Abstract
HIV-1 resistance towards integrase inhibitors is a potential threat of the success of HIV-1 combination treatment. G118R is a rare drug resistance mutation conferring pan-integrase resistance. Here, we describe the occurrence of G118R in a HIV-1 subtype-B-positive individual with major compliance problems, detected while the patient was on dolutegravir-based cART. We speculate the pre-selection of M184I/V aided the occurrence of G118R in this case, and discuss the robustness of dolutegravir-based therapies.
1. Introduction
Dolutegravir is a second-generation integrase inhibitor (InSTI), low in side-effects and drug-to-drug interactions, and with a once-daily formulation and a high genetic barrier for drug resistance development. Further, dolutegravir in two-drug regimens has proven to be non-inferior to the traditional three drug regimens in several randomized trials [1,2,3,4], and American, as well as European, guidelines now include DTG 2DRs for both treatment-naïve and virally suppressed patients.
The high genetic barrier to resistance for dolutegravir is partly explained by the requirement of more than one mutation in the integrase gene to reduce drug sensitivity. As an example, the Q148H/R/K alone does not reduce dolutegravir susceptibility notably. However, in combination with G140S and E138K, susceptibility is reduced 10–20-fold. Unlike the Q148H/R/K pathway, the G118R mutation alone confers high-level resistance, and is associated with a significant reduction of HIV replication capacity [5,6,7]. G118R is rarely observed; in a study of 1100 patients failing a INSTI-based regimen in France, it was not reported [8]. It has only been reported in a handful of cases failing dolutegravir [9,10,11,12,13,14,15], mostly non-B subtypes. At least in two of the cases, the selection was facilitated by the GGA natural polymorphism at codon 118 (1.5% prevalence) requiring only one mutation to change from GGA (G) to AGA (R). However, most clinical isolates harbor the GGC polymorphism at codon 118 (79% of all strains, and 91.8% of all subtype B), which requires two mutations, GGC to AGA, to change from G to R.
2. The Case Report
Here, we report the selection of pan-integrase resistance mutation G118R in a treatment-experienced 50-year-old male with a history of major compliance problems. The patient was infected with HIV-1 subtype B through blood products in the 1980s. He had been exposed to nucleotide-based therapies in the 1990s, and had previously failed NNRTI, protease, and first generation INSTI-based regimens, rendering the M184V mutation in the reverse-transcriptase gene. The patient failed Triumeq (abacavir, lamivudine, and dolutegravir) following years of non-compliance and documented viral loads of around 200 copies/mL on several occasions. In August 2020, the occurrence of the G118R mutation was documented, conferring pan-integrase resistance. Hereafter, cART was changed to Symtuza (cobicistat, darunavir emtricitabine, and TAF). The patient gained virus control. However, with the accumulated drug resistance, future therapeutic options are currently very limited. All genotypic drug resistance test were performed on viral RNA extracted from plasma. Sequences were obtained by standard RT-PCR targeting the integrase coding region of HIV-1, followed by Sanger sequencing [16], or targeting the region containing the protease and reverse transcriptase encoding genes using either the Viroseq kit as recommended by the manufacturer, or by using an in-house RT-PCR assay. GenBank accessions numbers (ON653600-03).
Treatment history and HIV status are shown in Figure 1. Figure 2 illustrates the evolution at site 118 of the integrase gene. Table 1 shows the three genotypic drug resistance tests performed during the course of antiretroviral treatment for this patient.
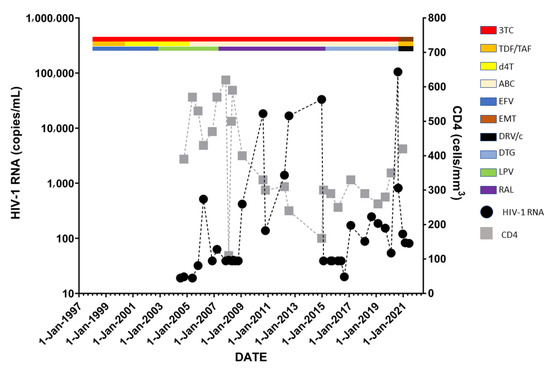
Figure 1.
Treatment history and HIV status with HIV-1 RNA in copies/mL and CD4 cell counts in cells/mm3.

Figure 2.
The evolution at site 118 of the integrase gene (highlighted in yellow) at nucleoside and amino acid level GGC (G) to AGA (R).

Table 1.
Results of genotypic drug resistance tests over the course of treatment.
