Abstract
The increasing number of patients with fatty liver disease is a major health problem. Fatty liver disease with metabolic dysfunction has been recognized as nonalcoholic fatty liver disease (NAFLD). Although there is no standard therapy for NAFLD, previous reports support the effect of sodium-glucose cotransporter 2 (SGLT2) inhibitors on NAFLD. Recently, fatty liver disease with metabolic dysfunction was proposed to be defined as a novel concept, “metabolic associated fatty liver disease (MAFLD)”, and it was proposed that new criteria for MAFLD diagnosis be established. To clarify the effect of SGLT2 inhibitors on MAFLD, we analyzed the efficacy of tofogliflozin in patients with MAFLD. We conducted a single-center, retrospective study to evaluate the efficacy of tofogliflozin in patients with MAFLD treated at Kyushu University Hospital between 2017 and 2019. Tofogliflozin was used to treat 18 patients with MAFLD. To determine the efficacy of tofogliflozin, we evaluated glucose metabolism, insulin resistance, liver injury, hepatic steatosis, and body composition three and six months after drug initiation. Although our study was a preliminary study because of some limitations (e.g., retrospective, observational, single-arm study, small sample size), we show that tofogliflozin could improve liver injury in patients with MAFLD by improving glucose metabolism and insulin resistance without causing muscle loss.
1. Introduction
Recently, the prevalence of obesity has increased worldwide, and the augmentation of patients with fatty liver disease is a major health problem. Previously, fatty liver disease with metabolic dysfunction was recognized as nonalcoholic fatty liver disease (NAFLD). NAFLD was diagnosed based on the presence of steatosis in more than 5% of hepatocytes in the absence of significant ongoing or recent alcohol consumption and other known causes of liver disease. Recently, fatty liver disease was proposed to be defined as a novel concept, “metabolic associated fatty liver disease (MAFLD)”, due to better knowledge of the relevance of metabolic dysfunction in the condition [1]. Furthermore, to accurately depict the pathogenesis of fatty liver diseases, new criteria for MAFLD diagnosis were proposed [2]. Contrary to NAFLD diagnosis based on exclusion criteria, MAFLD is diagnosed as the presence of both hepatic steatosis and metabolic dysregulation, including type 2 diabetes mellitus (T2DM). An appropriate pharmacological approach is required because no effective MAFLD therapy has been established. Novel medications for T2DM include sodium-glucose cotransporter 2 (SGLT2) inhibitors which inhibit the renal reuptake of glucose, consequently lowering blood glucose levels. Moreover, these drugs were recently reported to reduce body weight and improve hepatic steatosis in NAFLD patients. Because MAFLD is a novel concept and the criteria were recently proposed, the clinical research data regarding patients with MAFLD are insufficient. In this study, we evaluated the effect of SGLT2 inhibitors on patients with MAFLD diagnosed with the new criteria.
2. Methods
2.1. Patients
We conducted a single-center, retrospective study to evaluate the efficacy of tofogliflozin in patients with MAFLD treated at Kyushu University Hospital between 2017 and 2019. MAFLD was diagnosed using the criteria proposed as an international expert consensus statement [2]. Tofogliflozin 20 mg/day was adapted for patients with MAFLD with T2DM according to the therapeutic dose for T2DM approved in Japan. This study enrolled and retrospectively analyzed 18 patients with MAFLD treated with tofogliflozin. To determine the efficacy of tofogliflozin, we evaluated glucose metabolism, insulin resistance, liver injury, hepatic steatosis, and body composition three and six months after drug initiation. We employed ultrasonography using ARIETTA S70 (HITACHI, Tokyo, Japan) to assess the severity of hepatic steatosis and graded it as described previously [3]. Then, we measured the body fat mass, the proportion of body fat, and lean body mass using Inbody770 (Imbody Japan, Tokyo, Japan). We calculated the skeletal muscle index as the ratio of the appendicular skeletal muscle mass and the height squared (kg/m2).
