Influence of Aspirin on Hospital and Clinical Outcomes in Hepatocellular Carcinoma: Insights from National Data
Abstract
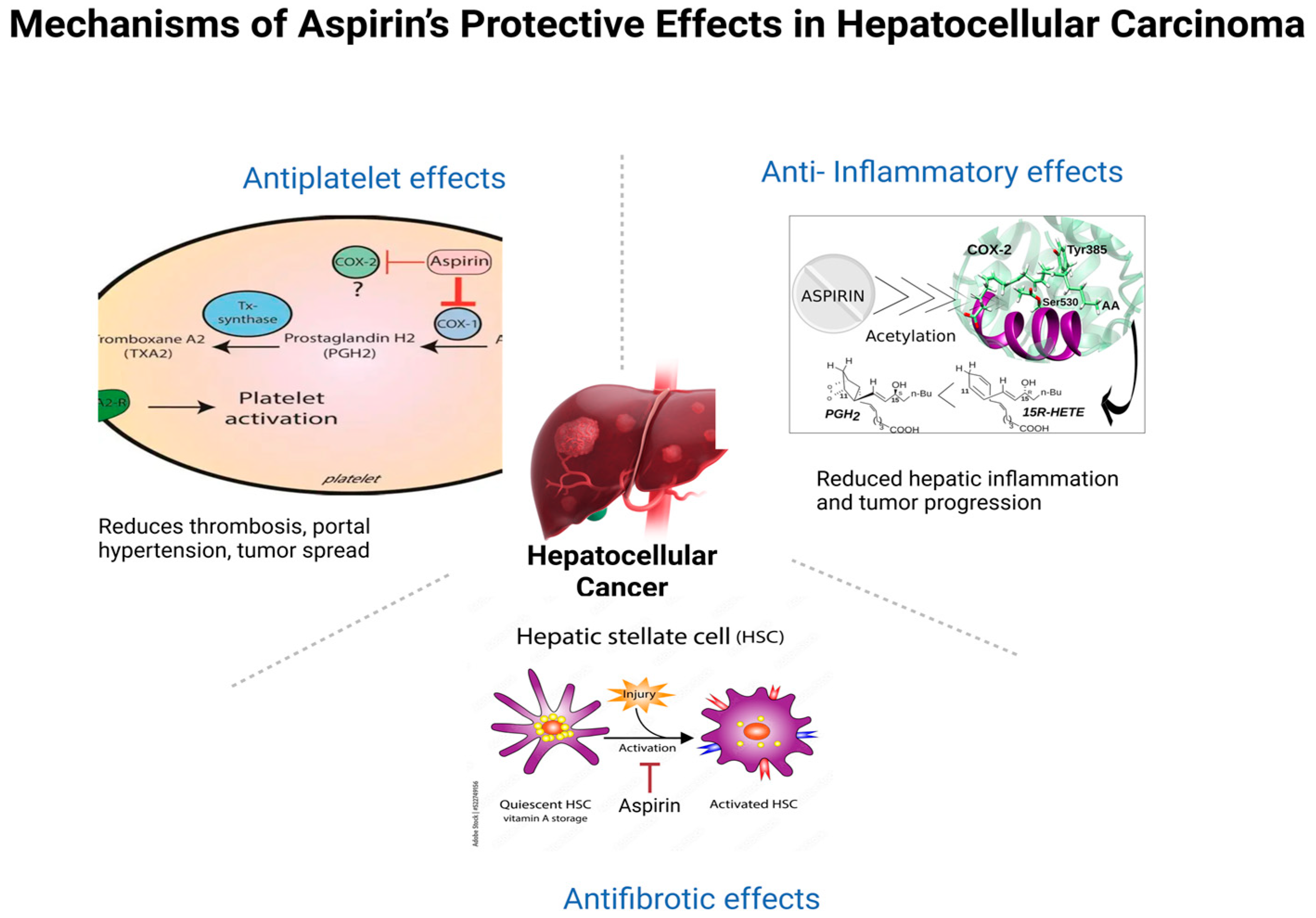
1. Introduction
2. Materials and Methods
2.1. Database
2.2. Study Population and Variables
2.3. Sensitivity Analysis
2.4. Statistical Analysis
3. Results
3.1. Patient and Hospital Characteristics
3.2. In Hospital Mortality and Morbidity Outcomes
4. Discussion
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HCC | Hepatocellular carcinoma |
| HBV | Hepatitis B virus |
| HCV | Hepatitis C virus |
| NAFLD | Non-alcoholic fatty liver disease |
| NASH | Non-alcoholic steatohepatitis |
| COX-2 | Cyclooxygenase-2 |
| ICD-10-CM | International Classification of Diseases, 10th Revision, Clinical Modification |
| IPTW | Inverse probability of treatment weighting |
| NIS | National Inpatient Sample |
| aOR | Adjusted odds ratio |
| GI | Gastrointestinal |
| ICU | Intensive care unit |
| HR | Hazard ratio |
| sHR | Subhazard ratio |
| AHRQ | Agency for Healthcare Research and Quality |
| HCUP | Healthcare Cost and Utilization Project |
| SMD | Standardized mean difference |
Appendix A

References
- Ferenci, P.; Fried, M.; Labrecque, D.; Bruix, J.; Sherman, M.; Omata, M.; Heathcote, J.; Piratsivuth, T.; Kew, M.; Otegbayo, J.A.; et al. Hepatocellular carcinoma (HCC): A global per-spective. J. Clin. Gastroenterol. 2010, 44, 239–245. [Google Scholar] [CrossRef]
- Ozakyol, A. Global epidemiology of hepatocellular carcinoma (HCC epidemiology). J. Gastrointest. Cancer 2017, 48, 238–240. [Google Scholar] [CrossRef] [PubMed]
- Petrick, J.L.; McGlynn, K.A. The changing epidemiology of primary liver cancer. Curr. Epidemiology Rep. 2019, 6, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.L.; Sidhu-Brar, S.; Woodman, R.; Chinnaratha, M.A. Regular aspirin use is associated with a reduced risk of hepatocellular carcinoma (HCC) in chronic liver disease: A systematic review and meta-analysis. J. Gastrointest. Cancer 2022, 54, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Simon, T.G.; Duberg, A.-S.; Aleman, S.; Chung, R.T.; Chan, A.T.; Ludvigsson, J.F. Association of aspirin with hepatocellular carcinoma and liver-related mortality. N. Engl. J. Med. 2020, 382, 1018–1028. [Google Scholar] [CrossRef]
