The New Horizon for Non-Metastatic dMMR Colorectal Cancer: A Systematic Review of the Adjuvant Chemoimmunotherapy and Neoadjuvant Immunotherapy Revolution
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Study Selection and Data Extraction
2.3. Protocol Registration
2.4. Data Synthesis
3. Results
3.1. Study Selection
3.2. Overview of Included Studies and Synthesized Findings
4. Discussion
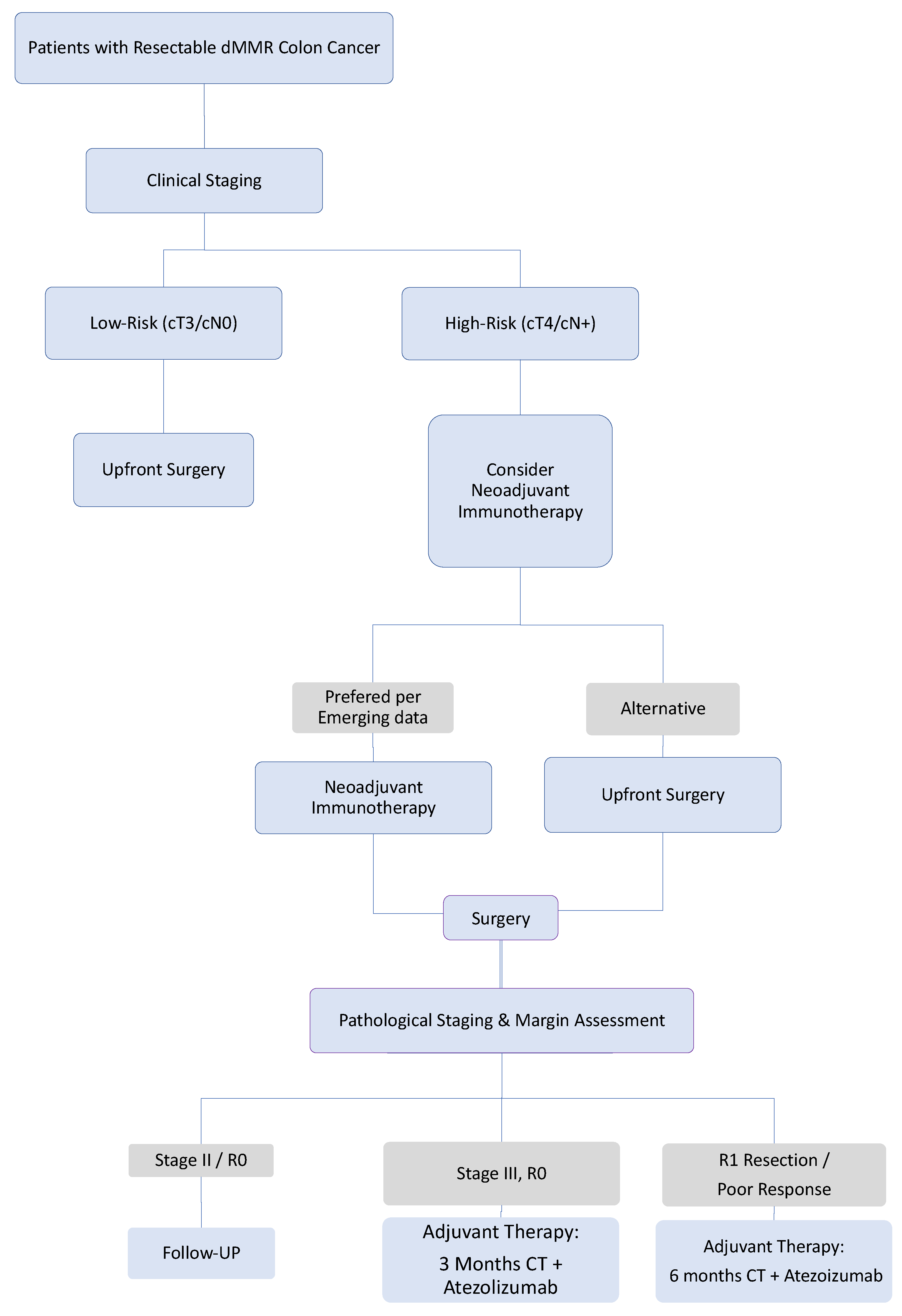
4.1. The Landmark ATOMIC Trial: Establishing a New Standard with Adjuvant Chemoimmunotherapy
4.2. The Paradigm Shift: Neoadjuvant Immunotherapy as a Potentially Transformative Strategy
4.3. Immunotherapy Alone in dMMR Stage III CRC
4.4. Limitations of the Current Evidence
4.5. Implications for Clinical Practice and Future Research
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviation | Explanation |
| 5-FU | 5-fluorouracil |
| ASCO | American Society of Clinical Oncology |
| ATOMIC | Adjuvant Trial of MImmunotherapy in Colon cancer |
| CAPEOX | Capecitabine and Oxaliplatin |
| CI | Confidence Interval |
| CRC | Colorectal Cancer |
| CR | Complete Response |
| CT | Chemotherapy |
| dMMR | Mismatch Repair-deficient |
| DFS | Disease-Free Survival |
| ESMO | European Society for Medical Oncology |
| FOLFOX | 5-Fluorouracil, Leucovorin, and Oxaliplatin |
| HR | Hazard Ratio |
| IDEA | International Duration Evaluation of Adjuvant Chemotherapy |
| LV5FU2 | Leucovorin and 5-Fluorouracil |
| MMR | Mismatch Repair |
| MPR | Major Pathological Response |
| MSI-H | Microsatellite Instability-High |
| MSS | Microsatellite Stable |
| NSCLC | Non-Small-Cell Lung Cancer |
| OS | Overall Survival |
| pCR | Pathological Complete Response |
| PD-1 | Programmed cell death protein 1 |
| PD-L1 | Programmed death-ligand 1 |
| PFS | Progression-Free Survival |
| pMMR | Proficient Mismatch Repair |
| PN | Peripheral Neuropathy |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| R0 | Complete Resection |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Moertel, C.G.; Fleming, T.R.; Macdonald, J.S.; Haller, D.G.; Laurie, J.A.; Goodman, P.J.; Ungerleider, J.S.; Emerson, W.A.; Tormey, D.C.; Glick, J.H.; et al. Levamisole and fluorouracil for adjuvant therapy of resected colon carcinoma. N. Engl. J. Med. 1990, 322, 352–358. [Google Scholar] [CrossRef]