2.2. Statistical Analysis
We analyzed the data using the JMP software package (v.15.1.0; SAS Institute, Inc., Cary, NC, USA). Continuous variables are expressed as median and interquartile range, or mean values and standard deviations. Significant differences were assessed using the paired-samples t-test or Mann–Whitney U test. A p-value of <0.05 was considered statistically significant.
3. Result
3.1. The Effect of Tofogliflozin on Glucose Metabolism and Hepatic Steatosis
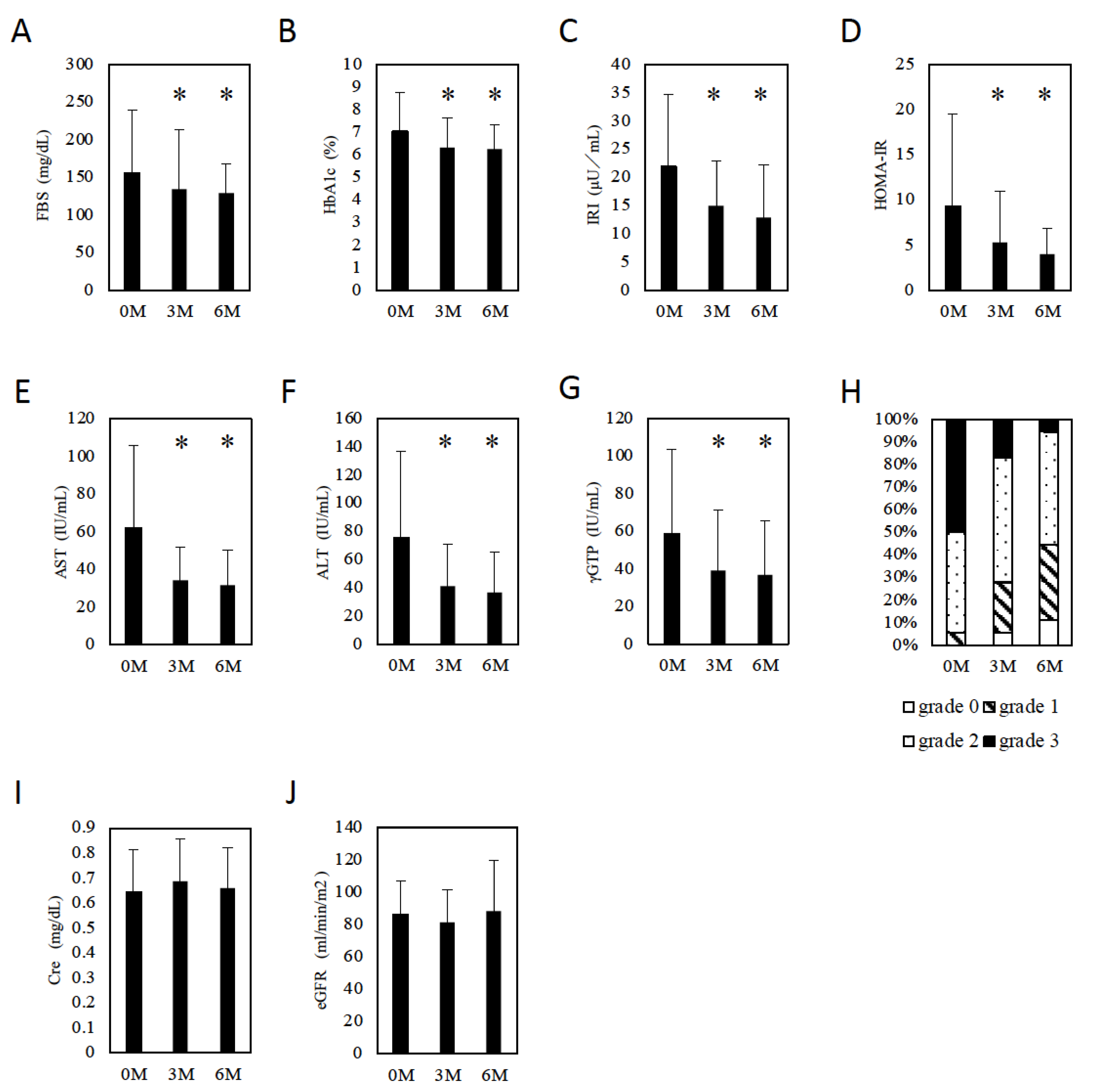
Of the 18 patients with MAFLD, 3 patients were infected with HBV, 1 patient was infected with HCV, and 1 patient was complicated with primary biliary cholangitis (PBC). One patient was treated with sulfonylurea, one patient was treated with biguanide, and one patient was treated with a DPP4 inhibitor before initiation of tofogliflozin. Other patients had been treated with only lifestyle modifications before initiation of tofogliflozin because their severities of T2DM were mild. These therapies were continued after initiation of tofogliflozin. At baseline, the patients with MAFLD had elevated aspartate aminotransferase (AST) (median 45 IU/L), alanine aminotransferase (ALT) (median 53 IU/L), and gamma-glutamyl transpeptidase (γ-GTP) (median 37 IU/L) levels, abnormal glucose metabolism (FBS: median 120 mg/dL, HbA1c: median 6.4%), a high body weight (BW) (median 68.0 kg), and a high body mass index (BMI) (median 26.8). To evaluate insulin resistance, we measured immunoreactive insulin (IRI) (median 19.6 IU/L) levels and calculated