Applications of Artificial Intelligence to Eosinophilic Esophagitis
Abstract
1. Introduction
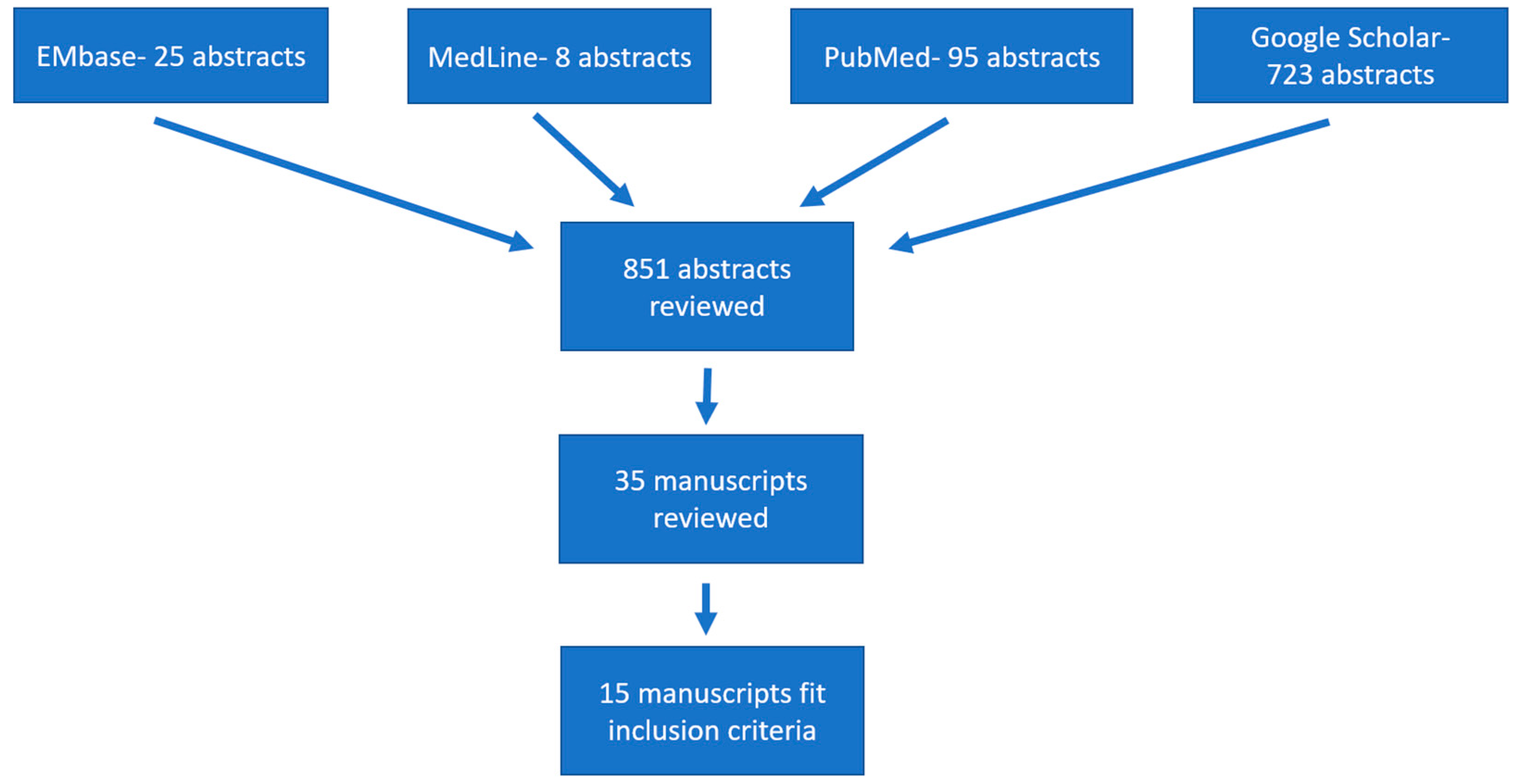
2. Materials and Methods
3. Results
4. AI for Biopsy Analysis
5. Endoscopic Imaging
6. Non-Invasive Diagnosis
7. Future Application
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Shaheen, N.J.; Mukkada, V.; Eichinger, C.S.; Schofield, H.; Todorova, L.; Falk, G.W. Natural history of eosinophilic esophagitis: A systematic review of epidemiology and disease course. Dis. Esophagus 2018, 31, doy015. [Google Scholar] [CrossRef] [PubMed]
- Mansoor, E.; Cooper, G.S. The 2010–2015 Prevalence of eosinophilic esophagitis in the USA: A population-based study. Dig. Dis. Sci. 2016, 61, 2928–2934. [Google Scholar] [CrossRef] [PubMed]
- Navarro, P.; Arias, Á.; Arias-González, L.; Laserna-Mendieta, E.J.; Ruiz-Ponce, M.; Lucendo, A.J. Systematic review with me-ta-analysis: The growing incidence and prevalence of eosinophilic oesophagitis in children and adults in population-based studies. Aliment. Pharm. Ther. 2019, 49, 1116–1125. [Google Scholar] [CrossRef] [PubMed]
- Edward, Y.; Philpott, H. Pathophysiology of Dysphagia in Eosinophilic Esophagitis: Causes, Consequences, and Management. Dig. Dis. Sci. 2022, 67, 1101–1115. [Google Scholar]
- Attwood, S.E.A.; Smyrk, T.C.; Demeester, T.R.; Jones, J.B. Esophageal eosinophilia with dysphagia. Am. J. Dig. Dis. 1993, 38, 109–116. [Google Scholar] [CrossRef]
- Schoepfer, A.M.; Safroneeva, E.; Bussmann, C.; Kuchen, T.; Portman, S.; Hansuwe, S.; Straumann. Delay in diagnosis of eosinophilic esophagitis increases risk for stricture formation in a time-dependent manner. Gastroenterology 2013, 145, 1230–1236. [Google Scholar] [CrossRef]
- Warners, M.J.; Nijuis, R.A.; Wijkerslooth, L.R.; Smout, A.J.; Bredenoord, A.J. The natural course of eosinophilic esophagitis and long-term consequences of undiagnosed disease in a large cohort. Am. J. Gastroenterol. 2018, 113, 836–844. [Google Scholar] [CrossRef]
- Votto, M.; Fasola, S.; Cilluffo, G.; Ferrante, G.; La Grutta, S.; Marseglia, G.L.; Licari, A. Cluster analysis of clinical data reveals three pediatric eosinophilic gastrointestinal disorder pheno-types. Pediatr. Allergy Immunol. 2022, 33, e13746. [Google Scholar] [CrossRef]
- Glatz, P.; Sandin, R.H.; Pedersen, N.L.; Bonamy, A.K.; Eriksson, L.I.; Granath, F. Association of anesthesia and surgery during childhood with long-term academic performance. JAMA Pediatr. 2017, 171, e163470. [Google Scholar] [CrossRef]
- Hirano, I. Future Directions in Eosinophilic Esophagitis. Gastrointest. Endosc. Clin. 2018, 28, 111–122. [Google Scholar] [CrossRef]
- Visaggi, P.; de Bortoli, N.; Barberio, B.; Savarino, V.; Oleas, R.; Rosi, E.M.; Marchi, S.; Ribolsi, M.; Savarino, E. Artificial Intelligence in the Diagnosis of Upper Gastrointestinal Diseases. J. Clin. Gastroenterol. 2021, 56, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Rajkomar, A.; Dean, J.; Kohane, I. Machine learning in medicine. N. Engl. J. Med. 2019, 380, 1347–1358. [Google Scholar] [CrossRef] [PubMed]
