, where carcasses were frequently contaminated with blood, faecal matter and other contaminants as seen in Figure 1 and noted by others [39]. From a systematic review, it was observed that in most studies on fresh meat treatment, the concentrations of OA were between 1–3% and are acceptable in the food industry [6]. Given the general contribution of AA and LA concentration change in the food industry [37,38].As a result of the contribution of AA and LA concentration change [40,41], a concentration of 5% as used in this study was expected to provide better results. While some traces of microbial reduction were observed around the gunshot pellet- and rifle bullet-inflicted wound areas, neither the water, 5% LA or 5% AA treatments proved to cause a meaningful reduction in the targeted microorganisms around the bullet wounds from the two killing methods. This could have been influenced by factors such as additional cross-contamination between AD and AC from the environment, carcass dressing, inter-carcass contact and manual handling resulting in an uneven distribution of microorganisms [42].
For TPC, this study found that although the use of water, LA and AA caused, to some extent, a drop in the log counts, the results were not conclusive in rifle-inflicted wounds where bruising was extensive and the colony count increased by 0.2 logs. This contrasts with the results of Saad and Hassanin [40], where it was suggested that the use of AA at 1–3% could be used effectively to reduce TPC on fresh carcasses compared to LA. Other studies rated LA (2%) as the most effective when applied on fresh meat to reduce the TPC compared to AA and water [9,11]. Kocharunchitt and Mellefont [43] concluded that LA (2%) alone may not provide sufficient TPC log reduction on carcass surfaces, unless it is used in combination with other OAs such as AA.
Irrespective of the treatment methods, the occurrence of coliforms on rifle bullet-inflicted wounds did not exceed log 5.3 (AD) and log 5.4 (AC). Nonetheless, the reduction potential of LA and AA in reducing coliform colonies was not clear from both killing methods. Although, the monitoring of coliforms as an indicator for hygiene slaughter remains important during slaughter [44], the application of these OA’s did not provide any difference (p > 0.05) in the reduction in coliforms between AD and AC. In the South African context, there are no limits of coliforms in the South African raw meat export standard.
The results in this investigation were consistent with conclusions [40] that a concentration of between 2.5 and 5% LA or AC could bring about some reduction in E. coli growth on fresh carcasses. Where reduction occurred, especially with rifle bullet-inflicted wounds, it was recorded AC (ave 0.5 logs) for LA and (ave 0.2 logs) for AA, where the carcass was dry and at low temperatures of between 1 and 5 °C. The results of this investigation show unexpected statistical differences (p < 0.05) on values, especially with regards to LA; the reasons for these differences could have been caused by the varying counts of samples that were included and excluded (Table 1) as noted by others [44]. E. coli is used as an indicator for the presence of faecal material from warm-blooded animals [17]. However, the consumption of food contaminated with pathogenic E. coli (Shiga toxin-producing E. coli-STEC) can cause serious gastrointestinal diseases, and it is therefore important to take appropriate hygienic measures to prevent contamination during slaughtering, processing and/or handling [27].
Others have reported the potential reduction in Salmonella on fresh carcasses when using LA and AA as being inconclusive [45]. It is further argued that to have a meaningful reduction in Salmonella, it could be beneficial to combine LA and AA and apply these OAs as a hurdle sequence during slaughter operations [46,47]. It is known that Salmonella strains could adapt to acidic environments at pH 5.0 to 6.0, which may lead to lower ineffective decontamination results [48].