HOMA-IR (median 6.0) (Table 1). Three and six months after tofogliflozin initiation, there was a significant decrease in FBS (3M: 134.1 ± 78.7 mg/dL, p = 0.0118 vs. baseline, 6M: 128.7 ± 39.4 mg/dL, p = 0.027 vs. baseline, Figure 1A) and HbA1c (3M: 6.3 ± 1.3%, p = 0.0002 vs. baseline, 6M: 6.2 ± 1.1%, p = 0.0008 vs. baseline, Figure 1B). Both IRI (3M: 14.9 ± 8.0 IU/L, p = 0.0016 vs. baseline, 6M: 12.9 ± 9.4 IU/L, p = 0.0127 vs. baseline, Figure 1C) and HOMA-IR (3M: 5.3 ± 5.7, p = 0.0057 vs. baseline, 6M: 4.0 ± 2.9, p = 0.03 vs. baseline, Figure 1D) decreased significantly. Similarly, AST (3M: 34.1 ± 17.8 IU/L, p = 0.0007 vs. baseline, 6M: 31.4 ± 18.9 IU/L, p = 0.0004 vs. baseline, Figure 1E), ALT (3M: 40.9 ± 30.1 IU/L, p = 0.0004 vs. baseline, 6M: 36.7 ± 28.5 IU/L, p = 0.0005 vs. baseline, Figure 1F), and γ-GTP (3M: 39.1 ± 32.4 IU/L, p = 0.0037 vs. baseline, 6M: 36.8 ± 29.0 IU/L, p = 0.0043 vs. baseline, Figure 1G) levels decreased significantly. Ultrasonography showed significant improvement in hepatic steatosis (p = 0.0016, Figure 1H). Regarding renal function, no significant differences in creatinine (Cre) (baseline, 3M, 6M: 0.64 ± 0.17, 0.69 ± 0.17, 0.66 ± 0.16, respectively, baseline vs. 3M, 6M: p = 0.0155, 0.2879, respectively, Figure 1I) or the estimated glomerular filtration rate (eGFR) (baseline, 3M, 6M: 86.4 ± 20.1, 81.5 ± 31.6, 88.4 ± 31.6, respectively, baseline vs. 3M, 6M: p = 0.0282, 0.5847, respectively, Figure 1J) were observed after drug initiation.

Table 1.
Profile and baseline characteristics of patients with MAFLD.