- Catalano, A.; Adorno, W.; Ehsan, L.; Shrivastava, A.; Barnes, B.H.; McGowan, E.C.; Moore, S.; Brown, D.; Syed, S. Mo1175 use of machine learning and computer vision to link eosinophilic esophagitis cellular patterns with clinical phenotypes and disease location. Gastroenterology 2020, 158, S-814. [Google Scholar] [CrossRef]
- Adorno, W., III; Catalano, A.; Ehsan, L.; von Eckstaedt, H.V.; Barnes, B.; McGowan, E.; Syed, S.; Brown, D.E. Advancing eosinophilic esophagitis diagnosis and phenotype assessment with deep learning computer vision. arXiv 2021, arXiv:2101.05326. [Google Scholar]
- Javaid, A.; Fernandes, P.; Adorno, W.; Catalano, A.; Ehsan, L.; von Eckstaedt, H.V.; Barnes, B.; Khan, M.; Raghavan, S.S.; McGowan, E.; et al. Deep learning tissue analysis diagnoses and predicts treatment response in eosinophilic esophagitis. medRxiv 2021. [Google Scholar] [CrossRef]
- Czyzewski, T.; Daniel, N.; Rochman, M.; Caldwell, J.M.; Osswald, G.A.; Collins, M.H.; Rothenberg, M.E.; Savir, Y. Machine learning approach for biopsy-based identification of eosinophilic esophagitis reveals importance of global features. IEEE Open J. Eng. Med. Biol. 2021, 2, 218–223. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, Q.; Xu, J.; Asad, Z.; Cui, C.; Correa, H.; Choksi, Y.; Hiremath, G.; Huo, Y. Eosinophilic esophagitis multi-label feature recognition on whole slide imaging using transfer learning. In Medical Imaging 2022: Digital and Computational Pathology; SPIE: San Diego, CA, USA, 2022; Volume 12039, pp. 277–284. [Google Scholar]
- Daniel, N.; Larey, A.; Aknin, E.; Osswald, G.A.; Caldwell, J.M.; Rochman, M.; Collins, M.H.; Yang, G.Y.; Arva, N.C.; Capocelli, K.E.; et al. PECNet: A deep multi-label segmentation network for eosinophilic esophagitis biopsy diagnostics. arXiv 2021, arXiv:2103.02015. [Google Scholar]
- Sallis, B.F.; Acar, U.; Hawthorne, K.; Babcock, S.J.; Kanagaratham, C.; Goldsmith, J.D.; Rosen, R.; Vanderhoof, J.A.; Nurko, S.; Fiebiger, E. A distinct esophageal mRNA pattern identifies eosinophilic esophagitis patients with food impactions. Front. Immunol. 2018, 9, 2059. [Google Scholar] [CrossRef]
- Sallis, B.F.; Erkert, L.; Moñino-Romero, S.; Acar, U.; Wu, R.; Konnikova, L.; Lexmond, W.S.; Hamilton, M.J.; Dunn, W.A.; Szepfalusi, Z.; et al. An algorithm for the classification of mRNA patterns in eosinophilic esophagitis: Integration of machine learning. J. Allergy Clin. Immunol. 2018, 141, 1354–1364.e9. [Google Scholar] [CrossRef]
- Lin, E.; Flygare, S.; Peterson, K.; Clayton, F.; Yandell, M. Using machine learning and RNA-seq to increase the accuracy and decrease the invasiveness of diagnosing eosinophilic esophagitis. J. Immunol. 2018, 200, 174-14. [Google Scholar]