4.2. Carcasses’ pH and Temperature
In this study a change in meat colour was observed in LA-treated carcasses, while carcasses exposed to AA had a strong vinegar smell after treatment. However, both quality trait changes were negated after overnight chilling similar to research conducted on fresh carcasses [49]. However, the appearance or colour change was not far from ordinary as game muscle carcasses tend to be much darker in general [50,51]. As expected, the initial pH measured immediately post exsanguination for helicopter (average pH 6.60) and rifle shot carcasses (average pH 6.55) were above >6, where the normal physiological pH is 7.1. Moreover, AC (helicopter), the pH measurements were all <6, and with the exception of two rifle shot carcasses that were within the typical Dark Firm Dry (DFD) range (pH > 6.09) [52]), all other pH measurements were <6. The two animals with AC pH > 6 could therefore have been exposed to stress, as shown by Carrasco-García and Pardío-Sedas [53] during the selection of specific animals (age, gender, etc.) as part of a game management plan. DFD is not only associated with quality aspects such as higher shear force, glycolytic potential and water-holding capacity [54], but also allows the growth of organisms [55]. The two carcasses with pH within the typical DFD range had TPC and coliform counts > 300, confirming the results of a study on black wildebeest (Connochaetes gnou) showing that DFD meat reached the microbial spoilage limit of 7 log cfu/g sooner than that of normal pH (pH ≤ 5.70) samples [29]. Similar to results obtained by others [56], it was evident that chilling played a role in the reduction in microbiological colonies. Factors such as carcass size, fatness of the carcass, chiller temperature, relative humidity and flow pattern of air influences the carcass chilling rate [57,58]. In relation to the use of organic acids such as LA and AA in a decontamination plan, the temperature of the OA mixture and the carcass temperature may influence the efficiency of the treatment [6].
4.3. Meat Safety
South Africa often export game meat to various countries and therefore have set criteria for the export of game meat in so-called Veterinary Procedural Notices (VPN 15) [59]. As indicated in Figure 2, the mean TPC counts were within the set limit of log 5.7/g, while for E. coli, some of the results exceeded the set limit of log 2.7/g (Figure 4). No limits are specified for coliforms. Despite the fact that LA and AA have potential to reduce organisms, it has been proven that acid-adapted cells are more resistant under stress conditions [60]. The microbiological counts in this study highlight the importance of good hygiene practices during harvesting (killing method and shot placement; bleeding), the transport of carcasses to the slaughter facility, slaughter (dressing. inspection) and chilling [61,62].
4.4. Limitation and Future Direction
The usage of OA as a microorganism decontaminant on fresh meat and other food products presents an opportunity to the meat industry [6]. However, the adoption of this intervention strategy should be carried out in a responsible manner so as to prevent the development of resistant strains of microorganisms. As confirmed by others [45], decontamination could be used as an added step of ensuring safer meat production. To ensure sustainable decontamination strategies, further investigations on the efficiency of using other physical decontaminants are required. Other researchers have identified the use of hot steam during slaughter, controlled chlorine usage, improved trimming, controlled pressured water at the final wash during slaughter and the training of slaughter operators on hygiene application during slaughter; their suitability on game carcasses should be evaluated further [63,64].
5. Conclusions
The use of OA could be as an added step on less contaminated carcasses. Severely contaminated carcasses should still be subjected to trimming at meat inspection before chilling. The study found that, as expected, shotgun pellets caused less body damage (as they targeted the head/higher neck region) with fewer microorganisms recorded compared to samples from rifle-killed carcasses that targeted the chest region. LA could be used to reduce the occurrence of Salmonella during slaughter. Various research reports suggest that the decontamination of carcasses at abattoirs by the use of LA could be a good introduction in meat production processes as the future for ensuring a much safer meat. Decontamination plans should be improving or adding a hurdle to control microbiological contamination during slaughter and growth. This is of importance with game animal slaughter where animals are killed in the field by using different killing methods, and carcasses, especially open wound areas, are exposed to contaminants during killing and slaughter. Contrary to what was expected, the results of this study were inconclusive on whether the application of water, 5% LA and 5% AA were effective in the reduction in TPC and coliforms. However, E. coli and Salmonella counts and occurrences were reduced by both OAs after chilling. Further investigations are required, which should consider different game species (smaller and larger animals); the use of different rifle calibres to try and reduce carcass damage and contamination, bruising and subsequent contamination; improved slaughter techniques (in-field and at the abattoir); organic acids other than the ones used for this study (single and in combination and at various concentrations); microbiological contamination before and after trimming of the affected parts of a carcass; organic acids’ exposure time; the effect of chilling; the relative humidity and air speed in chillers; the sensory effect of the addition of organic acids; microbiological shelf life and the use of treated meat in processed products. In addition, the assurance of proper hygiene training of proficient hunters, and slaughter and other supplementary staff on aspects such as personal hygiene, prevention of cross-contamination during slaughter and the preparation and application of OAs is of utmost importance in ensuring the safety of meat. Food industries including abattoirs should develop microbial monitoring programs by adopting and implementing hazard analysis programs. These should be coupled with the use of hygiene indicator organisms as a part of these monitoring systems.
