Abstract
Ten dairy farms supplying raw milk to the dairy industry were investigated to determine compliance with the safety criteria (plate count at 30 °C and somatic cell count) of Regulation (EC) 853/2004. The relationship of such criteria with lipid and protein percentages was also evaluated. The results demonstrated a great variability due to the different management systems of each dairy farm, with regard to the milking hygiene, the health status of the animals, and the prevention measures against mastitis. Moreover, in some dairy farms, the somatic cell counts were positively correlated with the quality components of raw milk as well as the plate count at 30 °C.
1. Introduction
Raw milk is characterized by good nutritional value thanks to a high content of proteins, vitamins, and trace elements. However, its composition can vary based on the dairy animal species, age, seasonality, the health status of the udder, lactation period, etc. [1]. Due to its natural components as well as low acidity, it represents an excellent growth medium for many microorganisms, which can develop and spread in the product, causing its spoilage and/or quality decline [2]. When it is secreted into the alveoli of the mammary gland, it can be considered sterile—not taking into account potential pathogens causing animal diseases, such as brucellosis, tuberculosis, etc.—but it can be contaminated during milking and/or handling by operators, or from the equipment and the environment [3]. Further sources of raw milk contamination are strictly associated with the animals, i.e., the accidental presence of feces, dirty udder, and clinical or sub-clinical mastitis [4].
Mastitis represents a food safety concern, and it is also one of the most common reasons for economic damage in cow herd management. It is caused by many microorganisms, such as Staphylococcus aureus, Streptococcus agalactiae, Corynebacterium bovis, and Mycoplasma spp., even if the most common species are Streptococcus uberis and Streptococcus dysgalactiae, as well as non-aureus staphylococci. The infection may also occur when the appropriate milking hygiene procedures are not applied by the operators taking care of the animals, and it can even become chronic, with flare-ups of clinical events [5]. Clinical mastitis is characterized by red, hot, and swollen mammary glands, while the subclinical form is seldom diagnosed in dairy animals because no or less visible signs are evident [6]. Generally, in the latter situation, milk production decreases and the somatic cell count (SCC) increases [7].
Somatic cells, naturally occurring in milk from the mammary gland epithelium, can increase during udder inflammation and, on such occasions, they mainly consist of polymorphonuclear leukocytes, macrophages, and/or lymphocytes. In animals affected by mastitis, leukocytes can increase by up to 99% [8]. Many factors other than mastitis can influence SCC in raw milk, such as the stage of lactation, age and breed of the animals, season, stress, and diurnal variation [9]. It is reported that the SCC increase causes a reduction in casein, fat, and lactose content of milk, consequently affecting the quality and yield of the derived dairy products [10].
According to Regulation (EC) 853/2004, raw milk must come from animals that do not show any symptoms of infectious diseases for humans or signs of diseases of the udder or the genital tract that could contaminate milk. Moreover, they must be compliant with two specific health requirements, i.e., the plate count at 30 °C and the SCC. The latter is established only for raw cow milk, and it corresponds to ≤400,000 cells/mL, while the plate count at 30 °C must be ≤100,000 CFU/mL. These two regulatory criteria are expressed as a rolling geometric average over a two- or three-month period for a plate count at 30 °C and SCC, respectively.
Many studies investigated the relationship of SCC with the physicochemical parameters of milk [11,12,13]. They reported that the SCC levels influenced the percentage of lactose, protein, lipid, or other raw milk components, showing a direct effect on cheesemaking and the production of dairy products. The objective of this study was the evaluation of compliance with the above-mentioned health requirements of raw milk samples collected over a year from ten dairy farms (named from A to L), which regularly supplied a dairy company located in the Abruzzo region (Central Italy). The relationship between such regulatory criteria with some quality parameters (lipid and protein contents) was also investigated.
2. Materials and Methods
The examined dairy farms were located in two provinces (Pescara and Teramo) of the Abruzzo region, and they were 20 to 102 km away from the dairy company. They were medium-small farms with a herd ranging from 30 to 50 animals (3–5 years old). They applied intensive farming, and feeding was mainly unifeed. The animals were Friesian or crossbreeds.
