Incidence of COVID-19 Symptom Rebound After Treatment with Remdesivir
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. Definition of Symptom Rebound
2.3. Outcome Assessment and Follow-Up
2.4. Statistical Analysis
3. Results
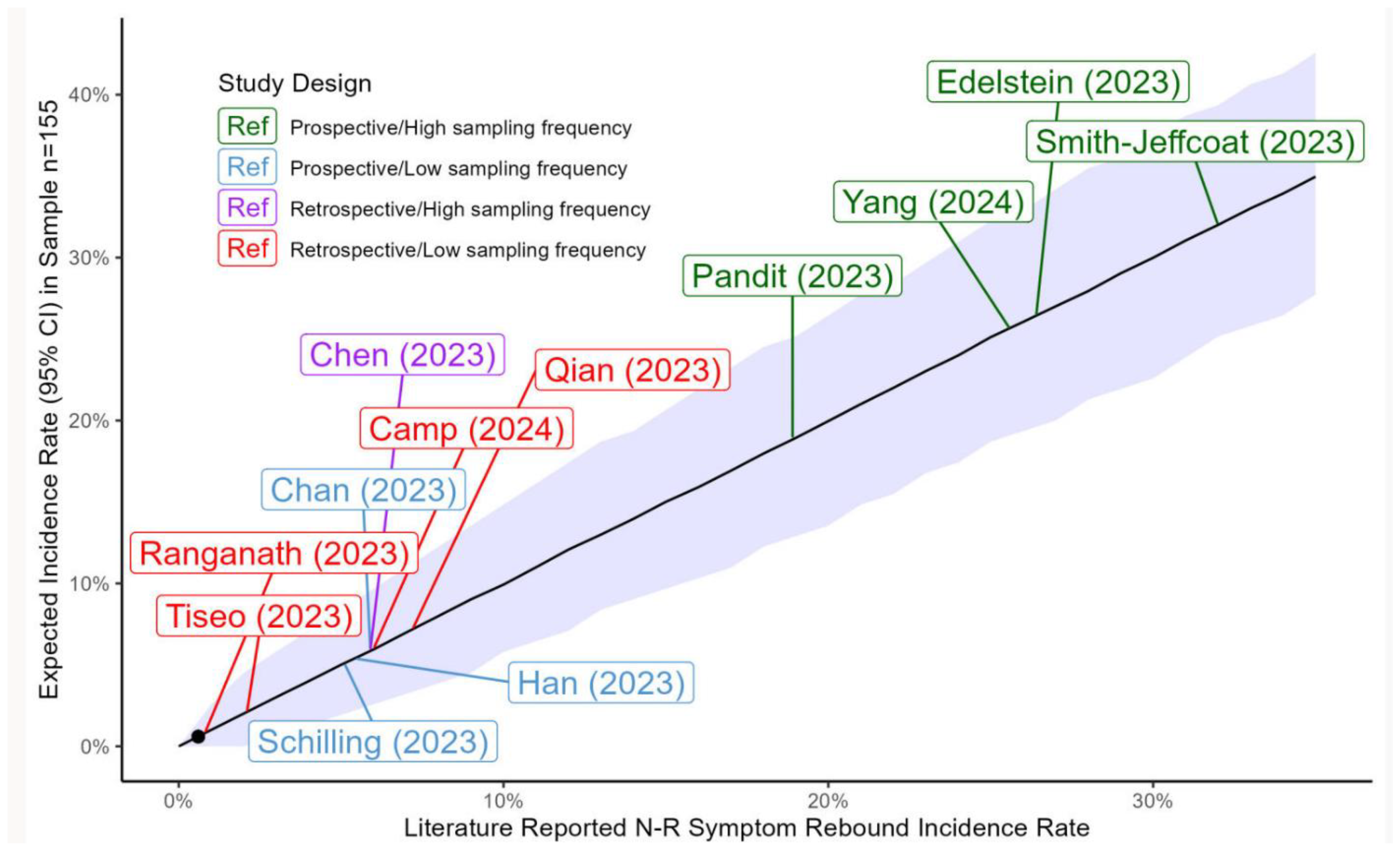
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ICU | Intensive care unit |
| EHR | Electronic health record |
| VA | Veterans Affairs |
References
- Charness, M.E.; Gupta, K.; Stack, G.; Strymish, J.; Adams, E.; Lindy, D.C.; Mohri, H.; Ho, D.D. Rebound of SARS-CoV-2 Infection after Nirmatrelvir-Ritonavir Treatment. N. Engl. J. Med. 2022, 387, 1045–1047. [Google Scholar] [CrossRef] [PubMed]
- Edelstein, G.E.; Boucau, J.; Uddin, R.; Marino, C.; Liew, M.Y.; Barry, M.; Choudhary, M.C.; Gilbert, R.F.; Reynolds, Z.; Li, Y.; et al. SARS-CoV-2 Virologic Rebound With Nirmatrelvir-Ritonavir Therapy: An Observational Study. Ann. Intern. Med. 2023, 176, 1577–1585. [Google Scholar] [CrossRef] [PubMed]
- Pandit, J.A.; Radin, J.M.; Chiang, D.C.; Spencer, E.G.; Pawelek, J.B.; Diwan, M.; Roumani, L.; Mina, M.J. The COVID-19 Rebound Study: A Prospective Cohort Study to Evaluate Viral and Symptom Rebound Differences in Participants Treated with Nirmatrelvir Plus Ritonavir Versus Untreated Controls. Clin. Infect. Dis. 2023, 77, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Smith-Jeffcoat, S.E.; Biddle, J.E.; Talbot, H.K.; Morrissey, K.G.; Stockwell, M.S.; Maldonado, Y.; McLean, H.Q.; Ellingson, K.D.; Bowman, N.M.; Asturias, E.; et al. Symptoms, viral loads, and rebound among COVID-19 outpatients treated with nirmatrelvir/ritonavir compared to propensity score matched untreated individuals. Clin. Infect. Dis. 2023, 78, 1175–1184. [Google Scholar] [CrossRef]
- Han, J.; Bae, S.; Jung, J.; Kim, M.J.; Chong, Y.P.; Lee, S.-O.; Choi, S.-H.; Kim, Y.S.; Chang, E.; Kim, S.-H. Clinical characteristics of COVID-19 rebound after nirmatrelvir-ritonavir or molnupiravir therapy: A prospective cohort study. Medicine 2023, 102, e35094. [Google Scholar] [CrossRef]
- Camp, D.; Caputo, M.; Echevarria, F.M.; Achenbach, C.J. Clinical rebound after treatment with nirmatrelvir/ritonavir in COVID-19. BMC Infect. Dis. 2024, 24, 963. [Google Scholar] [CrossRef]
- Nair, M.S.; Luck, M.I.; Huang, Y.; Sabo, Y.; Ho, D.D. Persistence of an infectious form of SARS-CoV-2 post protease inhibitor treatment of permissive cells in vitro. J. Infect. Dis. 2024, 231, e68–e76. [Google Scholar] [CrossRef]
- Wong, C.K.H.; Lau, J.J.; Au, I.C.H.; Lau, K.T.K.; Hung, I.F.N.; Peiris, M.; Leung, G.M.; Wu, J.T. Optimal timing of nirmatrelvir/ritonavir treatment after COVID-19 symptom onset or diagnosis: Target trial emulation. Nat. Commun. 2023, 14, 8377. [Google Scholar] [CrossRef]