- Strbkova, L.; Carson, B.; Vincent, T.; Vesely, P.; Chmelik, R. Automated interpretation of time-lapse quantitative phase image by machine learning to study cellular dynamics during epithelial–mesenchymal transition. J. Biomed. Opt. 2020, 25, 086502. [Google Scholar] [CrossRef] [PubMed]
- Römmele, C.; Mendel, R.; Rauber, D.; Rückert, T.; Byrne, M.; Palm, C.; Messmann, H.; Ebigbo, A. Endoscopic Diagnosis of Eosinophilic Esophagitis Using a deep Learning Algorithm. Endoscopy 2021, 53, OP14. [Google Scholar] [CrossRef]
- Guimarães, P.; Keller, A.; Fehlmann, T.; Lammert, F.; Casper, M. Deep learning-based detection of eosinophilic esophagitis. Endoscopy 2021, 54, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Okimoto, E.; Ishimura, N.; Adachi, K.; Kinoshita, Y.; Ishihara, S.; Tada, T. Application of Convolutional Neural Networks for Diagnosis of Eosinophilic Esophagitis Based on Endoscopic Imaging. J. Clin. Med. 2022, 11, 2529. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Gan, M.; Zhang, M.; Li, D. Adversarial convolutional network for esophageal tissue segmentation on OCT images. Biomed. Opt. Express 2020, 11, 3095. [Google Scholar] [CrossRef]
- Ryu, J.; Kang, D.; Do, D.; Osman, H.; Grant, C.N.; Giddings, S.; Baillargeon, A.; Gao, A.H.; Rosenberg, M.; Hesterberg, P.; et al. Tu1963 automated diagnosis of eosinophilic esophagitis from large images obtained by spectrally-encoded tethered capsule reflectance endomicroscopy. Gastrointest. Endosc. 2019, 89, AB633–AB634. [Google Scholar] [CrossRef]
- Collins, M.H.; Martin, L.J.; Alexander, E.S.; Boyd, J.T.; Sheridan, R.; He, H.; Pentiuk, S.; Putnam, P.E.; Abonia, J.P.; Mukkada, V.; et al. Newly developed and validated eosinophilic esophagitis histology scoring system and evidence that it outperforms peak eosinophil count for disease diagnosis and monitoring. Dis. Esophagus 2017, 30, 1–8. [Google Scholar] [CrossRef]
- Stucke, E.M.; Clarridge, K.E.; Collins, M.H.; Henderson, C.J.; Martin, L.J.; Rothenberg, M.E. Value of an additional review for eosinophil quantification in esophageal biopsies. J. Pediatr. Gastroenterol. Nutr. 2015, 61, 65–68. [Google Scholar] [CrossRef]
- Dellon, E.S.; Liacouras, C.A.; Molina-Infante, J.; Furuta, G.T.; Spergel, J.M.; Zevit, N.; Spechler, S.J.; Attwood, S.E.; Straumann, A.; Aceves, S.S.; et al. Updated International Consensus Diagnostic Criteria for Eosinophilic Esophagitis: Proceedings of the AGREE Conference. Gastroenterology 2018, 155, 1022–1033.e10. [Google Scholar] [CrossRef]
- Loizou, D.; Enav, B.; Komlodi-Pasztor, E.; Hider, P.; Kim-Chang, J.; Noonan, L.; Taber, T.; Kaushal, S.; Limgala, R.; Brown, M.; et al. A Pilot Study of Omalizumab in Eosinophilic Esophagitis. PLoS ONE 2015, 10, e0113483. [Google Scholar] [CrossRef]
- Gann, P.H.; Deaton, R.J.; McMahon, N.; Collins, M.H.; Dellon, E.S.; Hirano, I.; Hua, S.Y.; Rodriguez, C.; Harris, S. An anti–IL-13 antibody reverses epithelial-mesenchymal transition biomarkers in eosinophilic esophagitis: Phase 2 trial results. J. Allergy Clin. Immunol. 2020, 146, 367–376.e3. [Google Scholar] [CrossRef] [PubMed]
