Epidemiology and Genetic Characterization of Distinct Ebola Sudan Outbreaks in Uganda
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Sampling and Data Collection
3. Results
3.1. Epidemiological Analysis
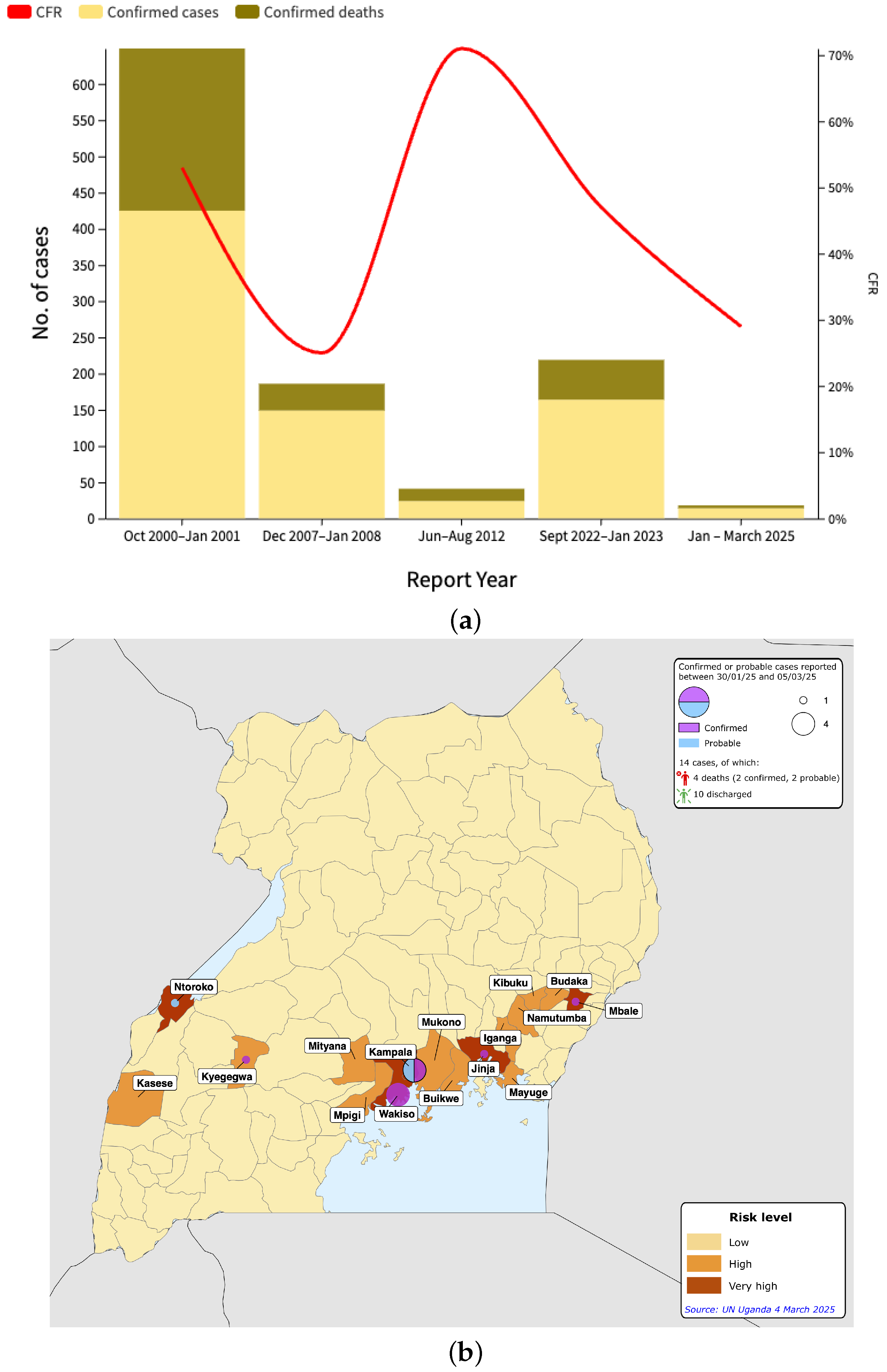
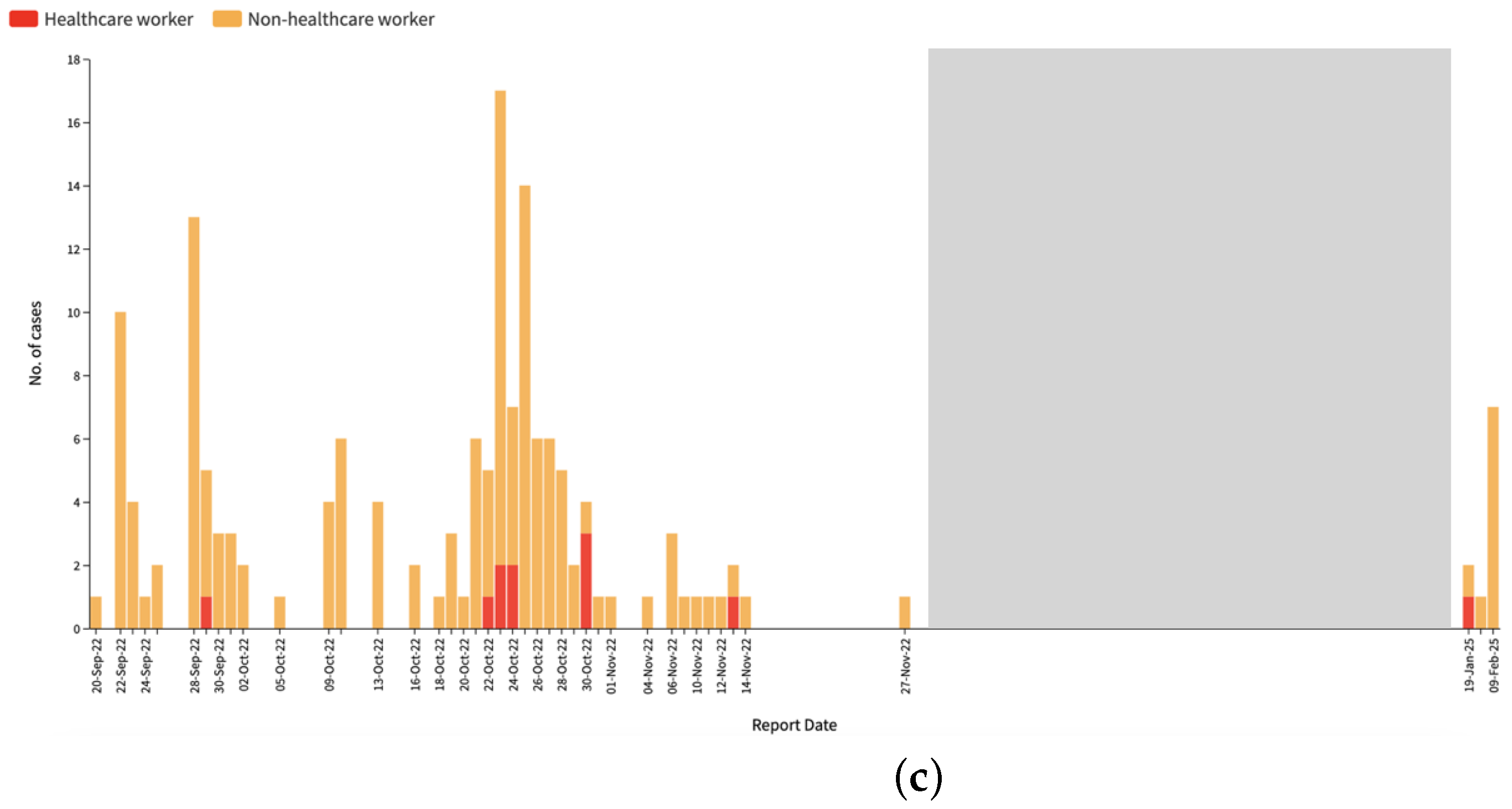
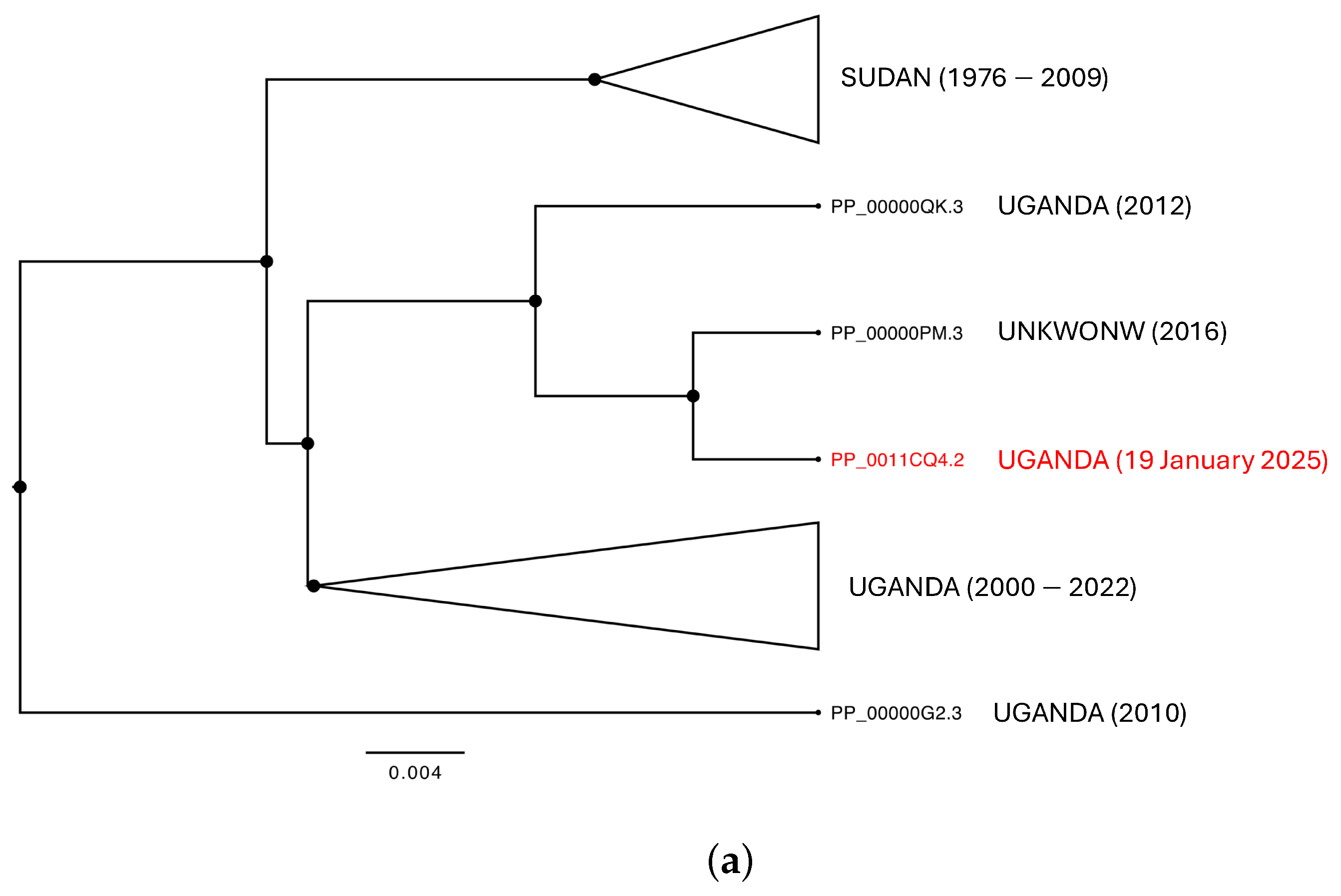
3.2. Phylogenomic Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Biedenkopf, N.; Bukreyev, A.; Chandran, K.; Di Paola, N.; Formenty, P.B.; Griffiths, A.; Hume, A.J.; Mühlberger, E.; Netesov, S.V.; Palacios, G.; et al. Renaming of genera Ebolavirus and Marburgvirus to Orthoebolavirus and Orthomarburgvirus, respectively, and introduction of binomial species names within family Filoviridae. Arch. Virol. 2023, 168, 220. [Google Scholar] [CrossRef] [PubMed]