- Memel, Z.N.; Arvind, A.; Moninuola, O.; Philpotts, L.; Chung, R.T.; Corey, K.E.; Simon, T.G. Aspirin use is associated with a reduced incidence of hepatocellular carcinoma: A systematic review and meta-analysis. Hepatol. Commun. 2021, 5, 133–143. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, M.; Liu, C.; Wang, W.; Shi, J.; Dang, S. Aspirin use and the risk of hepatocellular carcinoma: A meta-analysis. J. Clin. Gastroenterol. 2022, 56, e293–e302. [Google Scholar] [CrossRef]
- Lee, T.-Y.; Hsu, Y.-C.; Tseng, H.-C.; Yu, S.-H.; Lin, J.-T.; Wu, M.-S.; Wu, C.-Y. Association of daily aspirin therapy with risk of hepatocellular carcinoma in patients with chronic hepatitis B. JAMA Intern. Med. 2019, 179, 633–640. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, T.; Sun, Y.; Li, C.; Ding, X.; Zhu, Y.; Li, L.; Fan, Z. Systematic review and meta-analysis: Association of aspirin with incidence of hepatocellular carcinoma. Front. Pharmacol. 2022, 13, 764854. [Google Scholar] [CrossRef]
- Ma, S.; Qu, G.; Sun, C.; Liu, H.; Jiang, Y.; Li, N.; Wu, B.; Gao, J.; Feng, L.; Xie, P.; et al. and mortality of hepatocellular carcinoma? A GRADE-assessed systematic review and dose–response meta-analysis. Eur. J. Clin. Pharmacol. 2023, 79, 39–61. [Google Scholar] [CrossRef]
- Li, X.; Yu, Y.; Liu, L. Influence of aspirin use on clinical outcomes of patients with hepatocellular carcinoma: A meta-analysis. Clin. Res. Hepatol. Gastroenterol. 2021, 45, 101545. [Google Scholar] [CrossRef]
- Lee, C.-H.; Hsu, C.-Y.; Yen, T.-H.; Wu, T.-H.; Yu, M.-C.; Hsieh, S.-Y. Daily aspirin reduced the incidence of hepatocellular carcinoma and overall mortality in patients with cirrhosis. Cancers 2023, 15, 2946. [Google Scholar] [CrossRef] [PubMed]
- Dhaliwal, A.; Sohal, A.; Bains, K.; Chaudhry, H.; Singh, I.; Kalra, E.; Arora, K.; Dukovic, D.; Boiles, A.R. Impact of aspirin use on outcomes in patients with hepatocellular cancer: A nationwide analysis. World J. Oncol. 2023, 14, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Young, S.-H.; Chau, G.-Y.; Lee, I.-C.; Yeh, Y.-C.; Chao, Y.; Huo, T.-I.; Su, C.-W.; Lin, H.-C.; Hou, M.-C.; Lee, M.-H.; et al. Aspirin is associated with low recurrent risk in hepatitis B virus-related hepatocellular carcinoma patients after curative resection. J. Formos. Med. Assoc. 2020, 119, 218–229. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.H.; Hsu, R.J.; Wang, T.H.; Wu, C.T.; Huang, S.Y.; Hsu, C.Y.; Su, Y.C.; Hsu, W.L.; Liu, D.W. Aspirin decreases hepatocellular carcinoma risk in hepatitis C virus carriers: A nationwide cohort study. BMC Gastroenterol. 2020, 20, 6. [Google Scholar] [CrossRef]