- André, T.; Boni, C.; Mounedji-Boudiaf, L.; Navarro, M.; Tabernero, J.; Hickish, T.; Topham, C.; Zaninelli, M.; Clingan, P.; Bridgewater, J.; et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N. Engl. J. Med. 2004, 350, 2343–2351. [Google Scholar] [CrossRef] [PubMed]
- Grothey, A.; Sobrero, A.F.; Shields, A.F.; Yoshino, T.; Paul, J.; Taieb, J.; Souglakos, J.; Shi, Q.; Kerr, R.; Labianca, R.; et al. Duration of Adjuvant Chemotherapy for Stage III Colon Cancer. N. Engl. J. Med. 2018, 378, 1177–1188. [Google Scholar] [CrossRef]
- Johannet, P.; Rousseau, B.; Aghajanian, C.; Foote, M.B.; Diaz, L.A. Therapeutic targeting of mismatch repair-deficient cancers. Nat. Rev. Clin. Oncol. 2025, 22, 734–759. [Google Scholar] [CrossRef]
- André, T.; Shiu, K.K.; Kim, T.W.; Jensen, B.V.; Jensen, L.H.; Punt, C.J.A.; Smith, D.; Garcia-Carbonero, R.; Alcaide-Garcia, J.; Gibbs, P.; et al. Pembrolizumab versus chemotherapy in microsatellite instability-high or mismatch repair-deficient metastatic colorectal cancer: 5-year follow-up from the randomized phase III KEYNOTE-177 study. Ann. Oncol. 2025, 36, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Andre, T.; Elez, E.; Van Cutsem, E.; Jensen, L.H.; Bennouna, J.; Mendez, G.; Schenker, M.; de la Fouchardiere, C.; Limon, M.L.; Yoshino, T.; et al. Nivolumab plus Ipilimumab in Microsatellite-Instability-High Metastatic Colorectal Cancer. N. Engl. J. Med. 2024, 391, 2014–2026. [Google Scholar] [CrossRef]
- Sinicrope, F.A.; Ou, F.S.; Arnold, D.; Peters, W.; Behrens, R.J.; Lieu, C.H.; Matin, K.; Cohen, D.J.; Potter, S.L.; Frankel, W.L.; et al. Randomized trial of standard chemotherapy alone or combined with atezolizumab as adjuvant therapy for patients with stage III deficient DNA mismatch repair (dMMR) colon cancer (Alliance A021502; ATOMIC). J. Clin. Oncol. 2025, 43, LBA1. [Google Scholar] [CrossRef]