- Letafati, A.; Ardekani, O.S.; Karami, H.; Soleimani, M. Ebola virus disease: A narrative review. Microb. Pathog. 2023, 181, 106213. [Google Scholar] [CrossRef] [PubMed]
- Grard, G.; Biek, R.; Muyembe Tamfum, J.J.; Fair, J.; Wolfe, N.; Formenty, P.; Paweska, J.; Leroy, E. Emergence of divergent Zaire ebola virus strains in Democratic Republic of the Congo in 2007 and 2008. J. Infect. Dis. 2011, 204, S776–S784. [Google Scholar] [CrossRef]
- Kainulainen, M.H.; Harmon, J.R.; Whitesell, A.N.; Bergeron, É.; Karaaslan, E.; Cossaboom, C.M.; Malenfant, J.H.; Kofman, A.; Montgomery, J.M.; Choi, M.J.; et al. Recombinant Sudan virus and evaluation of humoral cross-reactivity between Ebola and Sudan virus glycoproteins after infection or rVSV-ΔG-ZEBOV-GP vaccination. Emerg. Microbes Infect. 2023, 12, 2265660. [Google Scholar] [CrossRef]
- Carroll, S.A.; Towner, J.S.; Sealy, T.K.; McMullan, L.K.; Khristova, M.L.; Burt, F.J.; Swanepoel, R.; Rollin, P.E.; Nichol, S.T. Molecular evolution of viruses of the family Filoviridae based on 97 whole-genome sequences. J. Virol. 2013, 87, 2608–2616. [Google Scholar] [CrossRef]
- CDC. Notes from the Field: Outbreak of Ebola Virus Disease Caused by Sudan ebolavirus—Uganda, August–October 2022. Available online: https://www.cdc.gov/mmwr/volumes/71/wr/mm7145a5.htm (accessed on 21 February 2025).