- Aktan, H.; Ozdemir, A.A.; Karaoğullarindan, Ü. Effect of aspirin use on survival in patients with hepatocellular carcinoma. Eur. J. Gastroenterol. Hepatol. 2023, 35, 1037–1043. [Google Scholar] [CrossRef]
- Lee, M.; Chung, G.E.; Oh, S.; Nam, J.Y.; Chang, Y.; Cho, H.; Ahn, H.; Cho, Y.Y.; Yoo, J.; Cho, Y.; et al. Antiplatelet therapy and the risk of hepatocellular carcinoma in chronic hepatitis B patients on antiviral treatment. Hepatology 2017, 66, 1556–1569. [Google Scholar] [CrossRef]
- Chen, H.; Cai, W.; Chu, E.S.H.; Tang, J.; Wong, C.-C.; Wong, S.H.; Sun, W.; Liang, Q.; Fang, J.; Sun, Z.; et al. Hepatic cyclooxygenase-2 overexpression induced spontaneous hepatocellular carcinoma formation in mice. Oncogene 2017, 36, 4415–4426. [Google Scholar] [CrossRef]
- Kern, M.A.; Schubert, D.; Sahi, D.; Schöneweiβ, M.M.; Moll, I.; Haugg, A.M.; Dienes, H.P.; Breuhahn, K.; Schirmacher, P. Proapoptotic and antiproliferative potential of selective cyclooxygenase-2 inhibitors in human liver tumor cells. Hepatology 2002, 36, 885–894. [Google Scholar] [CrossRef]
- Foderà, D.; D’Alessandro, N.; Cusimano, A.; Poma, P.; Notarbartolo, M.; Lampiasi, N.; Montalto, G.; Cervello, M. Induction of apoptosis and inhibition of cell growth in human hepatocellular carcinoma cells by COX-2 inhibitors. Ann. N. Y. Acad. Sci. 2004, 1028, 440–449. [Google Scholar] [CrossRef]
- Chen, G.; Li, X.; Yang, J.; Li, J.; Wang, X.; He, J.; Huang, Z. Prognostic significance of cyclooxygenase-2 expression in patients with hepatocellular carcinoma: A meta-analysis. Arch. Med. Sci. 2016, 12, 1110–1117. [Google Scholar] [CrossRef]
- Iannacone, M.; Sitia, G.; Isogawa, M.; Marchese, P.; Castro, M.G.; Lowenstein, P.R.; Chisari, F.V.; Ruggeri, Z.M.; Guidotti, L.G. Platelets mediate cytotoxic T lymphocyte-induced liver damage. Nat. Med. 2005, 11, 1167–1169. [Google Scholar] [CrossRef] [PubMed]
- Goubran, H.A.; Burnouf, T.; Stakiw, J.; Seghatchian, J. Platelet microparticle: A sensitive physiological “fine tuning” balancing factor in health and disease. Transfus. Apher. Sci. 2015, 52, 12–18. [Google Scholar] [CrossRef]
- Tripodi, A.; Mannucci, P.M. The coagulopathy of chronic liver disease. N. Engl. J. Med. 2011, 365, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Tripodi, A.; Salerno, F.; Chantarangkul, V.; Clerici, M.; Cazzaniga, M.; Primignani, M.; Mannucci, P.M. Evidence of normal thrombin generation in cirrhosis despite abnormal conventional coagulation tests. Hepatology 2005, 41, 553–558. [Google Scholar] [CrossRef] [PubMed]