Author Contributions
Conceptualization, D.V.N., J.L.B. and L.C.H.; methodology, D.V.N., J.L.B., P.A.G. and L.C.H.; analysis, D.V.N.; data curation, D.V.N., J.L.B. and L.C.H.; writing, review and editing, D.V.N., J.L.B., P.A.G. and L.C.H.; supervision, J.L.B. and L.C.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research is supported by the South African Research Chairs Initiative (SARChI) and partly funded by the South African Department of Science and Technology (UID number: 84633), as administered by the National Research Foundation (NRF) of South Africa, and partly by the Department of Trade and Industry’s THRIP program (THRIP/64/19/04/2017) with Wildlife Ranching South Africa as a partner and by Stellenbosch University. Any opinions, findings and conclusions or recommendations expressed in this material are those of the author(s), and the National Research Foundation does not accept any liability in this regard.
Institutional Review Board Statement
The study was conducted in accordance with the Tshwane University of Technology, and approved by the Animal Research Ethics Committee of Tshwane University of Technology (Proposal code AREC 2019/03/002).
Informed Consent Statement
Not applicable.
Data Availability Statement
Reasonable requests for data can be sourced from the corresponding author.
Acknowledgments
The authors thank Ramaco game ranch in Mokopane, South Africa, for the provision of game meat animals, proficient hunters and slaughter facilities for the purpose of this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Van der Merwe, M.; Hoffman, L.C.; Jooste, P.J.; Calitz, F.J. The hygiene practices of three systems of game meat production in South Africa in terms of animal class and health compliance. Meat Sci. 2013, 94, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Gouws, P.A.; Shange, N.; Hoffman, L.C. The microbial quality of black wildebeest (Connochaetes gnou) carcasses processed in a South African abattoir. In Game Meat Hygiene: Food Safety and Security; Wageningen Academic Publishers: Wageningen, The Netherlands, 2017; pp. 379–395. [Google Scholar]
- Taylor, A.; Lindsey, P.; Davies-Mostert, H.; Goodman, P. An Assessment of the Economic, Social and Conservation Value of the Wildlife Ranching Industry and Its Potential to Support the Green Economy in South Africa; The Endangered Wildlife Trust: Johannesburg, South Africa, 2016; pp. 96–109. Available online: http://www.rhinoresourcecenter.com/pdf_files/146/1462014538.pdf (accessed on 2 March 2022).
- IUCN SSC Antelope Specialist Group. Aepyceros melampus. The IUCN Red List of Threatened Species 2016: E.T550A50180828. Available online: https://www.iucnredlist.org/species/550/50180828 (accessed on 10 September 2022).