- Leroy, E.M.; Kumulungui, B.; Pourrut, X.; Rouquet, P.; Hassanin, A.; Yaba, P.; Délicat, A.; Paweska, J.T.; Gonzalez, J.P.; Swanepoel, R. Fruit bats as reservoirs of Ebola virus. Nature 2005, 438, 575–576. [Google Scholar] [CrossRef]
- Singh, R.K.; Dhama, K.; Malik, Y.S.; Ramakrishnan, M.A.; Karthik, K.; Khandia, R.; Tiwari, R.; Munjal, A.; Saminathan, M.; Sachan, S.; et al. Ebola virus–epidemiology, diagnosis, and control: Threat to humans, lessons learnt, and preparedness plans—An update on its 40 year’s journey. Vet. Q. 2017, 37, 98–135. [Google Scholar] [CrossRef]
- Subissi, L.; Keita, M.; Mesfin, S.; Rezza, G.; Diallo, B.; Van Gucht, S.; Musa, E.O.; Yoti, Z.; Keita, S.; Djingarey, M.H.; et al. Ebola virus transmission caused by persistently infected survivors of the 2014–2016 outbreak in West Africa. J. Infect. Dis. 2018, 218, S287–S291. [Google Scholar] [CrossRef]
- CDC. Ebola; Outbreak History. Available online: https://www.cdc.gov/ebola/outbreaks/index.html (accessed on 9 February 2025).
- World Health Organization. Sudan Virus Disease in Uganda. Disease Outbreak News, 1 February 2025. Available online: https://www.who.int/emergencies/disease-outbreak-news/item/2025-DON555 (accessed on 9 February 2025).
- Branda, F.; Mahal, A.; Maruotti, A.; Pierini, M.; Mazzoli, S. The challenges of open data for future epidemic preparedness: The experience of the 2022 Ebolavirus outbreak in Uganda. Front. Pharmacol. 2023, 14, 1101894. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Okonechnikov, K.; Golosova, O.; Fursov, M.; Team, U. Unipro UGENE: A unified bioinformatics toolkit. Bioinformatics 2012, 28, 1166–1167. [Google Scholar] [CrossRef] [PubMed]
- Drummond, A.J.; Rambaut, A. BEAST: Bayesian evolutionary analysis by sampling trees. Bmc Evol. Biol. 2007, 7, 214. [Google Scholar] [CrossRef] [PubMed]
- Kass, R.E.; Raftery, A.E. Bayes factors. J. Am. Stat. Assoc. 1995, 90, 773–795. [Google Scholar] [CrossRef]
- Rambaut, A.; Drummond, A.J.; Xie, D.; Baele, G.; Suchard, M.A. Posterior summarization in Bayesian phylogenetics using Tracer 1.7. Syst. Biol. 2018, 67, 901–904. [Google Scholar] [CrossRef]
- Peakall, R.; Smouse, P.E. GENALEX 6: Genetic analysis in Excel. Population genetic software for teaching and research. Mol. Ecol. Notes 2006, 6, 288–295. [Google Scholar] [CrossRef]
- Paradis, E.; Claude, J.; Strimmer, K. APE: Analyses of phylogenetics and evolution in R language. Bioinformatics 2004, 20, 289–290. [Google Scholar] [CrossRef]
- Kabami, Z.; Ario, A.R.; Harris, J.R.; Ninsiima, M.; Ahirirwe, S.R.; Ocero, J.R.A.; Atwine, D.; Mwebesa, H.G.; Kyabayinze, D.J.; Muruta, A.N.; et al. Ebola disease outbreak caused by the Sudan virus in Uganda, 2022: A descriptive epidemiological study. Lancet Glob. Health 2024, 12, e1684–e1692. [Google Scholar] [CrossRef]
- Dahl, B.A. CDC’s response to the 2014–2016 Ebola epidemic—Guinea, Liberia, and Sierra Leone. MMWR Suppl. 2016, 65, 12–20. [Google Scholar] [CrossRef]
- World Health Organization. Sudan Virus Disease in Uganda. Disease Outbreak News, 8 March 2025. Available online: https://www.who.int/emergencies/disease-outbreak-news/item/2025-DON558 (accessed on 10 March 2025).