- Esmaeili, S.; Owens, K.; Wagoner, J.; Polyak, S.J.; White, J.M.; Schiffer, J.T. A unifying model to explain frequent SARS-CoV-2 rebound after nirmatrelvir treatment and limited prophylactic efficacy. Nat. Commun. 2024, 15, 5478. [Google Scholar] [CrossRef]
- Du, Z.; Wang, L.; Bai, Y.; Liu, Y.; Lau, E.H.; Galvani, A.P.; Krug, R.M.; Cowling, B.J.; Meyers, L.A. A retrospective cohort study of Paxlovid efficacy depending on treatment time in hospitalized COVID-19 patients. eLife 2024, 13, e89801. [Google Scholar] [CrossRef]
- Phan, T.; Ribeiro, R.M.; Edelstein, G.E.; Boucau, J.; Uddin, R.; Marino, C.; Liew, M.Y.; Barry, M.; Choudhary, M.C.; Tien, D.; et al. Modeling suggests SARS-CoV-2 rebound after nirmatrelvir-ritonavir treatment is driven by target cell preservation coupled with incomplete viral clearance. J. Virol. 2025, 99, e0162324. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, G.; Rovito, R.; Bono, V.; Bonara, P.; Bai, F.; Monforte, A.D. Immunologic characterization of a patient with clinical and virologic rebound upon Nirmatrelvir/Ritonavir treatment: The unfortunate epilogue of COVID-19. Clin. Microbiol. Infect. 2023, 29, 660–662. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.S.; Caubel, P.; Rusnak, J.M. Nirmatrelvir–Ritonavir and Viral Load Rebound in COVID-19. N. Engl. J. Med. 2022, 387, 1047–1049. [Google Scholar] [CrossRef]
- Yang, Z.; Xu, Y.; Zheng, R.; Ye, L.; Lv, G.; Cao, Z.; Han, R.; Li, M.; Zhu, Y.; Cao, Q.; et al. COVID-19 Rebound After VV116 vs Nirmatrelvir-Ritonavir Treatment: A Randomized Clinical Trial. JAMA Netw. Open 2024, 7, e241765. [Google Scholar] [CrossRef]
- Schilling, W.H.K.; Jittamala, P.; Watson, J.A.; Boyd, S.; Luvira, V.; Siripoon, T.; Ngamprasertchai, T.; Batty, E.M.; Cruz, C.; Callery, J.J.; et al. Antiviral efficacy of molnupiravir versus ritonavir-boosted nirmatrelvir in patients with early symptomatic COVID-19 (PLATCOV): An open-label, phase 2, randomised, controlled, adaptive trial. Lancet Infect. Dis. 2023, 24, 36–45. [Google Scholar] [CrossRef]
- Chan, G.C.K.; Lui, G.C.Y.; Wong, C.N.S.; Yip, S.S.T.; Li, T.C.M.; Cheung, C.S.K.; Sze, R.K.H.; Szeto, C.C.; Chow, K.M. Safety Profile and Clinical and Virological Outcomes of Nirmatrelvir-Ritonavir Treatment in Patients With Advanced Chronic Kidney Disease and Coronavirus Disease 2019. Clin. Infect. Dis. 2023, 77, 1406–1412. [Google Scholar] [CrossRef]