- Chalabi, M.; Verschoor, Y.L.; Tan, P.B.; Balduzzi, S.; Van Lent, A.U.; Grootscholten, C.; Dokter, S.; Büller, N.V.; Grotenhuis, B.A.; Kuhlmann, K.; et al. Neoadjuvant Immunotherapy in Locally Advanced Mismatch Repair–Deficient Colon Cancer. N. Engl. J. Med. 2024, 390, 1949–1958. [Google Scholar] [CrossRef]
- Wang, F.; Chen, G.; Qiu, M.; Ma, J.; Mo, X.; Liu, H.; Li, Y.; Ding, P.; Wan, X.; Hu, Y.; et al. Neoadjuvant Treatment of IBI310 Plus Sintilimab in Locally Advanced MSI-H/dMMR Colon Cancer: A Randomized Phase 1b Study. Cancer Cell 2025, 43, 1958–1967. [Google Scholar] [CrossRef]
- de Gooyer, P.G.; Verschoor, Y.L.; van den Dungen, L.D.; Balduzzi, S.; Marsman, H.A.; Geukes Foppen, M.H.; Grootscholten, C.; Dokter, S.; den Hartog, A.G.; Verbeek, W.H.; et al. Neoadjuvant nivolumab and relatlimab in locally advanced MMR-deficient colon cancer: A phase 2 trial. Nat. Med. 2024, 30, 3284–3290. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Kang, L.; Zhang, J.; Wu, Z.; Wang, H.; Huang, M.; Lan, P.; Wu, X.; Wang, C.; Cao, W.; et al. Neoadjuvant PD-1 blockade with toripalimab, with or without celecoxib, in mismatch repair-deficient or microsatellite instability-high, locally advanced, colorectal cancer (PICC): A single-centre, parallel-group, non-comparative, randomised, phase 2 trial. Lancet Gastroenterol. Hepatol. 2022, 7, 38–48. [Google Scholar] [CrossRef]
- Shiu, K.K.; Jiang, Y.; Saunders, M.; Seligmann, J.F.; Iveson, T.; Wilson, R.H.; Graham, J.S.; Khan, K.H.; Militello, A.M.; Irvine, S.; et al. NEOPRISM-CRC: Neoadjuvant pembrolizumab stratified to tumour mutation burden for high-risk stage 2 or stage 3 deficient-MMR/MSI-high colorectal cancer. J. Clin. Oncol. 2024, 42, 3500. [Google Scholar] [CrossRef]
- de la Fouchardiere, C.; Zaanan, A.; Cohen, R.; Le Sourd, S.M.; Tougeron, D.; Soularue, E.; Dubreuil, O.; Williet, N.; Samalin-Scalzi, E.; Piessen, G.; et al. IMHOTEP phase II trial of neoadjuvant pembrolizumab in dMMR/MSI localized cancers: Results of the digestive non-colorectal cancer cohorts. Ann. Oncol. 2024, 35, S899–S900. [Google Scholar] [CrossRef]