- Hirano, I.; Moy, N.; Heckman, M.G.; Thomas, C.S.; Gonsalves, N.; Achem, S.R. Endoscopic assessment of the oesophageal features of eosinophilic oesophagitis: Validation of a novel classification and grading system. Gut 2012, 62, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Visaggi, P.; Barberio, B.; Gregori, D.; Azzolina, D.; Martinato, M.; Hassan, C.; Sharma, P.; Savarino, E.; de Bortoli, N. Systematic review with meta-analysis: Artificial intelligence in the diagnosis of oesophageal diseases. Aliment. Pharmacol. Ther. 2022, 55, 528–540. [Google Scholar] [CrossRef] [PubMed]
- Liacouras, C.A.; Spergel, J.M.; Ruchelli, E.; Verma, R.; Mascarenhas, M.; Semeao, E.; Flick, J.; Kelly, J.; Brown–Whitehorn, T.; Mamula, P.; et al. Eosinophilic Esophagitis: A 10-Year Experience in 381 Children. Clin. Gastroenterol. Hepatol. 2005, 3, 1198–1206. [Google Scholar] [CrossRef]
- Liu, Z.; Xi, J.; Tse, M.; Myers, A.C.; Li, X.; Pasricha, P.J.; Yu, S. 426 Allergic Inflammation-Induced Structural and Functional Changes in Esophageal Epithelium in a Guinea Pig Model of Eosinophilic Esophagitis. Gastroenterology 2014, 146, S-92. [Google Scholar] [CrossRef]
- Kang, D.; Do, D.; Ryu, J.; Grant, C.N.; Giddings, S.L.; Rosenberg, M.; Hesterberg, P.E.; Yuan, Q.; Garber, J.J.; Katz, A.J.; et al. A miniaturized, tethered, spectrally-encoded confocal endomicroscopy capsule. Lasers Surg. Med. 2019, 51, 452–458. [Google Scholar] [CrossRef]
- Kumagai, Y.; Takubo, K.; Sato, T.; Ishikawa, H.; Yamamoto, E.; Ishiguro, T.; Hatano, S.; Toyomasu, Y.; Kawada, K.; Matsuyama, T.; et al. AI analysis and modified type classification for endocytoscopic observation of esophageal lesions. Dis. Esophagus 2022. [Google Scholar] [CrossRef]
- Wechsler, J.B.; Bolton, S.M.; Amsden, K.; Wershil, B.K.; Hirano, I.; Kagalwalla, A.F. Eosinophilic Esophagitis Reference Score Accu-rately Identifies Disease Activity and Treatment Effects in Children. Clin. Gastroenterol. Hepatol. 2018, 16, 1056–1063. [Google Scholar] [CrossRef]
- Furuta, G.T.; Kagalwalla, A.F.; Lee, J.J.; Alumkal, P.; Maybruck, B.T.; Fillon, S.; Masterson, J.C.; Ochkur, S.; Protheroe, C.; Moore, W.; et al. The oesophageal string test: A novel, minimally invasive method measures mucosal inflammation in eosinophilic oesophagitis. Gut 2012, 62, 1395–1405. [Google Scholar] [CrossRef]
- Katzka, D.A.; Geno, D.M.; Ravi, A.; Smyrk, T.C.; Lao-Sirieix, P.; Miramedi, A.; Debiram, I.; O’Donovan, M.; Kita, H.; Kephart, G.M.; et al. Accuracy, Safety, and Tolerability of Tissue Collection by Cytosponge vs Endoscopy for Evaluation of Eosinophilic Esophagitis. Clin. Gastroenterol. Hepatol. 2014, 13, 77–83.e2. [Google Scholar] [CrossRef]