3. Discussion
Our case highlights the risk of developing pan-integrase resistance in patients with non-complacence and non-optimal NRTI backbones. The M184V/I is one of the most common NRTI-associated mutations in HIV-1 infected patients, and is typically selected in patients failing lamivudine (3TC) or emtricitabine (FTC). The presence of M184V/I reduces the susceptibility to these drugs by more than 100-fold, and additionally causes impaired efficacy to abacavir (ABC). Although a large study from five European cohorts did not find excess viral failure upon switching to Triumeq (abacavir/lamivudin/dolutegravir) in patients with documented M184V/I mutation [17], such studies may be biased by a relative low observation time (288 days), and biased towards switching highly compliant patients to a single tablet regimen. We speculate that the pre-selection of M184V may have facilitated the development of G118R in this case. A very similar case has been reported [18]. In line with these considerations, the TANGO trial [19] found the combination of M184V/I and at least one TAM and previous failure on an INSTI-based regimen to predict failure upon switching to lamivudine and dolutegravir, highlighting the importance of an optimal NRTI backbone for virologic success. Although the overall finding from the NADIA trial [15] was noninferiority between dolutegravir and ritonavir boosted darunavir (darunavir/r) in combination with either tenofovir or zidovudine as second-line treatment, four patients failing dolutegravir had confirmed major drug resistance mutations versus none failing darunavir/r. Two failed with the G118R mutation, suggesting darunavir/r may be safer long-term in the setting of NRTI resistance and adherence problems. However, data beyond 48-weeks are needed to confirm this. Thus, drug history and previous drug resistance tests should be carefully revised before changing antiretroviral treatment to a dolutegravir-based regimen.
Author Contributions
Conceptualization, J.G. and H.M.; methodology, writing—original draft preparation, H.M.; writing—review and editing, H.M., J.G., L.F. and J.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
No appliable.
Informed Consent Statement
Written informed consent has been obtained from the patient to publish this paper.
Data Availability Statement
HIV-1 sequences have been submitted to GenBank accessions numbers ON653600-03.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cahn, P.; Madero, J.S.; Arribas, J.R.; Antinori, A.; Ortiz, R.; Clarke, A.E.; Hung, C.C.; Rockstroh, J.K.; Girard, P.M.; Sievers, J.; et al. Dolutegravir plus lamivudine versus dolutegravir plus tenofovir disoproxil fumarate and emtricitabine in antiretroviral-naive adults with HIV-1 infection (GEMINI-1 and GEMINI-2): Week 48 results from two multicentre, double-blind, randomised, non-inferiority, phase 3 trials. Lancet 2019, 393, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Cahn, P.; Andrade-Villanueva, J.; Arribas, J.R.; Gatell, J.M.; Lama, J.R.; Norton, M.; Patterson, P.; Sierra Madero, J.; Sued, O.; Figueroa, M.I.; et al. Dual therapy with lopinavir and ritonavir plus lamivudine versus triple therapy with lopinavir and ritonavir plus two nucleoside reverse transcriptase inhibitors in antiretroviral-therapy-naive adults with HIV-1 infection: 48 week results of the randomised, open label, non-inferiority GARDEL trial. Lancet Infect. Dis. 2014, 14, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Cahn, P.; Rolon, M.J.; Figueroa, M.I.; Gun, A.; Patterson, P.; Sued, O. Dolutegravir-lamivudine as initial therapy in HIV-1 infected, ARV-naive patients, 48-week results of the PADDLE (Pilot Antiretroviral Design with Dolutegravir LamivudinE) study. J. Int. AIDS Soc. 2017, 20, 21678. [Google Scholar] [CrossRef] [PubMed]
- Taiwo, B.O.; Zheng, L.; Stefanescu, A.; Nyaku, A.; Bezins, B.; Wallis, C.L.; Godfrey, C.; Sax, P.E.; Acosta, E.; Haas, D.; et al. ACTG A5353: A Pilot Study of Dolutegravir Plus Lamivudine for Initial Treatment of Human Immunodeficiency Virus-1 (HIV-1)-infected Participants with HIV-1 RNA <500,000 Copies/mL. Clin. Infect. Dis. 2018, 66, 1689–1697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quashie, P.K.; Mesplede, T.; Han, Y.S.; Oliveira, M.; Singhroy, D.N.; Fujiwara, T.; Underwood, M.R.; Wainberg, M.A. Characterization of the R263K mutation in HIV-1 integrase that confers low-level resistance to the second-generation integrase strand transfer inhibitor dolutegravir. J. Virol. 2012, 86, 2696–2705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quashie, P.K.; Mesplede, T.; Han, Y.S.; Veres, T.; Osman, N.; Hassounah, S.; Sloan, R.D.; Xu, H.T.; Wainberg, M.A. Biochemical analysis of the role of G118R-linked dolutegravir drug resistance substitutions in HIV-1 integrase. Antimicrob. Agents Chemother. 2013, 57, 6223–6235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quashie, P.K.; Oliviera, M.; Veres, T.; Osman, N.; Han, Y.S.; Hassounah, S.; Lie, Y.; Huang, W.; Mesplede, T.; Wainberg, M.A. Differential effects of the G118R, H51Y, and E138K resistance substitutions in different subtypes of HIV integrase. J. Virol. 2015, 89, 3163–3175. [Google Scholar] [CrossRef] [Green Version]