- Gatt, A.; Riddell, A.; Calvaruso, V.; Tuddenham, E.G.; Makris, M.; Burroughs, A.K. Enhanced thrombin generation in patients with cirrhosis-induced coagulopathy. J. Thromb. Haemost. 2010, 8, 1994–2000. [Google Scholar] [CrossRef]
- Davì, G.; Ferro, D.; Basili, S.; Iuliano, L.; Basili, S.; Iuliano, L.; Camastra, C.; Giammarresi, C.; Santarone, S.; Rocca, B.; et al. Increased thromboxane metabolites excretion in liver cirrhosis. Thromb. Haemost. 1998, 79, 747–751. [Google Scholar] [CrossRef]
- Panasiuk, A.; Prokopowicz, D.; Zak, J.; Matowicka-Karna, J.; Osada, J.; Wysocka, J. Activation of blood platelets in chronic hepatitis and liver cirrhosis: P-selectin ex-pression on blood platelets and secretory activity of beta-thromboglobulin and platelet factor-4. Hepatogastroenterology 2001, 48, 818–822. [Google Scholar]
- Lisman, T.; Bongers, T.N.; Adelmeijer, J.; Janssen, H.L.; de Maat, M.P.; de Groot, P.G.; Leebeek, F.W. Elevated levels of von Willebrand factor in cirrhosis support platelet adhesion despite reduced functional capacity. Hepatology 2006, 44, 53–61. [Google Scholar] [CrossRef]
- Hayashi, T.; Shibata, M.; Oe, S.; Miyagawa, K.; Honma, Y.; Harada, M. Antiplatelet therapy improves the prognosis of patients with hepatocellular carcinoma. Cancers 2020, 12, 3215. [Google Scholar] [CrossRef]

| Baseline Characteristics | HCC Hospitalizations with Aspirin Use (%) | HCC Hospitalizations Without Aspirin Use (%) | p Value | |
|---|---|---|---|---|
| Age (in years) | 69.84 | 65.30 | - | |
| Sex | Male | 78.70 | 74.07 | <0.001 |
| Female | 21.30 | 25.93 | ||
| Race | White | 62.07 | 54.89 | <0.001 |
| Black | 17.42 | 14.80 | ||
| Hispanic | 12.23 | 18.42 | ||
| Others | 8.28 | 11.89 | ||
| Quartile of median household income for zip code | 0−25th | 32.31 | 32.23 | 0.892 |
| 26th−50th | 24.87 | 25.24 | ||
| 51st−75th | 23.03 | 23.09 | ||
| 76th−100th | 19.79 | 19.43 | ||
| Primary payer | Medicare | 68.28 | 53.62 | <0.001 |
| Medicaid | 11.21 | 19.07 | ||
| Private | 15.74 | 20.34 | ||
| Others | 4.76 | 6.97 | ||
| Hospital teaching status and location | Rural | 3.90 | 3.78 | 0.004 |
| Urban non-teaching | 12 | 13.66 | ||
| Urban teaching | 84.10 | 82.56 | ||
| Hospital bed-size | Small | 15.70 | 15.08 | 0.464 |
| Medium | 24.55 | 24.38 | ||
| Large | 59.74 | 60.54 | ||
| Hospital region | North-east | 18.68 | 19.39 | <0.001 |
| Mid-west | 24.42 | 17.79 | ||
| South | 36.72 | 37.95 | ||
| West | 20.18 | 24.88 | ||
| HCC Hospitalizations with Aspirin Use | HCC Hospitalizations Without Aspirin Use | Multivariate Adjusted Odds Ratio [OR] */Coefficient | 95% Confidence Interval | p Value | |