- Cercek, A.; Foote, M.B.; Rousseau, B.; Smith, J.J.; Shia, J.; Sinopoli, J.; Weiss, J.; Lumish, M.; Temple, L.; Patel, M.; et al. Nonoperative Management of Mismatch Repair–Deficient Tumors. N. Engl. J. Med. 2025, 392, 2297–2308. [Google Scholar] [CrossRef]
- Sargent, D.J.; Marsoni, S.; Monges, G.; Thibodeau, S.N.; Labianca, R.; Hamilton, S.R.; French, A.J.; Kabat, B.; Foster, N.R.; Torri, V.; et al. Defective mismatch repair as a predictive marker for lack of efficacy of fluorouracil-based adjuvant therapy in colon cancer. J. Clin. Oncol. 2010, 28, 3219–3226. [Google Scholar] [CrossRef]
- Forde, P.M.; Spicer, J.D.; Provencio, M.; Mitsudomi, T.; Awad, M.M.; Wang, C.; Lu, S.; Felip, E.; Swanson, S.J.; Brahmer, J.R.; et al. Overall Survival with Neoadjuvant Nivolumab plus Chemotherapy in Lung Cancer. N. Engl. J. Med. 2025, 392, 2047–2058. [Google Scholar] [CrossRef] [PubMed]
- Blank, C.U.; Lucas, M.W.; Scolyer, R.A.; Van De Wiel, B.A.; Menzies, A.M.; Lopez-Yurda, M.; Hoeijmakers, L.L.; Saw, R.P.; Lijnsvelt, J.M.; Maher, N.G.; et al. Neoadjuvant Nivolumab and Ipilimumab in Resectable Stage III Melanoma. N. Engl. J. Med. 2024, 391, 1696–1708. [Google Scholar] [CrossRef]
- Chalabi, M.; Fanchi, L.F.; Dijkstra, K.K.; Van den Berg, J.G.; Aalbers, A.G.; Sikorska, K.; Lopez-Yurda, M.; Grootscholten, C.; Beets, G.L.; Snaebjornsson, P.; et al. Neoadjuvant immunotherapy leads to pathological responses in MMR-proficient and MMR-deficient early-stage colon cancers. Nat Med. 2020, 26, 566–576. [Google Scholar] [CrossRef] [PubMed]
- Forde, P.M.; Spicer, J.; Lu, S.; Provencio, M.; Mitsudomi, T.; Awad, M.M.; Felip, E.; Broderick, S.R.; Brahmer, J.R.; Swanson, S.J.; et al. Neoadjuvant Nivolumab plus Chemotherapy in Resectable Lung Cancer. N. Engl. J. Med. 2022, 386, 1973–1985. [Google Scholar] [CrossRef]
- Liu, J.; Blake, S.J.; Yong, M.C.R.; Harjunpää, H.; Ngiow, S.F.; Takeda, K.; Young, A.; O’Donnell, J.S.; Allen, S.; Smyth, M.J.; et al. Improved Efficacy of Neoadjuvant Compared to Adjuvant Immune Checkpoint Blockade in Non-Small Cell Lung Cancer. Cancer Cell 2021, 39, 593–595. [Google Scholar] [CrossRef]