- Bekker, J.L.; Hoffman, L.C.; Jooste, P.J. Essential food safety management points in the supply chain of game meat in South Africa. In Game Meat Hygiene in Focus; Springer: Berlin/Heidelberg, Germany, 2011; pp. 39–65. [Google Scholar]
- Nkosi, D.V.; Bekker, J.L.; Hoffman, L.C. The Use of Organic Acids (Lactic and Acetic) as a Microbial Decontaminant during the Slaughter of Meat Animal Species: A Review. Foods 2021, 10, 2293. [Google Scholar] [CrossRef] [PubMed]
- Sohaib, M.; Anjum, F.M.; Arshad, M.S.; Rahman, U.U. Postharvest intervention technologies for safety enhancement of meat and meat based products; a critical review. J. Food Sci. Technol. 2016, 53, 19–30. [Google Scholar] [CrossRef]
- Wardhana, D.K. Risk Factors for Bacterial Contamination of Bovine Meat during Slaughter in Ten Indonesian Abattoirs. Vet. Med. Int. 2019, 2019, 2707064. [Google Scholar]
- Van Ba, H.; Seo, H.-W.; Pil-Nam, S.; Kim, Y.-S.; Park, B.Y.; Moon, S.-S.; Kang, S.-J.; Choi, Y.-M.; Kim, J.-H. The effects of pre-and post-slaughter spray application with organic acids on microbial population reductions on beef carcasses. Meat Sci. 2018, 137, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Hernandez, A.; Brashears, M.M.; Sanchez-Plata, M.X. Efficacy of lactic acid, lactic acid–acetic acid blends, and peracetic acid to reduce Salmonella on chicken parts under simulated commercial processing conditions. J. Food Prot. 2018, 81, 17–24. [Google Scholar] [CrossRef]
- Sallam, K.I.; Abd-Elghany, S.M.; Hussein, M.A.; Imre, K.; Morar, A.; Morshdy, A.E.; Sayed-Ahmed, M.Z. Microbial decontamination of beef carcass surfaces by lactic acid, acetic acid, and trisodium phosphate sprays. BioMed Res. Int. 2020, 2020, 2324358. [Google Scholar] [CrossRef] [PubMed]
- Capita, R.; Alonso-Calleja, C.; Sierra, M.; Moreno, B.; del Camino García-Fernández, M. Effect of trisodium phosphate solutions washing on the sensory evaluation of poultry meat. Meat Sci. 2000, 55, 471–474. [Google Scholar] [CrossRef]
- South Africa. Hygiene Managers Manual Poultry Training: Module 2 Poultry Processing; Government Gazette: Pretoria, South Africa, 2006. Available online: http://www.nda.agric.za/Vetweb/VPH/Manuals/PoultryManual.pdf (accessed on 3 March 2022).
- Dan, S.D.; Mihaiu, M.; Reget, O.; Oltean, D.; Tabaran, A. Influence on week organic acids on pathogens on swine carcasses. Lucr. Stiintifice-Med. Veterinara. Univ. Stiinte Agric. Si Med. Vet. “Ion Ionescu Brad” Iasi 2017, 60, 265–273. [Google Scholar]
- Han, J.; Luo, X.; Zhang, Y.; Zhu, L.; Mao, Y.; Dong, P.; Yang, X.; Liang, R.; Hopkins, D.L.; Zhang, Y. Effects of spraying lactic acid and peroxyacetic acid on the bacterial decontamination and bacterial composition of beef carcasses. Meat Sci. 2020, 164, 108104. [Google Scholar] [CrossRef] [PubMed]
- SANS. The Limits and Associated Risks for Domestic Water South African National Standard: Version 3, No 982; Government Gazette: Pretoria, South Africa, 2017. Available online: https://cer.org.za/ (accessed on 6 July 2022).