- Haddad, A.J.; Hachem, R.Y.; Moussa, M.; Jiang, Y.; Dagher, H.R.; Chaftari, P.; Chaftari, A.-M.; Raad, I.I. Comparing Molnupiravir to Nirmatrelvir/Ritonavir (Paxlovid) in the Treatment of Mild-to-Moderate COVID-19 in Immunocompromised Cancer Patients. Cancers 2024, 16, 1055. [Google Scholar] [CrossRef]
- Qian, G.; Wang, X.; Patel, N.J.; Kawano, Y.; Fu, X.; Cook, C.E.; Vanni, K.M.M.; Kowalski, E.N.; Banasiak, E.P.; Bade, K.J.; et al. Outcomes with and without outpatient SARS-CoV-2 treatment for patients with COVID-19 and systemic autoimmune rheumatic diseases: A retrospective cohort study. Lancet Rheumatol. 2023, 5, e139–e150. [Google Scholar] [CrossRef]
- Deo, R.; Choudhary, M.C.; Moser, C.; Ritz, J.; Daar, E.S.; Wohl, D.A.; Greninger, A.L.; Eron, J.J.; Currier, J.S.; Hughes, M.D.; et al. Symptom and Viral Rebound in Untreated SARS-CoV-2 Infection. Ann. Intern. Med. 2023, 176, 348–354. [Google Scholar] [CrossRef]
- Smith, D.M.; Li, J.Z.; Moser, C.; Yeh, E.; Currier, J.S.; Chew, K.W.; Hughes, M.D.; Team, A.-A.S. Recurrence of Symptoms Following a 2-Day Symptom Free Period in Patients With COVID-19. JAMA Netw. Open 2022, 5, e2238867. [Google Scholar] [CrossRef]
- Durstenfeld, M.S.; Peluso, M.J.; Lin, F.; Peyser, N.D.; Isasi, C.; Carton, T.W.; Henrich, T.J.; Deeks, S.G.; Olgin, J.E.; Pletcher, M.J.; et al. Association of nirmatrelvir for acute SARS-CoV-2 infection with subsequent Long COVID symptoms in an observational cohort study. J. Med. Virol. 2024, 96, e29333. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.Y.; Wang, J.T.; Chang, S.Y.; Hung, C.C.; Fang, C.T.; Cheng, A.; Liu, W.D.; Huang, Y.S.; Lin, K.Y.; Sun, H.Y.; et al. Factors associated with viral rebound among COVID-19 patients receiving oral antivirals. J. Formos. Med. Assoc. 2023, 122, 766–775. [Google Scholar] [CrossRef]
- Hay, J.A.; Kissler, S.M.; Fauver, J.R.; Mack, C.; Tai, C.G.; Samant, R.M.; Connolly, S.; Anderson, D.J.; Khullar, G.; MacKay, M.; et al. Quantifying the impact of immune history and variant on SARS-CoV-2 viral kinetics and infection rebound: A retrospective cohort study. eLife 2022, 11, e81849. [Google Scholar] [CrossRef]
- Ranganath, N.; O’Horo, J.C.; Challener, D.W.; Tulledge-Scheitel, S.M.; Pike, M.L.; O’Brien, M.; Razonable, R.R.; Shah, A. Rebound Phenomenon After Nirmatrelvir/Ritonavir Treatment of Coronavirus Disease 2019 (COVID-19) in High-Risk Persons. Clin. Infect. Dis. 2023, 76, e537–e539. [Google Scholar] [CrossRef]
- Tiseo, G.; Barbieri, C.; Galfo, V.; Occhineri, S.; Matucci, T.; Almerigogna, F.; Kalo, J.; Sponga, P.; Cesaretti, M.; Marchetti, G.; et al. Efficacy and Safety of Nirmatrelvir/Ritonavir, Molnupiravir, and Remdesivir in a Real-World Cohort of Outpatients with COVID-19 at High Risk of Progression: The PISA Outpatient Clinic Experience. Infect. Dis. Ther. 2023, 12, 257–271. [Google Scholar] [CrossRef]