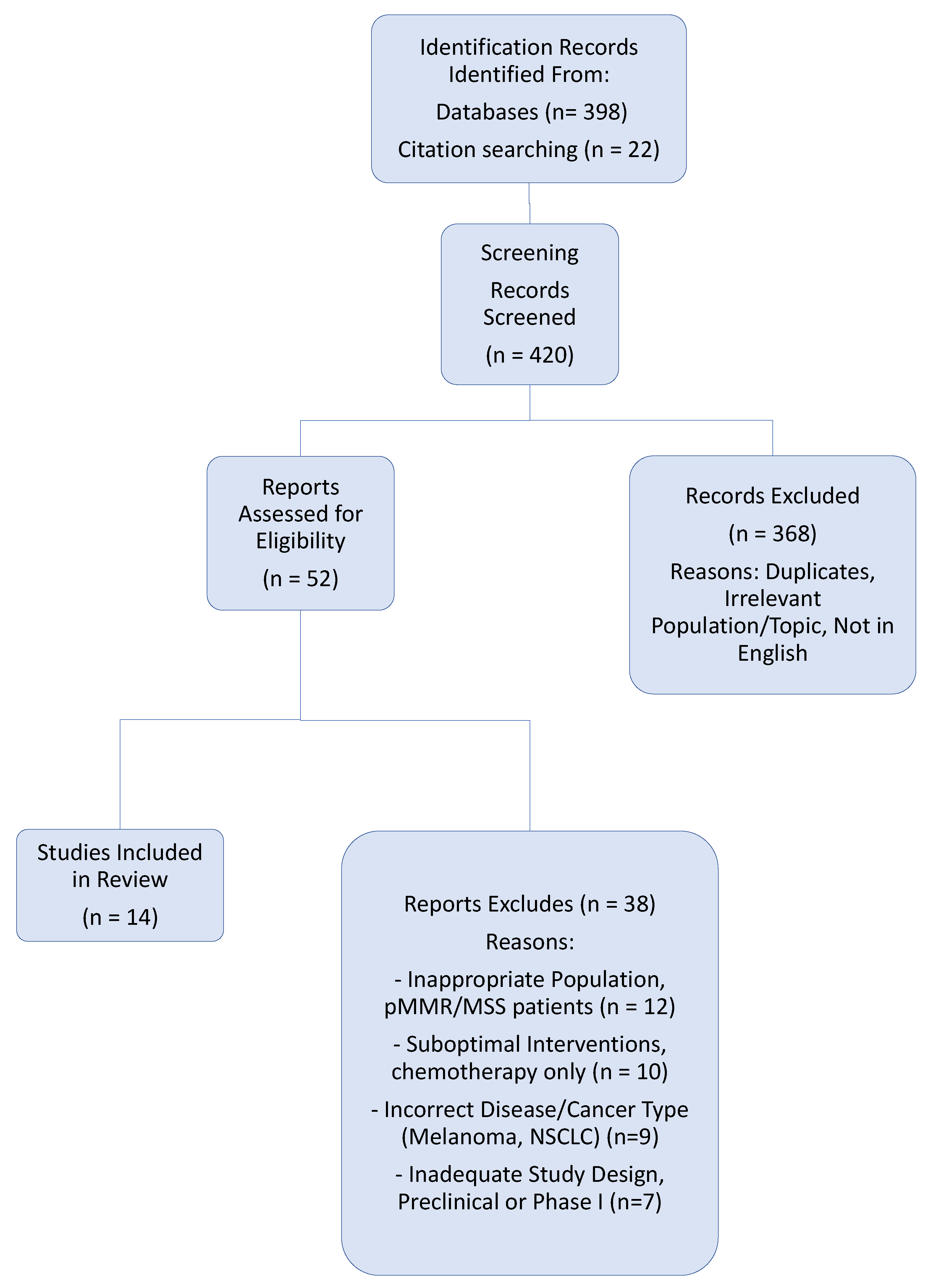
| Trial (Phase) | Year of Publication | Patient Population | Investigation vs. Comparator | 3-Year DFS | Key Grade 3/4 Adverse Events |
|---|---|---|---|---|---|
| Moertel et al. (III) [2] | 1990 | Resected stage III CRC (n = 1296) | Fluorouracil + levamisole vs. Observation | 65% vs. 50% | Nausea (5%), vomiting (2%), diarrhea (7%), stomatitis (3%), dermatitis (1%), leukopenia (3%) |
| MOSAIC (III) [3] | 2004 | Resected stage II/III colon cancer (n = 2246); Included dMMR (n = 95) | FOLFOX vs. LV5FU2 | 78.2% vs. 72.9% (HR 0.76) | Sensory Neuropathy: 12.4% (FOLFOX4) vs. 0% (LV5FU2) |
| IDEA (Meta-Analysis) [4] | 2018 | Resected low-risk stage III CRC | 3 months (CAPEOX/FOLFOX) vs. 6 months (CAPEOX/FOLFOX) | 75.9% vs. 74.8% (HR 1.07) | Sensory Neuropathy: 24.2% (3-mo) vs. 36.9% (6-mo) |
| Resected high-risk stage III CRC | 3 months (CAPEOX/FOLFOX) vs. 6 months (CAPEOX/FOLFOX) | 62.7% vs. 64.4% (HR 1) | |||
| ATOMIC (III) [8] | 2025 | R0-resected stage III dMMR CRC (n = 712) | Atezolizumab + mFOLFOX6, then Atezolizumab vs. mFOLFOX6 | 86.4% vs. 76.6% (HR 0.50) | Higher rates of PN (19% vs. 15%), hypothyroidism (20.5% vs. 0%), diarrhea (60.1% vs. 12.1%) neutropenia (43% vs. 36%) (Atezo arm) |
| # | Reference/Study Status (Trial Name) | Study Design | Study Population (n) | Intervention | Key Outcomes (DFS, pCR) |
|---|---|---|---|---|---|
| 1 | Sinicrope et al. ASCO 2025 (ATOMIC) [8] | Randomized Phase 3 Trial | Resected stage III dMMR CRC, R0 resected (n = 712) | mFOLFOX6 + Atezolizumab vs. mFOLFOX6 (adjuvant) | 3-year DFS: 86.4% vs. 76.6% (HR 0.50). Potential new standard. |
| 2 | Chalabi et al. NEJM 2024 (NICHE-2) [9] | Phase 2 Trial | Locally advanced dMMR CRC (n = 115) | Nivolumab + Ipilimumab (neoadjuvant, 2 cycles) | pCR: 68%; Major Pathological Response (MPR): 95%; 3-year DFS: 100%. |
| 3 | Xu et al., 2024 [10] | Phase 1b Trial | Early MSI-H/dMMR CRC | IBI310 (anti-CTLA-4) + Sintilimab (anti-PD-1) (neoadjuvant) | Promising pCR rates. |