All dairy farms used an automatic milking machine; the milk samples were collected daily and stored at 8 °C before transport to the dairy company. For the determination of the safety criteria of Regulation (EC) 853/2004, the raw milk samples were taken twice a month from each dairy farm, according to the regulatory sampling frequency. The results were expressed as a rolling geometric average over a two- (bimester) or three-month (trimester) period for plate count at 30 °C and SCC, respectively. Further, the protein and lipid contents were calculated for trimesters.
The samples were put into sterile plastic tubes, where they were kept under refrigeration conditions (4 °C) until their arrival at the laboratory. Specifically, the determination of the quality parameters was performed by the in-house laboratory joined to the dairy company, while the compliance with the regulatory criteria was verified by an officially accredited laboratory.
The lipid and protein contents were determined with a Milkoscan FT2 (Foss Italia S.r.l., Padova, Italy). The method was based on the differential absorption of infrared waves by the different milk components, and the results were expressed as percentages. The analyses of the safety criteria were performed according to the reference methods reported in the Regulation (EU) 2019/627, i.e., EN ISO 4833-1:2013 and EN ISO 13366-1:2008 for plate count at 30 °C and SCC, respectively.
The statistical analysis was carried out by using GraphPad InStat, Version 3.0 (San Diego, CA, USA). All data were assessed for normality by the Kolmogorov–Smirnov assay. Since the samples were not normally distributed, nonparametric methods (Kruskal–Wallis and Dunn’s Multiple Comparisons tests) were selected to compare the different dairy farms, with no distinction among trimesters or bimesters. Then, all the investigated parameters from each dairy farm were also compared to observe statistically significant differences (p < 0.05) among trimesters for SCC, lipid, and protein values, and bimesters for plate count at 30 °C. The SCC correlation with lipid, protein, and plate count at 30 °C was studied by the multiple regression model. The results were statistically significant when R2 was not lower than 0.25.
3. Results
The results of the regulatory criteria are shown in Figure 1 and Figure 2, while the mean values of the quality parameters are reported in Table 1.
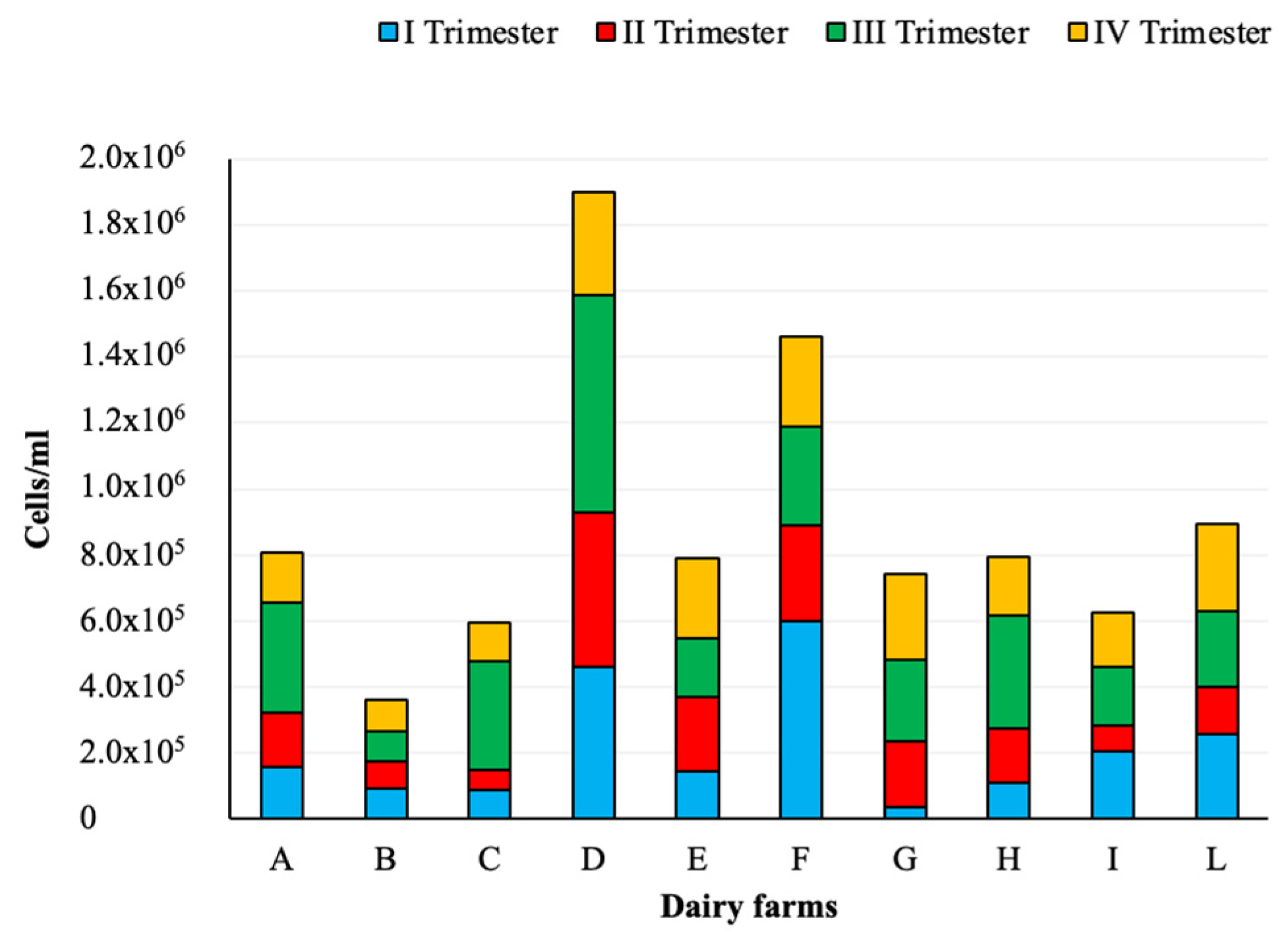
Figure 1.
Somatic cell counts in raw milk samples collected from the investigated dairy farms.
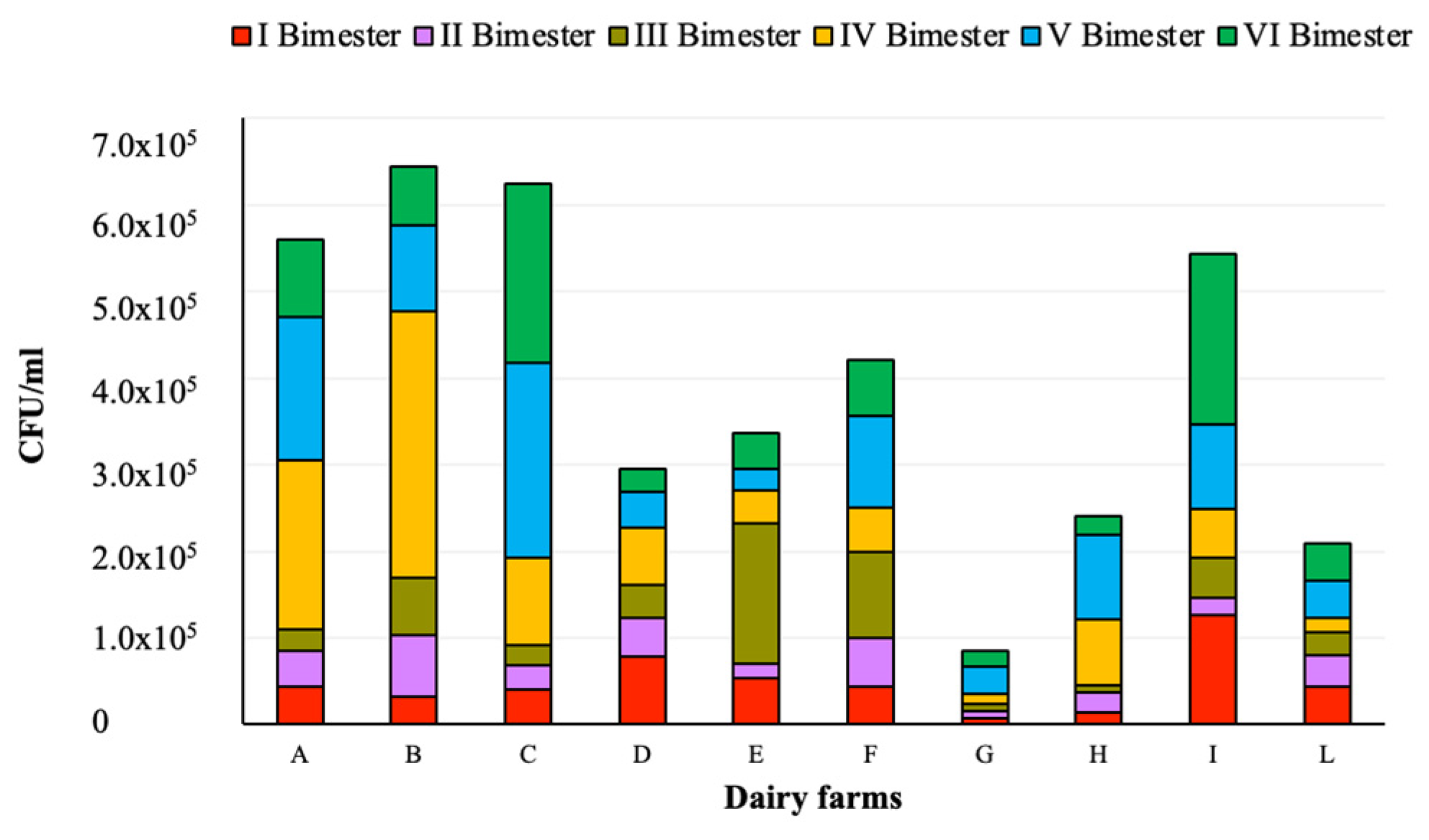
Figure 2.