- World Health Organization (African Region). Weekly Bulletins on Outbreaks and Other Emergencies. Available online: https://iris.who.int/bitstream/handle/10665/380693/OEW9-240202032025.pdf (accessed on 4 April 2025).
- Nanyondo, S.J.; Nakato, S.; Franklin, J.; Kwiringira, A.; Malikisi, M.; Kesande, M.; Wailagala, A.; Suubi, R.; Byonanebye, D.M.; Katwesigye, E.; et al. Implementation of an infection prevention and control response strategy to combat the Sudan Virus Disease outbreak in an urban setting, the Kampala Metropolitan area, Uganda, 2022. BMC Infect. Dis. 2025, 25, 317. [Google Scholar] [CrossRef]
- Fischer, W.A., II; Weber, D.J.; Wohl, D.A. Personal protective equipment: Protecting health care providers in an Ebola outbreak. Clin. Ther. 2015, 37, 2402–2410. [Google Scholar] [CrossRef]
- ReliefWeb. Uganda|Sudan (Ebola) Virus Disease Outbreak and EU Response-DG ECHO Daily Map|26/03/2025. Available online: https://erccportal.jrc.ec.europa.eu/ECHO-Products/Maps#/maps/5237 (accessed on 4 April 2025).
- CDC. Ebola Outbreak Caused by Sudan Virus in Uganda. Available online: https://www.cdc.gov/han/2025/han00521.html (accessed on 22 February 2025).
- Fisher-Hoch, S.P.; Brammer, T.L.; Trappier, S.G.; Hutwagner, L.C.; Farrar, B.B.; Suyu, L.R.; Brown, B.G.; Hermann, L.M.; Perez-Oronoz, G.I.; Goldsmith, C.S.; et al. Pathogenic Potential of Filoviruses: Role of Geographic Origin of Primate Host and Virus Strain. J. Infect. Dis. 1992, 166, 753–763. [Google Scholar] [CrossRef] [PubMed]

| Indicator | 2025 Outbreak (as of 5 March 2025) | 2022–2023 Outbreak |
|---|---|---|
| Confirmed Cases | 12 | 142 |
| Probable Cases | 2 | 22 |
| Total Cases | 14 | 164 |
| Confirmed Deaths | 2 | 55 |
| Probable Deaths | 2 | 22 |
| Total Deaths | 4 | 77 |
| CFR | 29% | 47% (overall), 39% (confirmed) |
| Affected Districts | Jinja, Kampala, Kyegegwe, Mbale, Ntoroko, Wakiso | Bunyangabu, Jinja, Kagadi, Kampala, Kassanda, Kyegegwa, Masaka, Mubende, Wakiso |
| Duration | 30 January 2025–ongoing | 20 September 2022–11 January 2023 |
| Mean Age of Cases | 27 years | 20–29 years (most affected) |
| Gender Distribution | 55% Male | 59% Male |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Branda, F.; Ciccozzi, M.; Scarpa, F. Epidemiology and Genetic Characterization of Distinct Ebola Sudan Outbreaks in Uganda. Infect. Dis. Rep. 2025, 17, 44. https://doi.org/10.3390/idr17030044
Branda F, Ciccozzi M, Scarpa F. Epidemiology and Genetic Characterization of Distinct Ebola Sudan Outbreaks in Uganda. Infectious Disease Reports. 2025; 17(3):44. https://doi.org/10.3390/idr17030044
Chicago/Turabian StyleBranda, Francesco, Massimo Ciccozzi, and Fabio Scarpa. 2025. "Epidemiology and Genetic Characterization of Distinct Ebola Sudan Outbreaks in Uganda" Infectious Disease Reports 17, no. 3: 44. https://doi.org/10.3390/idr17030044
APA StyleBranda, F., Ciccozzi, M., & Scarpa, F. (2025). Epidemiology and Genetic Characterization of Distinct Ebola Sudan Outbreaks in Uganda. Infectious Disease Reports, 17(3), 44. https://doi.org/10.3390/idr17030044