- Morris, D.W.; Stucke, E.M.; Martin, L.J.; Abonia, J.P.; Mukkada, V.A.; Putnam, P.E.; Rothenberg, M.E.; Fulkerson, P.C. Eosinophil progenitor levels are increased in patients with active pediatric eosinophilic esophagitis. J. Allergy Clin. Immunol. 2016, 138, 915–918.e5. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lingblom, C.; Albinsson, S.; Johansson, L.; Larsson, H.; Wennerås, C. Patient-reported outcomes and blood-based parameters identify response to treatment in eosino-philic esophagitis. Dig. Dis. Sci. 2021, 66, 1556–1564. [Google Scholar] [CrossRef] [PubMed]
- Wechsler, J.B.; Ackerman, S.J.; Chehade, M.; Amsden, K.; Riffle, M.E.; Wang, M.; Du, J.; Kleinjan, M.L.; Alumkal, P.; Gray, E.; et al. Noninvasive biomarkers identify eosinophilic esophagitis: A prospective longitudinal study in children. Allergy 2021, 76, 3755–3765. [Google Scholar] [CrossRef] [PubMed]
- Kahwash, B.M.; Jaramillo, L.; Smith, B.; Kruszewski, P.; Ramilo, O.; Erwin, E.A. Peripheral Blood Microarray Analysis in Pediatric Patients with Eosinophilic Esophagitis. J. Allergy Clin. Immunol. 2019, 143, AB135. [Google Scholar] [CrossRef]
- Tisdale, A.; Cutillo, C.M.; Nathan, R.; Russo, P.; Laraway, B.; Haendel, M.; Nowak, D.; Hasche, C.; Chan, C.-H.; Griese, E.; et al. The IDeaS initiative: Pilot study to assess the impact of rare diseases on patients and healthcare systems. Orphanet J. Rare Dis. 2021, 16, 1–18. [Google Scholar] [CrossRef]
- Hiremath, G.; Sun, L.; Correa, H.; Acra, S.; Collins, M.H.; Bonis, P.; Arva, N.C.; Capocelli, K.E.; Falk, G.W.; King, E.; et al. Development and Validation of Web-Based Tool to Predict Lamina Propria Fibrosis in Eosinophilic Esophagitis. Am. J. Gastroenterol. 2021, 117, 272–279. [Google Scholar] [CrossRef]
- Mennini, M.; Tambucci, R.; Riccardi, C.; Rea, F.; De Angelis, P.; Fiocchi, A.; Assa’Ad, A. Eosinophilic Esophagitis and Microbiota: State of the Art. Front. Immunol. 2021, 12, 595762. [Google Scholar] [CrossRef]
- Patchett, B.; Nriagu, B.; Mavraj, G.; Schulman, E. A015 decoding allergic poly-sensitization with machine learning. Ann. Allergy Asthma Immunol. 2020, 125, S4. [Google Scholar] [CrossRef]
- Neethirajan, S.; Weng, X.; Tah, A.; Cordero, J.; Ragavan, K. Nano-biosensor platforms for detecting food allergens—New trends. Sens. Bio-Sens. Res. 2018, 18, 13–30. [Google Scholar] [CrossRef]
- Adamson, A.S.; Smith, A. Machine Learning and Health Care Disparities in Dermatology. JAMA Dermatol. 2018, 154, 1247–1248. [Google Scholar] [CrossRef]
- Finlayson, S.G.; Bowers, J.D.; Ito, J.; Zittrain, J.L.; Beam, A.L.; Kohane, I.S. Adversarial attacks on medical machine learning. Science 2019, 363, 1287–1289. [Google Scholar] [CrossRef] [PubMed]
| Study | Type | Outcome | AI Model | Data and Sample Size | Study Results/Validation Cohort | QUADAS Quality Assessment (Strong Study Is > 6) |
|---|---|---|---|---|---|---|