- Marcelin, A.G.; Charpentier, C.; Bellecave, P.; Abdi, B.; Chaix, M.L.; Ferre, V.; Raymond, S.; Fofana, D.; Bocket, L.; Mirand, A.; et al. Factors associated with the emergence of integrase resistance mutations in patients failing dual or triple integrase inhibitor-based regimens in a French national survey. J. Antimicrob. Chemother. 2021, 76, 2400–2406. [Google Scholar] [CrossRef] [PubMed]
- Brenner, B.G.; Thomas, R.; Blanco, J.L.; Ibanescu, R.I.; Oliveira, M.; Mesplede, T.; Golubkov, O.; Roger, M.; Garcia, F.; Martinez, E.; et al. Development of a G118R mutation in HIV-1 integrase following a switch to dolutegravir monotherapy leading to cross-resistance to integrase inhibitors. J. Antimicrob. Chemother. 2016, 71, 1948–1953. [Google Scholar] [CrossRef] [PubMed]
- Blanco, J.L.; Marcelin, A.G.; Katlama, C.; Martinez, E. Dolutegravir resistance mutations: Lessons from monotherapy studies. Curr. Opin. Infect. Dis. 2018, 31, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Blanco, J.L.; Oldenbuettel, C.; Thomas, R.; Mallolas, J.; Wolf, E.; Brenner, B.; Spinner, C.D.; Wainberg, M.A.; Martinez, E. Pathways of resistance in subjects failing dolutegravir monotherapy. In Proceedings of the Twenty-Fourth Conference on Retroviruses and Opportunistic Infections, Seatle, WA, USA, 13–16 February 2017. [Google Scholar]
- Vavro, C.; Ruel, T.; Wiznia, A.; Montañez, N.; Nangle, K.; Horton, J.; Buchanan, A.M.; Stewart, E.L.; Palumbo, P. Emergence of resistance in HIV-1 Integrase (IN) following dolutegravir (DTG) treatment in 6 to 18 year old participants enrolled in the P1093 study (abstract THPEB114). Antimicrob. Agents Chemother. 2021, 66, e01645-21. [Google Scholar]
- Wang, R.; Horton, J.; Hopking, J.; King, K.; Smith, K.Y.; Aboud, M.; Wynne, B.; Sievers, J.; Underwood, M. Resistance through week 48 in the DAWNING study comparing dolutegravir (DTG) plus 2 nucleoside RT inhibitors (NRTIs) compared with lopinavir/ritonavir (LPV/r) plus 2 NRTIs in second-line treatment (Abstract THPEB071). In Proceedings of the 22nd International AIDS Conference, Amsterdam, The Netherlands, 23–27 July 2018. [Google Scholar]
- Marcelin, A.G.; Grude, M.; Charpentier, C.; Bellecave, P.; Le Guen, L.; Pallier, C.; Raymond, S.; Mirand, A.; Bocket, L.; Fofana, D.B.; et al. Resistance to integrase inhibitors: A national study in HIV-1-infected treatment-naive and -experienced patients. J. Antimicrob. Chemother. 2019, 74, 1368–1375. [Google Scholar] [CrossRef] [PubMed]
- Paton, N.I.; Musaazi, J.; Kityo, C.; Walimbwa, S.; Hoppe, A.; Balyegisawa, A.; Kaimal, A.; Mirembe, G.; Tukamushabe, P.; Ategeka, G.; et al. Dolutegravir or Darunavir in Combination with Zidovudine or Tenofovir to Treat HIV. N. Engl. J. Med. 2021, 385, 330–341. [Google Scholar] [CrossRef] [PubMed]
- Fonager, J.; Larsson, J.T.; Hussing, C.; Neess Engsig, F.; Nielsen, C.; Fischer, T.K. Identification of minority resistance mutations in the HIV-1 integrase coding region using next generation sequencing. J. Clin. Virol. 2015, 73, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Olearo, F.; Nguyen, H.; Bonnet, F.; Yerly, S.; Wandeler, G.; Stoeckle, M.; Cavassini, M.; Scherrer, A.; Costagiola, D.; Schmid, P.; et al. Impact of the M184V/I Mutation on the Efficacy of Abacavir/Lamivudine/Dolutegravir Therapy in HIV Treatment-Experienced Patients. Open Forum Infect. Dis. 2019, 6, ofz330. [Google Scholar] [CrossRef] [PubMed]
- Braun, D.L.; Scheier, T.; Ledermann, U.; Flepp, M.; Metzner, K.J.; Boni, J.; Gunthard, H.F. Emergence of Resistance to Integrase Strand Transfer Inhibitors during Dolutegravir Containing Triple-Therapy in a Treatment-Experienced Patient with Pre-Existing M184V/I Mutation. Viruses 2020, 12, 1330. [Google Scholar] [CrossRef] [PubMed]
- Borghetti, A.; Giacomelli, A.; Borghi, V.; Ciccullo, A.; Dusina, A.; Fabbiani, M.; Rusconi, S.; Zazzi, M.; Mussini, C.; Di Giambenedetto, S. Nucleoside Reverse-Transcriptase Inhibitor Resistance Mutations Predict Virological Failure in Human Immunodeficiency Virus-Positive Patients during Lamivudine Plus Dolutegravir Maintenance Therapy in Clinical Practice. Open Forum Infect. Dis. 2021, 8, ofab103. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).