- Yang, X.; Tran, F.; Wolters, T. Microbial ecology of decontaminated and not decontaminated beef carcasses. J. Food Res. 2017, 6, 85–91. [Google Scholar] [CrossRef]
- Neethling, J.; Hoffman, L.; Muller, M. Factors influencing the flavour of game meat: A review. Meat Sci. 2016, 113, 139–153. [Google Scholar] [CrossRef] [PubMed]
- Hilbig, J.; Loeffler, M.; Herrmann, K.; Weiss, J. Application of exopolysaccharide-forming lactic acid bacteria in cooked ham model systems. Food Res. Int. 2019, 119, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Barcenilla, C.; Ducic, M.; López, M.; Prieto, M.; Álvarez-Ordóñez, A. Application of lactic acid bacteria for the biopreservation of meat products: A systematic review. Meat Sci. 2022, 183, 108661. [Google Scholar] [CrossRef]
- Aykın-Dinçer, E.; Ergin, F.; Küçükçetin, A. Reduction of Salmonella enterica in Turkey breast slices kept under aerobic and vacuum conditions by application of lactic acid, a bacteriophage, and ultrasound. J. Food Saf. 2021, e12923. [Google Scholar] [CrossRef]
- Omori, Y.; Miake, K.; Nakamura, H.; Kage-Nakadai, E.; Nishikawa, Y. Influence of lactic acid and post-treatment recovery time on the heat resistance of Listeria monocytogenes. Int. J. Food Microbiol. 2017, 257, 10–18. [Google Scholar] [CrossRef]
- Casas, D.E.; Vargas, D.A.; Randazzo, E.; Lynn, D.; Echeverry, A.; Brashears, M.M.; Sanchez-Plata, M.X.; Miller, M.F. In-Plant Validation of Novel On-Site Ozone Generation Technology (Bio-Safe) Compared to Lactic Acid Beef Carcasses and Trim Using Natural Microbiota and Salmonella and E. coli O157: H7 Surrogate Enumeration. Foods 2021, 10, 1002. [Google Scholar] [CrossRef]
- Pohlman, F.; Dias-Morse, P.; Pinidiya, D. Product safety and color characteristics of ground beef processed from beef trimmings treated with peroxyacetic acid alone or followed by novel organic acids. J. Microbiol. Biotechnol. Food Sci. 2019, 2019, 93–101. [Google Scholar] [CrossRef]
- South Africa. Meat Safety Act (Act 40 of 2000). Available online: https://www.gov.za/ (accessed on 6 July 2022).
- Leygonie, C.; Britz, T.J.; Hoffman, L.C. Oxidative stability of previously frozen ostrich Muscularis iliofibularis packaged under different modified atmospheric conditions. Int. J. Food Sci. Technol. 2011, 46, 1171–1178. [Google Scholar] [CrossRef]
- Shange, N.; Makasi, T.N.; Gouws, P.A.; Hoffman, L.C. The influence of normal and high ultimate muscle pH on the microbiology and colour stability of previously frozen black wildebeest (Connochaetes gnou) meat. Meat Sci. 2018, 135, 14–19. [Google Scholar] [CrossRef]
- Leygonie, C.; Hoffman, L.C. Effect of different combinations of freezing and thawing rates on the shelf-life and oxidative stability of ostrich moon steaks (M. femorotibialis medius) under retail display conditions. Foods 2020, 9, 1624. [Google Scholar] [CrossRef] [PubMed]
- Shange, N.; Gouws, P.; Hoffman, L.C. Changes in pH, colour and the microbiology of black wildebeest (Connochaetes gnou) longissimus thoracis et lumborum (LTL) muscle with normal and high (DFD) muscle pH. Meat Sci. 2019, 147, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Zhang, W.; Rajput, N.; Khan, M.A.; Li, C.-B.; Zhou, G.-H. Effect of multiple freeze–thaw cycles on the quality of chicken breast meat. Food Chem. 2015, 173, 808–814. [Google Scholar] [CrossRef] [PubMed]
- Standarová, E.; Vorlová, L.; Gallas, L. Distribution of biogenic amines and polyamines in the pheasant meat. Maso 2012, 1, 51–54. [Google Scholar]
- Anderson, B.; Horder, J. The Australian Carcass Bruise Scoring System [visual appraisal of damage]. Qld. Agric. J. 1979, 105, 281–287. [Google Scholar]