Plate count at 30 °C in raw milk samples collected from the investigated dairy farms.

Table 1.
Lipid and protein contents (mean ± S.D., %) in raw milk samples collected from the investigated dairy farms.
A total of 23 (9.6%) out of 240 samples exceeded the regulatory limit for SCC. The maximum value (2,351,000 cells/mL) was found in a sample collected from dairy farm D, which reported some non-compliant samples in all four trimesters. Non-complaint samples were detected in all the other dairy farms, except for B and I. Considering SCC results, not distinguished for trimesters, the highest numbers were detected in dairy farms D and F, while the lowest were found in B. The results of nonparametric ANOVA tests showed a statistically significant difference between dairy farm B and D and between B and E (p < 0.05), and, also, between B and F as well as between B and L (p < 0.01). The comparison of SCC results obtained in the four trimesters from each dairy farm showed no statistically significant difference (p > 0.05), except in dairy farm G, between I and III and IV trimesters (p < 0.01), and in dairy farm H, between I and III trimesters (p < 0.05).
With regard to the plate count at 30 °C, 37 (15.4%) out of 240 samples exceeded the maximum limit (100,000 CFU/mL). The lowest levels were found in dairy farm G, showing a statistically significant difference (p < 0.001) from B, F, and I, and also with A (p < 0.01) and C (p < 0.05). Moreover, the values found in I and II trimesters in samples collected from dairy farm I differed significantly (p < 0.05), as well as those obtained in I and IV trimesters from dairy farm L.
A lower variability related to the quality parameters was observed (Table 1). They showed statistically significant differences (not considering trimesters) only between D and E (p < 0.01) and D and I (p < 0.05) for lipids, while the protein percentage detected in raw milk samples from dairy farm I differed significantly (p < 0.01) from A, C, and F, but also, G and H (p < 0.05).
The results of the multiple regression analysis showed a positive correlation (R2 = 0.33, p < 0.001) of SCC with lipid percentages found in samples of dairy farm L, and a quite significant positive correlation (R2 = 0.24, p < 0.01) in dairy farm G. The latter dairy farm also showed a positive correlation (R2 = 0.58, p < 0.0001) between SCC and proteins. A positive correlation also emerged between SCC and plate count at 30 °C in the samples from dairy farms G (R2 = 0.39, p < 0.001), H (R2 = 0.34, p < 0.001), and L (R2 = 0.38, p < 0.001).
4. Discussion
The first objective of this study was the evaluation of ten different dairy farms with respect to the safety criteria fixed by Regulation (EC) 853/2004. The majority of them resulted as compliant, demonstrating both good animal health conditions and milking hygiene. Conversely, when samples exceed the regulatory criteria, the milk collection from the corresponding dairy farm should be suspended until the results are returned to regular, and the farmers have to take measures to correct such a situation.