| 4 | de Gooyer et al. Nat Med 2024 (NICHE-3) [11] | Phase 2 Trial | Locally advanced dMMR CRC (n = 59) | Nivolumab + Relatlimab (neoadjuvant, 4 cycles) | pCR: 68%; MPR: 92%. |
| 5 | Hu et al. Lancet Gastro Hep 2022 (PICC) [12] | Randomized Phase 2 Trial | Locally advanced dMMR CRC (n = 28) | Toripalimab + Celecoxib (neoadjuvant) vs. Toripalimab | pCR: 88% (combo) vs. 65% (mono). |
| 6 | Shiu et al., 2024 (NEOPRISM-CRC) [13] | Phase 2 Trial | Stage II–III dMMR CRC (n = 32) | Pembrolizumab (neoadjuvant, 3 cycles) | pCR: 53% |
| 7 | de la Fouchardière et al. Ann Oncol 2024 (IMHOTEP) [14] | Phase 2 Trial | CRC Cohort: Localized dMMR CRC cancer (n = 87) | Pembrolizumab (neoadjuvant, 1–2 cycles) | pCR: 53.8% |
| 8 | Cercek et al. NEJM 2025 (Cohort-1) [15] | Phase 2 Trial | Locally Advanced dMMR Rectal Cancer (n = 50) | Dostarlimab (neoadjuvant, 6 months) | pCR: 74%; 2-year Event-Free Survival: 92%. |
| 9 | André et al. NEJM 2004 (MOSAIC) [3] | Randomized Phase 3 Trial | Resected stage II/III CRC (n = 2246); Included dMMR (n = 95) | FOLFOX4 vs. LV5FU2 (adjuvant) | Established FOLFOX as adjuvant standard; less benefit in dMMR. |
| 10 | Grothey et al. NEJM 2018 (IDEA) [4] | Meta-Analysis | Resected stage III CRC | 3 months vs. 6 months of (CAPEOX/FOLFOX) (adjuvant) | Non-inferiority of 3 months for low-risk disease; less neuropathy. |
| 11 | Moertel et al. NEJM 1990 [2] | Randomized Phase 3 Trial | Resected stage III CRC (n = 1296) | 5-FU + Levamisole vs. Observation (adjuvant) | Foundation of adjuvant chemotherapy; 33% reduction in death risk. |
| 12 | Sargent et al. JCO 2010 [16] | Pooled Analysis | Resected stage II/III CRC | 5-FU-based adjuvant therapy (adjuvant) | dMMR is a predictive marker for lack of benefit from 5-FU. |
| 13 | André et al. Ann Oncol 2025 (KEYNOTE-177) [6] | Randomized Phase 3 Trial | Metastatic MSI-H/dMMR CRC | Pembrolizumab vs. Chemotherapy | Pivotal proof of efficacy of immunotherapy in metastatic dMMR CRC. |
| 14 | Andre et al. NEJM 2024 (CheckMate 8HW) [7] | Randomized Phase 3 Trial | Metastatic MSI-H/dMMR CRC | Nivolumab plus Ipilimumab vs. Chemotherapy | - |