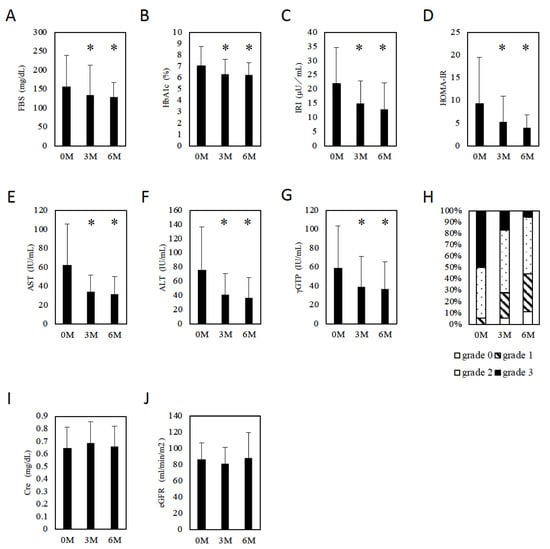
Figure 1.
The effect of tofogliflozin. (A) FBS changes in patients with MAFLD. (B) HbA1c changes in patients with MAFLD. (C) IRI changes in patients with MAFLD. (D) HOMA-IR changes in patients with MAFLD. (E) AST changes in patients with MAFLD. (F) ALT changes in patients with MAFLD. (G) Changes in γ-GTP in patients with MAFLD. (H) Changes in US grades in patients with MAFLD. (I) Cre changes in patients with MAFLD. (J) eGFR changes in patients with MAFLD. Data represent means ± SD. * p < 0.05 vs. 0 M.
3.2. The Effect of Tofogliflozin on Body Composition
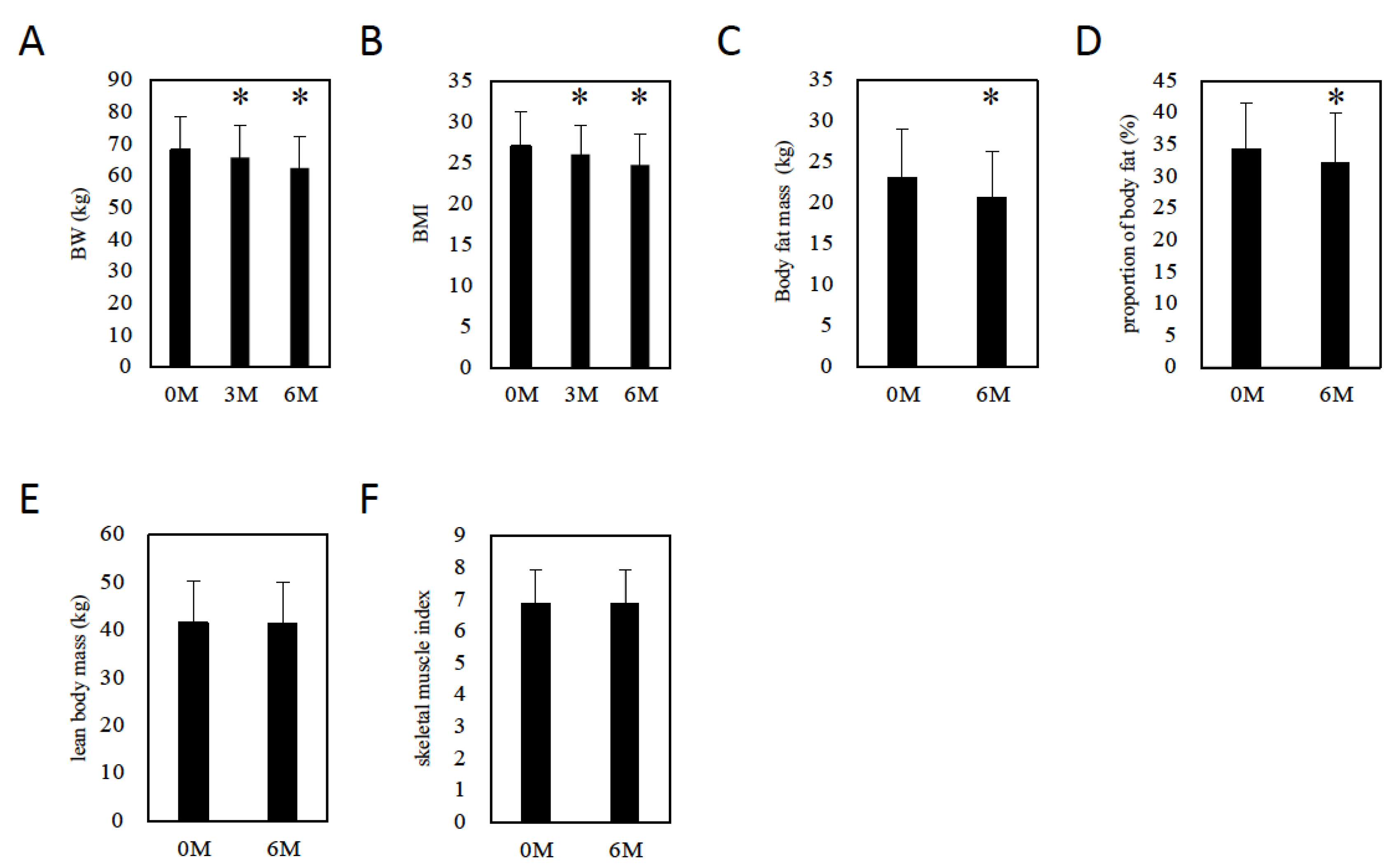
Six months after initiation of tofogliflozin, BW (3M: 65.8 ± 10.2 kg, p = 0.0013 vs. baseline, 6M: 62.6 ± 9.7 kg, p = 0.0002 vs. baseline, Figure 2A) and BMI (3M: 26.0 ± 3.7, p = 0.0016 vs. baseline, 6M: 24.7 ± 3.7, p = 0.0002 vs. baseline, Figure 2B) decreased. To evaluate the effect of tofogliflozin on body fat and muscle mass, we measured body