The variability of the results of both plate count at 30 °C and SCC among the examined dairy farms could be explained by their different management systems, especially regarding the milking procedures. The dairy farms where low values were detected, independently from bimesters/trimesters, applied some good hygiene practices, such as proper cleaning of the teats and udder before milking, their disinfection at the end of the procedure, wearing of gloves by operators, appropriate storage temperature of milk, as well as other measures related to the treatment of mastitis during the non-lactating period, and/or the implementation of regular mastitis prevention. By contrast, some dairy farms resulted in occasionally non-compliant results and, therefore, they should improve the organization of the farm, starting from the veterinary care for suspected cases of illness, especially related to mastitis, the hygiene of animals and environment, and the correct cleaning of the milking apparatus, as it is well known that milk residues on the surfaces of used equipment can favor the growth of microorganisms.
Other studies reported non-compliant results with regard to the safety criteria of raw milk. Bogdanovičová et al. [14] evaluated the microbiological quality of raw milk collected from 41 dairy farms located in the Czech Republic and found that the plate count at 30 °C exceeded the regulatory limit in 13% of samples, while the highest SCC value corresponded to 990,000 cells/mL. Similarly, Bolzoni et al. [15] observed that 29% out of 5200 dairy farms exceeded the SCC limit one or more times a year, but the parameter returned below the limit within 3 months. However, a further 27% of non-compliant dairy farms remained in such condition even after this period.
The positive correlation of SCC with lipid and protein content confirmed the results of other authors [16,17,18], which also found that in milk with high SCC values, the percentage of protein or fat increased. In particular, Garcia et al. [11] reported that the increase in lipid together with SCC could be linked to a general decrease in milk production and, therefore, the results could be concentrated. However, fat is the milk component that shows the highest variation due to cow breed, lactation period, and animal diet. With regard to proteins, it has been supposed that the plasma proteins can migrate to the site of inflammation, also in case of mastitis, and as a consequence, they increase the total protein concentration of milk. Further studies are necessary to understand the influence of SCC on milk components, as fat, protein, and casein are fundamental in the dairy industry and represent the main matrix for cheese production.
The positive correlation of SCC with the plate count at 30 °C in dairy farms G, H, and L, characterized by low values of both parameters, underlined that the two regulatory criteria can sometimes be associated. The SCC levels indicate the health status of the animals, especially regarding subclinical mastitis, while the values of the plate count at 30 °C reveal the hygiene practices applied on the dairy farm. It must be underlined that the farmers declared no health problems related to clinical mastitis or other diseases affecting the animals during the considered period. Further, Erdem et al. [19] investigated the influence of the hygiene status of dairy cows on SCC and milk components and found that it was crucial to obtain high-quality raw milk.
5. Conclusions
The present study highlighted that most raw milk samples were compliant with the regulatory criteria (90.4% and 84.6% for SCC and plate count at 30 °C, respectively). By contrast, when non-compliant samples were found, the competent authority suspended the raw milk collection and required appropriate corrective actions by the corresponding dairy farm. The positive SCC correlation with the other investigated parameters in some dairy farms should also be considered, especially for its influence on the quality of the product.
Author Contributions
Conceptualization, P.V. and M.S.; investigation, P.V. and M.S.; data curation, M.S.; statistical software, P.V.; writing—original draft preparation, P.V. and M.S.; writing—review and editing, P.V. and M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Malek dos Reis, C.B.; Barreiro, J.R.; Mestieri, L.; Porcionato, M.A.F.; Santos, M.V. Effect of somatic cell count and mastitis pathogens on milk composition in Gyr cows. BMC Vet. Res. 2013, 9, 67. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S. Microbiological Considerations: Pasteurized Milk. Int. J. Dairy Sci. 2015, 10, 206–218. [Google Scholar] [CrossRef]