| Study (Tumor Location) | Patient Population | Neoadjuvant Immunotherapy Regimen | pCR | Other Efficacy | Grade ≥ 3 Adverse Events |
|---|---|---|---|---|---|
| NICHE-2 [9] (Colon) | Stage cT3-4/cNany dMMR CRC (n = 115) | Nivolumab + ipilimumab (2 cycles) | 68% | MPR: 95%; 3-year DFS: 100% | 4% |
| NICHE-3 [11] (Colon) | Stage cT2-4/cNany dMMR CRC (n = 59) | Nivolumab + Relatlimab (4 cycles) | 68% | MPR: 92% | Not Specified (Mostly G1-2) |
| Hu et al. [12] (Colon/Rectum) | Stage cT3 4/cN+ dMMR CRC | Toripalimab + Celecoxib (6 cycles) | 88% | - | Not Specified (59% G1-2) |
| Toripalimab (6 cycles) | 65% | - | Not Specified | ||
| NEOPRISM-CRC (Colon/Rectum) [13] | Stage II–III dMMR CRC (n = 32) | Pembrolizumab (3 cycles) | 53% | - | 0% |
| IMOTHEP [14] (Colon/Rectum) | CRC cohort: Stage cT2-4/cNany dMMR CRC (n = 87) | Pembrolizumab (1–2 cycles) | 53.8% | - | 9% |
| Cercek et al. [15] (Rectum) | Locally Advanced dMMR Rectal Cancer (n = 50) | Dostarlimab (6 months) | 74% | 2-yr DFS: 92% | 5% (Mostly G1-2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ismaili, N. The New Horizon for Non-Metastatic dMMR Colorectal Cancer: A Systematic Review of the Adjuvant Chemoimmunotherapy and Neoadjuvant Immunotherapy Revolution. Gastroenterol. Insights 2025, 16, 43. https://doi.org/10.3390/gastroent16040043
Ismaili N. The New Horizon for Non-Metastatic dMMR Colorectal Cancer: A Systematic Review of the Adjuvant Chemoimmunotherapy and Neoadjuvant Immunotherapy Revolution. Gastroenterology Insights. 2025; 16(4):43. https://doi.org/10.3390/gastroent16040043
Chicago/Turabian StyleIsmaili, Nabil. 2025. "The New Horizon for Non-Metastatic dMMR Colorectal Cancer: A Systematic Review of the Adjuvant Chemoimmunotherapy and Neoadjuvant Immunotherapy Revolution" Gastroenterology Insights 16, no. 4: 43. https://doi.org/10.3390/gastroent16040043
APA StyleIsmaili, N. (2025). The New Horizon for Non-Metastatic dMMR Colorectal Cancer: A Systematic Review of the Adjuvant Chemoimmunotherapy and Neoadjuvant Immunotherapy Revolution. Gastroenterology Insights, 16(4), 43. https://doi.org/10.3390/gastroent16040043