composition using Inbody770 six months after drug initiation. Body fat mass (baseline, 6M: 23 ± 6.1 kg, 21 ± 5.5 kg, respectively, p = 0.0004, Figure 2C) and proportion of body fat (baseline, 6M: 34 ± 7.3%, 32 ± 7.8%, respectively, p = 0.0005, Figure 2D) decreased significantly. However, lean body mass (baseline, 6M: 42 ± 8.6 kg, 41 ± 8.4 kg, respectively, p = 0.7718, Figure 2E) and the skeletal muscle index (baseline, 6M: 7 ± 1.1, 7 ± 1.0, respectively, p = 0.8977, Figure 2F) did not change six months after drug initiation.
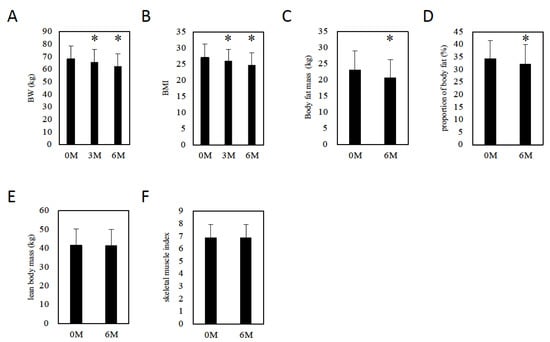
Figure 2.
The effect of tofogliflozin on body composition. (A) Changes in body weight in patients with MAFLD. (B) BMI changes in patients with MAFLD. (C) Changes in body fat mass in patients with MAFLD. (D) Changes in the proportion of body fat in patients with MAFLD. (E) Changes in lean body mass in patients with MAFLD. (F) Changes in skeletal muscle index in patients with MAFLD. Data represent means ± SD. * p < 0.05 vs. 0 M.