- Chambers, P.; Grandin, T.; Heinz, G.; Srisuvan, T. Effects of stress and injury on meat and by-product quality. In Guidelines for Humane Handling, Transport and Slaughter of Livestock FAO Corporate Document Repository; Food and Agricultural Organization: Geneva, Switzerland, 2004; pp. 6–10. [Google Scholar]
- Carlson, B.A.; Geornaras, I.; Yoon, Y.; Scanga, J.A.; Sofos, J.N.; Smith, G.C.; Belk, K.E. Studies to evaluate chemicals and conditions with low-pressure applications for reducing microbial counts on cattle hides. J. Food Prot. 2008, 71, 1343–1348. [Google Scholar] [CrossRef]
- Buncic, S.; Sofos, J. Interventions to control Salmonella contamination during poultry, cattle and pig slaughter. Food Res. Int. 2012, 45, 641–655. [Google Scholar] [CrossRef]
- Yeh, Y.; De Moura, F.; Van Den Broek, K.; De Mello, A. Effect of ultraviolet light, organic acids, and bacteriophage on Salmonella populations in ground beef. Meat Sci. 2018, 139, 44–48. [Google Scholar] [CrossRef]
- Kim, J.H.; Hur, S.J.; Yim, D.G. Monitoring of microbial contaminants of beef, pork, and chicken in HACCP implemented meat processing plants of Korea. Korean J. Food Sci. Anim. Resour. 2018, 38, 282–290. [Google Scholar]
- Schlegelova, J.; Nápravnıková, E.; Dendis, M.; Horvath, R.; Benedık, J.; Babak, V.; Klımová, E.; Navratilova, P.; Šustáčková, A. Beef carcass contamination in a slaughterhouse and prevalence of resistance to antimicrobial drugs in isolates of selected microbial species. Meat Sci. 2004, 66, 557–565. [Google Scholar] [CrossRef]
- Tibesso, G.; Hiko, A. Effect of pre-slaughter animal handling on the physicochemical and microbiological quality of beef in selected municipal abattoirs, Oromia Reginal State, Ethiopia. EC Vet. Sci. Res. Artic. 2019, 4, 202–212. [Google Scholar]
- Saad, S.M.; Hassanin, F.S.; Salem, A.M.; Saleh, E.A.E. Efficiency of some organic acids as decontaminants in sheep carcasses. Benha Vet. Med. J. 2020, 38, 116–119. [Google Scholar]
- Ciríaco, M.; Moura-Alves, M.; Silva, R.; Pinto, I.; Saraiva, C.M.; Esteves, A. Decontamination of Pig Carcasses with Organic Acids. Proceedings 2020, 70, 63. [Google Scholar]
- Alnajrani, M.; Hanlon, K.; English, A.; Fermin, K.; Brashears, M.M.; Echeverry, A. Comparing the recovery of indicator microorganisms from beef trimmings using swabbing, rinsing, and grinding methodologies. Meat Muscle Biol. 2018, 2. [Google Scholar] [CrossRef]
- Kocharunchitt, C.; Mellefont, L.; Bowman, J.P.; Ross, T. Application of chlorine dioxide and peroxyacetic acid during spray chilling as a potential antimicrobial intervention for beef carcasses. Food Microbiol. 2020, 87, 103355. [Google Scholar] [CrossRef]
- Membré, J.-M.; Laroche, M.; Magras, C. Assessment of levels of bacterial contamination of large wild game meat in Europe. Food Microbiol. 2011, 28, 1072–1079. [Google Scholar] [CrossRef]
- Castro, V.S.; Mutz, Y.d.S.; Rosario, D.K.A.; Cunha-Neto, A.; Figueiredo, E.E.d.S.; Conte-Junior, C.A. Inactivation of Multi-Drug Resistant Non-Typhoidal Salmonella and Wild-Type Escherichia coli STEC Using Organic Acids: A Potential Alternative to the Food Industry. Pathogens 2020, 9, 849. [Google Scholar] [CrossRef]
- Dittoe, D.K.; Feye, K.M.; Peyton, B.; Worlie, D.; Draper, M.J.; Ricke, S.C. The addition of ViriditecTM aqueous ozone to peracetic acid as an antimicrobial spray increases air quality while maintaining Salmonella Typhimurium, non-pathogenic Escherichia coli, and Campylobacter jejuni reduction on whole carcasses. Front. Microbiol. 2019, 9, 3180. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, M.; Cosby, D.; Cox, N.; Thippareddi, H. Efficacy of peroxy acetic acid in reducing Salmonella and Campylobacter spp. populations on chicken breast fillets. Poult. Sci. 2020, 99, 2655–2661. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Zhu, L.; Niu, L.; Luo, X.; Dong, P. The acid tolerance responses of the Salmonella strains isolated from beef processing plants. Food Microbiol. 2022, 104, 103977. [Google Scholar] [CrossRef]