| Catalano et al., 2020 [13] | Poster | Eosinophil quantification (PEC and average eosinophil count), diagnosis, prediction of treatment response | U-net segmentation convolutional neural network (CNN) | 91 biopsies (36 from patients with EoE) | Diagnostic accuracy of 96% with an average error of + 0.16 eosinophils per HPF, standard deviation of 1.20. They also found high average eosinophil counts were associated with response to four to six food elimination diets and higher numbers of eosinophils in the mid vs. distal esophagus. | 7 |
| Adorno III et al., 2021 [14] | Manuscript | Eosinophil quantification (PEC, average eosinophil count, percent patches with 0, >5, 10, 15 eosinophils, average eosinophil size), diagnosis, prediction of treatment response | Compared 11 CNNs: U-Net vs. Res. U-Net vs. R2U-Net vs. Attn. U-Net with various test set dice coefficients | Biopsies from 101 patients (44 with EoE) | Diagnostic accuracy of 99.0%, 100% sensitivity, and 98% specificity. Higher maximum eosinophils in 4/6 FED responders over PPI and steroid responders. | 9 |
| Javaid et al., 2021 [15] | Manuscript | Eosinophil quantification (PEC), diagnosis, prediction of treatment response | U-net and VGG16 CNNs | Biopsies from 77 patients (36 with EoE) | Diagnostic accuracy of 99.9%, eosinophil quantification SD of −0.3 Eos/HPF; 4/6 FED responders had higher PEC. | 10 |
| Czyzewski et al., 2021 [16] | Manuscript | Diagnosis from biopsy images using deep CNN, determine which image size is ideal | ResNet50 (Deep CNN) | 420 biopsy images (210 with EoE) | Diagnostic accuracy of 85%, 448 × 448 pixels downscaled to 224 × 224 performed better than 224 × 224 pixel images, suggesting that global features contribute to model. | 10 |
| Shi et al., 2022 [17] | Manuscript | Diagnosis from biopsy through data augmentation using small dataset | ResNet50 and Bit-M CNNs | 202 biopsy images from 15 EoE patients compared to 404 normal biopsies | Diagnostic accuracy of 62%, ResNet50 outperformed Bit-M, limited data successfully augmented by random flipping, increasing contrast, and weight to training loss function. | 8 |
| Daniel et al., 2021 [18] | Manuscript | Eosinophil quantification (PEC), diagnosis, intact vs. not intact eosinophils | U-net | Biopsies from 23 patients with EoE | Diagnostic accuracy of 95%, distinguishes intact vs. not intact eosinophils with 98.8% accuracy. | 10 |
| Sallis et al., 2018, #1 [19] | Manuscript | Diagnosis of EoE vs. GERD vs. controls using RNA transcripts | Random Forest | Biopsies from 113 patients, (38 with EoE) | Diagnostic accuracy of 85% in patients with equivocal histology, created p (EoE) score that predicts diagnosis with AUC 0.985. | 10 |
| Sallis et al., 2018, #2 [20] | Manuscript | Identify patients with history of food impaction using RNA transcripts | Random Forest | Biopsies from 215 EoE patients, (26 with food impaction) | Predicts food impaction with 93% sensitivity and 100% specificity | 10 |