4. Discussion
In this study, we investigated the efficacy of tofogliflozin in patients with MAFLD. We demonstrated that tofogliflozin improved glucose metabolism, insulin resistance, liver enzyme elevation, and hepatic steatosis. The decrease in AST and ALT indicates the improvement in liver injury. We considered that improving glucose metabolism and insulin resistance would alleviate hepatic steatosis and consequently reduce liver injury. SGLT2 inhibitors have previously been reported to improve liver function tests and hepatic steatosis in NAFLD patients [4,5,6,7,8]. The proposed criteria for MAFLD are based on histological, imaging, or blood biomarker evidence of fat accumulation in the liver (hepatic steatosis) in addition to one of the following three criteria: overweight/obesity, presence of type 2 diabetes mellitus (T2DM), or evidence of metabolic dysregulation. The last criterion is defined by the presence of at least two metabolic risk abnormalities, such as a high waist circumference, hypertension, a high level of plasma triglycerides, a low level of plasma HDL cholesterol, prediabetes, elevated HOMA-IR, and a high level of plasma high-sensitivity C-reactive protein [2]. Because NAFLD is diagnosed based on exclusion criteria and MAFLD is diagnosed regardless of the presence of other liver diseases, patients with MAFLD include patients with other liver diseases. In this study, although some patients were complicated with other liver diseases such as HBV, HCV, and PBC, we discovered a similar effect on MAFLD diagnosed by the new criteria. In this study, the enrolled patients were diagnosed with MAFLD based on the presence of both liver steatosis and T2DM. Because SGLT2 inhibitors were reported to act on NAFLD via several mechanisms [9], it is possible that SGLT2 inhibitors are effective against MAFLD without T2DM. In future study, the availability of SGLT2 in patients with MAFLD without T2DM should be investigated.
Furthermore, tofogliflozin reduced fat mass without causing muscle loss. Loss of skeletal muscle mass and strength is known as sarcopenia. Sarcopenia has frequently been associated with chronic liver disease, including NAFLD, and related to liver fibrosis [10]. Although the pathogenesis of sarcopenia is not fully understood, insulin resistance and chronic inflammation have been linked to sarcopenia [11]. Conversely, sarcopenia has been identified as an independent risk factor for NAFLD-related liver fibrosis [12]. Therefore, muscle loss may be unfavorable for T2DM and MAFLD. The renal excretion of glucose caused by the SGLT2 inhibitors could enhance the catabolic process and induce protein degradation in skeletal muscles; however, we discovered that tofogliflozin did not affect muscle mass. Komiya et al. reported that ipragliflozin, one of the SGLT2 inhibitors, increased insulin-induced Akt phosphorylation in skeletal muscle [13]. The Akt/mTORC1 pathways have been identified as crucial regulators of skeletal muscle mass [14], whereas Akt can suppress FOXO, which promotes protein degradation [15]. Moreover, it has been discovered that Akt regulates muscle mass by coordinating both the mTORC1 and FOXO1 pathways [16], and insulin was recognized as a potent anabolic signal in proteins [17]. The anabolic effect of improving insulin resistance may predominate the catabolic effect of SGLT2 inhibitors by enhancing Akt/mTORC1 and suppressing FOXO1.
The limitations of this study are the small sample size, the heterogeneity of the patients, the fact it was a retrospective, single-arm study, and the lack of analysis for other medications. Because the diagnosis of MAFLD is based on novel inclusion criteria, patients with MAFLD are more heterogenous than those with NAFLD. Consistently, the baseline characteristics showed the heterogeneity of the patients in this study. Because it was a single-arm study, the improvement in MAFLD may be due to the lifestyle modifications and placebo effect. However, most patients were resistant to lifestyle intervention before initiation of tofogliflozin; we considered that the improvement was mainly due to the effect of tofogliflozin. Moreover, we could not evaluate the potential effects of other antidiabetics because only a few patients were administered other antidiabetic drugs. Although this study was a preliminary study because of these limitations, the effects of tofogliflozin were statistically significant despite the small sample size and heterogeneity of the patients. We consider these findings to provide important insights into research on MAFLD. Randomized, controlled trials with a larger sample size are required to establish SGLT2 inhibitors as a standard therapy for MAFLD.
Conclusively, tofogliflozin could improve liver injury in patients with MAFLD by improving glucose metabolism and insulin resistance without causing muscle loss. These findings support the use of SGLT2 inhibitors for MAFLD therapy. However, we need further confirmation and investigation, including randomized, controlled trials with larger samples, in order to demonstrate the effect of tofogliflozin on MAFLD.