- Bennett, C.L.; Magagnoli, J.; Gundabolu, K.; Georgantopoulos, P.; Lebby, A.; Watson, G.; Knopf, K.; Martin, L.; Carson, K.R.; Hrushesky, W.J.; et al. A SONAR report on Nirmatrelvir/ritonavir-associated rebound COVID-19: Using new databases for evaluating new diseases. PLoS ONE 2024, 19, e0308205. [Google Scholar] [CrossRef]
- Li, M.; Zhang, Q.S.; Liu, X.L.; Wang, H.L.; Liu, W. Adverse Events Associated with Nirmatrelvir/Ritonavir: A Pharmacovigilance Analysis Based on FAERS. Pharmaceuticals 2022, 15, 1455. [Google Scholar] [CrossRef]
| Directly Observed for 15–20 Days | Directly Observed for <15 Days | Total Cohort | |
|---|---|---|---|
| N | 80 | 75 | 155 |
| Age (years ± SD) | 75.2 ± 11.6 | 75.0 ± 12.2 | 75.1 ± 11.9 |
| Male | 74 (93%) | 73 (97%) | 147 (95%) |
| Symptom rebound assessment through Day 20 of COVID diagnosis | Multiple nursing and clinician notes per day. | Multiple nursing and clinician notes per day until discharged. | |
| Symptom rebound assessment after discharge | N/A | Clinician notes from home-based primary care, repeated clinic visits, community care notes, and secure messages. | |
| Total directly observed follow-up (Days ± SD) | 19.5 ± 1.4 | 6 ± 3.9 | 13 ± 7.4 |
| Duration of remdesivir treatment | 3.9 ± 1.5 | 3.9 ± 1.4 | 3.9 ± 1.4 |
| Start time of remdesivir treatment from symptom onset | 0.40 ± 0.6 | 0.44 ± 0.9 | 0.43 ± 0.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gupta, K.; O’Brien, W.J.; Strymish, J.; Chen, A.; Linsenmeyer, K.; Madjarov, R.; Charness, M.E. Incidence of COVID-19 Symptom Rebound After Treatment with Remdesivir. Infect. Dis. Rep. 2025, 17, 43. https://doi.org/10.3390/idr17030043
Gupta K, O’Brien WJ, Strymish J, Chen A, Linsenmeyer K, Madjarov R, Charness ME. Incidence of COVID-19 Symptom Rebound After Treatment with Remdesivir. Infectious Disease Reports. 2025; 17(3):43. https://doi.org/10.3390/idr17030043
Chicago/Turabian StyleGupta, Kalpana, William J. O’Brien, Judith Strymish, Anna Chen, Katherine Linsenmeyer, Rebecca Madjarov, and Michael E. Charness. 2025. "Incidence of COVID-19 Symptom Rebound After Treatment with Remdesivir" Infectious Disease Reports 17, no. 3: 43. https://doi.org/10.3390/idr17030043
APA StyleGupta, K., O’Brien, W. J., Strymish, J., Chen, A., Linsenmeyer, K., Madjarov, R., & Charness, M. E. (2025). Incidence of COVID-19 Symptom Rebound After Treatment with Remdesivir. Infectious Disease Reports, 17(3), 43. https://doi.org/10.3390/idr17030043


_Rachiotis.png)