| Lin et al., 2018 [21] | Abstract | Diagosis using RNA transcipts and buccal biopsies | Not clear | Not clear | “EoE status can be predicted using buccal epithelial tissue biopsies…[enabling] more accurate diagnosis of EoE using less invasive and lower-cost biopsy protocols” | 3 |
| Strbkova et al., 2020 [22] | Manuscript | Using time lapse images to classify cells as entering endothelial–mesenchymal transition (EMT) in real time, which correlates with strictures in EoE. | Compared 19 AI models, including various decision trees, k-nearest neighbor neural networks, and supervised vector machines | 180 cells monitored every 5 min over 48 h period | Models averaged 98% accuracy at predicting cells going through EMT, improving future studies on stricturing EoE. | 10 |
| Rommele et al., 2021 [23] | Abstract | Endoscopic diagnosis | ResNet CNN, comparing image only vs. image with EREFS score augmented | 1272 endoscopic white light images (410 EoE) | EREFS-augmented model was strongest, with sensitivity, specificity, and F1-score of 0.85, 0.95, and 0.86, respectively. | 8 |
| Guimaraes et al., 2022 [24] | Abstract | Endoscopic diagnosis (EoE vs. Candida vs. control) | CNN with deep Taylor decomposition | 484 endoscopic images from 134 patients | Diagnostic accuracy of 92%, sensitivity of 87%, specificity of 94%, better than endoscopists. | 8 |
| Okimoto et al., 2019 [25] | Manuscript | Endoscopic diagnosis | ResNet50 | 2384 endoscopic images (1192 EoE) | 95% accuracy, 91% sensitivity, and 97% specificity. | 10 |
| Wang et al., 2020 [26] | Manuscript | Automatic segmentation of esophageal OCT images from guinea pigs with EoE, diagnosis by layer width | Several CNNs including Segnet, U-net, pix2pix, and adversarial convoluted network (ACN) | 1100 OCT images from five healthy and two animals with EoE | ACN outperformed other models, with 97% accuracy at segmenting esophageal tissue layers, basal layer significantly larger in EoE guinea pigs. | 10 |
| Ryu et al., 2019 [27] | Abstract | EoE diagnosis using images from tethered capsule using reflectance endomicroscopy | CNN | 2000 images with labeled regions of hypereosinophilia | 86% accurate at identifying HPF-sized images positive or negative. | 6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smith, E.R.; Shah, J. Applications of Artificial Intelligence to Eosinophilic Esophagitis. Gastroenterol. Insights 2022, 13, 218-227. https://doi.org/10.3390/gastroent13030022
Smith ER, Shah J. Applications of Artificial Intelligence to Eosinophilic Esophagitis. Gastroenterology Insights. 2022; 13(3):218-227. https://doi.org/10.3390/gastroent13030022
Chicago/Turabian StyleSmith, Eric Reuben, and Jay Shah. 2022. "Applications of Artificial Intelligence to Eosinophilic Esophagitis" Gastroenterology Insights 13, no. 3: 218-227. https://doi.org/10.3390/gastroent13030022
APA StyleSmith, E. R., & Shah, J. (2022). Applications of Artificial Intelligence to Eosinophilic Esophagitis. Gastroenterology Insights, 13(3), 218-227. https://doi.org/10.3390/gastroent13030022