- Zhang, Y.; Mao, Y.; Li, K.; Luo, X.; Hopkins, D.L. Effect of carcass chilling on the palatability traits and safety of fresh red meat. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1676–1704. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, L.; Muller, M.; Schutte, D.W.; Calitz, F.; Crafford, K. Consumer expectations, perceptions and purchasing of South African game meat. S. Afr. J. Wildl. Res.-24-Mon. Delayed Open Access 2005, 35, 33–42. [Google Scholar]
- Neethling, N.E.; Suman, S.P.; Sigge, G.O.; Hoffman, L.C. Muscle-specific colour stability of blesbok (Damaliscus pygargus phillipsi) meat. Meat Sci. 2016, 119, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Peruzy, M.; Murru, N.; Smaldone, G.; Proroga, Y.; Cristiano, D.; Fioretti, A.; Anastasio, A. Hygiene evaluation and microbiological hazards of hunted wild boar carcasses. Food Control 2022, 135, 108782. [Google Scholar] [CrossRef]
- Carrasco-García, A.A.; Pardío-Sedas, V.T.; León-Banda, G.G.; Ahuja-Aguirre, C.; Paredes-Ramos, P.; Hernández-Cruz, B.C.; Murillo, V.V. Effect of stress during slaughter on carcass characteristics and meat quality in tropical beef cattle. Asian-Australas. J. Anim. Sci. 2020, 33, 1656. [Google Scholar] [CrossRef]
- Ijaz, M.; Li, X.; Zhang, D.; Hussain, Z.; Ren, C.; Bai, Y.; Zheng, X. Association between meat color of DFD beef and other quality attributes. Meat Sci. 2020, 161, 107954. [Google Scholar] [CrossRef]
- Newton, K.; Gill, C. The microbiology of DFD fresh meats: A review. Meat Sci. 1981, 5, 223–232. [Google Scholar] [CrossRef]
- Yu, S.L.; Cooke, P.; Tu, S.I. Effects of chilling on sampling of bacteria attached to swine carcasses. Lett. Appl. Microbiol. 2001, 32, 205–210. [Google Scholar] [CrossRef]
- Smulders, F.; Toldra, F.; Flores, J.; Prieto, M. New technologies for meat and meat products. Utrecht Audet Tijdschr. 1992, 182, 186–188. [Google Scholar]
- Savell, J.; Mueller, S.; Baird, B. The chilling of carcasses. Meat Sci. 2005, 70, 449–459. [Google Scholar] [CrossRef]
- VPN/15/2010/01; Standard for Microbiological Monitoring of Meat Process Hygiene and Cleaning. Government Gazette: Pretoria, South Africa, 2010. Available online: https://www.nda.agric.za/v (accessed on 6 July 2022).
- Ben Braïek, O.; Smaoui, S. Chemistry, safety, and challenges of the use of organic acids and their derivative salts in meat preservation. J. Food Qual. 2021, 2021, 6653190. [Google Scholar] [CrossRef]
- Atanassova, V.; Apelt, J.; Reich, F.; Klein, G. Microbiological quality of freshly shot game in Germany. Meat Sci. 2008, 78, 414–419. [Google Scholar] [CrossRef]
- Avagnina, A.; Nucera, D.; Grassi, M.A.; Ferroglio, E.; Dalmasso, A.; Civera, T. The microbiological conditions of carcasses from large game animals in Italy. Meat Sci. 2012, 91, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Hochreutener, M.; Zweifel, C.; Corti, S.; Stephan, R. Effect of a commercial steam-vacuuming treatment implemented after slaughtering for the decontamination of cattle carcasses. Ital. J. Food Saf. 2017, 6, 6864. [Google Scholar] [CrossRef]
- Kure, C.F.; Axelsson, L.; Carlehög, M.; Måge, I.; Jensen, M.R.; Holck, A. The effects of a pilot-scale steam decontamination system on the hygiene and sensory quality of chicken carcasses. Food Control 2020, 109, 106948. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).