|---|---|---|---|---|---|
| Mortality | 5.2% | 10.09% | 0.58 ** | 0.50–0.68 | <0.001 |
| Length of stay | 5.42 days | 6.39 days | −0.83 | (−0.98)–(−0.68) | <0.001 |
| Total hospital charge | 80,310 $ | 95,098 $ | −6330 | (−9797)–(−2863) | <0.001 |
| Outcomes of Hepatocellular Carcinoma | With Aspirin Use (%) | Without Aspirin Use (%) | Multivariate Adjusted Odds Ratio [aOR] * | p-Value |
|---|---|---|---|---|
| Acute liver failure | 4.0 | 7.39 | 0.65 [0.55–0.78] | <0.001 |
| Hepatic encephalopathy | 1.17 | 2.61 | 0.59 [0.43–0.80] | 0.003 |
| Variceal Bleeding | 2.77 | 4.90 | 0.82 [0.66–1.02] | 0.075 |
| Portal vein thrombosis | 10.51 | 14.45 | 0.89 [0.79–1.01] | 0.066 |
| Ascites | 28.0 | 42.87 | 0.68 [0.64–0.72] | <0.001 |
| Spontaneous bacterial peritonitis | 1.81 | 4.27 | 0.58 [0.47–0.71] | <0.001 |
| Gastrointestinal Bleeding | 3.46 | 2.59 | 1.04 [0.84–1.24] | 0.569 |
| Obstructive Jaundice | 2.94 | 3.01 | 1.09 [0.87–1.36] | 0.468 |
| Sepsis | 9.39 | 14.09 | 0.70 [0.62–0.79] | <0.001 |
| ICU Admission | 3.55 | 6.45 | 0.62 [0.52–0.75] | <0.001 |
| Acute Kidney Injury | 27.46 | 31.75 | 0.81 [0.74–0.88] | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ginjupalli, M.; Bandaru, P.; Sharma, A.R.; Jayakumar, J.; Forlemu, R.N.S.M.; Wakil, A.; Forlemu, A.; Reddy, M. Influence of Aspirin on Hospital and Clinical Outcomes in Hepatocellular Carcinoma: Insights from National Data. Gastroenterol. Insights 2025, 16, 33. https://doi.org/10.3390/gastroent16030033
Ginjupalli M, Bandaru P, Sharma AR, Jayakumar J, Forlemu RNSM, Wakil A, Forlemu A, Reddy M. Influence of Aspirin on Hospital and Clinical Outcomes in Hepatocellular Carcinoma: Insights from National Data. Gastroenterology Insights. 2025; 16(3):33. https://doi.org/10.3390/gastroent16030033
Chicago/Turabian StyleGinjupalli, Manasa, Praneeth Bandaru, Anuj Raj Sharma, Jayalekshmi Jayakumar, Raissa Nana Sede Mbakop Forlemu, Ali Wakil, Arnold Forlemu, and Madhavi Reddy. 2025. "Influence of Aspirin on Hospital and Clinical Outcomes in Hepatocellular Carcinoma: Insights from National Data" Gastroenterology Insights 16, no. 3: 33. https://doi.org/10.3390/gastroent16030033
APA StyleGinjupalli, M., Bandaru, P., Sharma, A. R., Jayakumar, J., Forlemu, R. N. S. M., Wakil, A., Forlemu, A., & Reddy, M. (2025). Influence of Aspirin on Hospital and Clinical Outcomes in Hepatocellular Carcinoma: Insights from National Data. Gastroenterology Insights, 16(3), 33. https://doi.org/10.3390/gastroent16030033