- Ritota, M.; Di Costanzo, M.G.; Mattera, M.; Manzi, P. New trends for the evaluation of heat treatments of milk. J. Anal. Methods Chem 2017, 2017, 1864832. [Google Scholar] [CrossRef] [PubMed]
- Ürkek, B.; Şengül, M.; Erkaya, T.; Aksakal, V. Prevalence and comparing of some microbiological properties, somatic cell count and antibiotic residue of organic and conventional raw milk produced in Turkey. Korean J. Food Sci. Anim. Resour. 2017, 37, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Abebe, R.; Hatiya, H.; Abera, M.; Megersa, B.; Asmare, K. Bovine mastitis: Prevalence, risk factors and isolation of Staphylococcus aureus in dairy herds at Hawassa milk shed, South Ethiopia. BMC Vet. Res. 2016, 12, 270. [Google Scholar] [CrossRef] [PubMed]
- Moroni, P.; Nydam, D.; Ospina, P.A.; Scillieri-Smith, J.C.; Virkler, P.D.; Watters, R.D.; Wellcome, F.L.; Zurakowki, M.J.; Ducharme, N.G.; Yeager, A.E. Diseases of the Teats and Udder. In Rebhun’s Diseases of Dairy Cattle, 3rd ed.; Peek, S.F., Divers, T.J., Eds.; Elsevier: St. Louis, MO, USA, 2018; Chapter 8; pp. 389–465. [Google Scholar] [CrossRef]
- Costa, A.; Neglia, G.; Campanile, G.; De Marchi, M. Milk somatic cell count and its relationship with milk yield and quality traits in Italian water buffaloes. J. Dairy Sci. 2020, 103, 5485–5494. [Google Scholar] [CrossRef] [PubMed]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards). Scientific Opinion on the public health risk related to the consumption of raw drinking milk. EFSA J. 2015, 13, 3940. [Google Scholar] [CrossRef]
- Sharma, N.; Singh, N.K.; Bhadwal, M.S. Relationship of somatic cell count and mastitis: An overview. Asian-Aust. J. Anim. Sci. 2011, 24, 429–438. [Google Scholar] [CrossRef]
- Guariglia, B.A.D.; dos Santos, P.A.; de Souza Araújo, L.; Giovannini, C.I.; Neves, R.B.S.; Nicolau, E.S.; da Silva, M.A.P. Effect of the somatic cell count on physicochemical components of milk from crossbred cows. Afr. J. Biotechnol. 2015, 14, 1519–1524. [Google Scholar] [CrossRef] [Green Version]
- Garcia, R.R.; Maion, V.B.; de Almeida, K.M.; de Santana, E.H.W.; Costa, M.R.; Fagnani, R.; Ludovico, A. Relationship between somatic cell counts and milk production and composition in Jersey cows. Rev. Salud. Anim. 2015, 37, 137–142. [Google Scholar] [CrossRef]
- Musayeva, K.; Sederevičius, A.; Želvytė, R.; Monkevičienė, I.; Beliavska-Aleksiejūnė, D.; Stankevičius, R. Relationship between somatic cell count and milk casein level obtained by two different methods. Czech J. Food Sci. 2016, 34, 47–51. [Google Scholar] [CrossRef]
- Oravcová, M.; Mačuhová, L.; Tančin, V. The relationship between somatic cells and milk traits, and their variation in dairy sheep breeds in Slovakia. J. Animal Feed Sci. 2018, 27, 97–104. [Google Scholar] [CrossRef]
- Bogdanovičová, K.; Vyletělová-Klimešová, M.; Babák, V.; Kalhotka, L.; Koláčková, I.; Karpíšková, R. Microbiological quality of raw milk in the Czech Republic. Czech J. Food Sci. 2016, 34, 189–196. [Google Scholar] [CrossRef]
- Bolzoni, G.; Marcolini, A.; Buffoli, E. End of the derogations to Regulation (EC) 853/2004 for cow’s milk in Italy. Italian J. Food Sci. 2015, 27, 118–125. [Google Scholar] [CrossRef]
- Ramos, T.M.; Costa, F.F.; Pinto, I.S.B.; Pinto, S.M.; Abreu, L.R. Effect of somatic cell count on bovine milk protein fractions. J. Anal. Bioanal. Tech. 2015, 6, 1000269. [Google Scholar] [CrossRef]
- Moslehishad, M.; Hamid, E.; Mehdi, A. Chemical and electrophoretic properties of Holstein cow milk as affected by somatic cell count. Int. J. Dairy Technol. 2020, 63, 512–515. [Google Scholar] [CrossRef]
- Cinar, M.; Serbester, U.; Ceyhan, A.; Gorgulu, M. Effect of somatic cell count on milk yield and composition of first and second lactation dairy cows. Italian J. Anim. Sci. 2015, 14, 105–108. [Google Scholar] [CrossRef] [Green Version]
- Erdem, H.; Okuyucu, I.C. Influence of hygiene status of cows on somatic cell count and milk components during summer season. Large Anim. Rev. 2019, 25, 7–10. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).