Author Contributions
T.G., M.K. (Motoyuki Kohjima), M.T. (Masatake Tanaka), M.K. (Masaki Kato), and Y.O. designed this study. M.K. (Motoyuki Kohjima), K.I., S.T., T.A., M.T. (Motoi Takahashi), M.K. (Miho Kurokawa), and H.S. assisted with data analyses. M.K. (Motoyuki Kohjima), M.T. (Masatake Tanaka), and Y.O. contributed to the analysis and interpretation of the data. M.K. (Motoyuki Kohjima), M.T. (Masatake Tanaka), and Y.O. assisted in preparing the manuscript and critically reviewed it. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported, in part, by the Takeda Science Foundation, Smoking Research Foundation, and JSPS KAKENHI (Grant Numbers: JP17K09430, JP18H05039, JP19H01054, JP19K17496, JP20K22877, JP20H04949).
Institutional Review Board Statement
This study was approved by the Ethics Committee of Kyushu University Hospital (No.29-432).
Informed Consent Statement
This study was approved by the Ethics Committee of Kyushu University Hospital, and the requirement for written informed consent was waived because of the retrospective study design.
Data Availability Statement
The data collected and/or analyzed during this study are available from the corresponding author on reasonable request.
Conflicts of Interest
Yoshihiro Ogawa received research support from Kowa. Takeshi Goya, Koji Imoto, Shigeki Tashiro, Tomomi Aoyagi, Motoi Takahashi, Miho Kurokawa, Hideo Suzuki, Masatake Tanaka, Masaki Kato, and Motoyuki Kohjima declare no conflicts of interest. The authors are responsible for the content and writing of this article.
References
- Eslam, M.; Sanyal, A.J.; George, J. International Consensus Panel. MAFLD: A consensus-driven proposed nomenclature for met-abolic associated fatty liver disease. Gastroenterology 2020, 158, 1999–2014. [Google Scholar] [CrossRef] [PubMed]
- Eslam, M.; Newsome, P.N.; Sarin, S.K.; Anstee, Q.M.; Targher, G.; Romero-Gomez, M.; Zelber-Sagi, S.; Wong, V.W.; Dufour, J.F.; Schattenberg, J.M.; et al. A new definition for metabolic dys-function-associated fatty liver disease: An international expert consensus statement. J. Heptol. 2020, 73, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Saadeh, S.; Younossi, Z.M.; Remer, E.M.; Gramlich, T.; Ong, J.P.; Hurley, M.; Mullen, K.D.; Cooper, J.N.; Sheridan, M.J. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology 2002, 123, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Ito, D.; Shimizu, S.; Inoue, K.; Saito, D.; Yanagisawa, M.; Inukai, K.; Akiyama, Y.; Morimoto, Y.; Noda, M.; Shimada, A. Comparison of Ipragliflozin and Pioglitazone Effects on Nonalcoholic Fatty Liver Disease in Patients With Type 2 Diabetes: A Randomized, 24-Week, Open-Label, Active-Controlled Trial. Diabetes Care 2017, 40, 1364–1372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shibuya, T.; Fushimi, N.; Kawai, M.; Yoshida, Y.; Hachiya, H.; Ito, S.; Kawai, H.; Ohashi, N.; Mori, A. Luseogliflozin improves liver fat deposition compared to metformin in type 2 diabetes patients with non-alcoholic fatty liver disease: A prospective randomized controlled pilot study. Diabetes Obes. Metab. 2017, 20, 438–442. [Google Scholar] [CrossRef] [PubMed]
- Kuchay, M.S.; Krishan, S.; Mishra, S.K.; Farooqui, K.J.; Singh, M.K.; Wasir, J.S.; Bansal, B.; Kaur, P.; Jevalikar, G.; Gill, H.K.; et al. Effect of Empagliflozin on Liver Fat in Patients With Type 2 Diabetes and Nonalcoholic Fatty Liver Disease: A Randomized Controlled Trial (E-LIFT Trial). Diabetes Care 2018, 41, 1801–1808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eriksson, J.W.; Lundkvist, P.; Jansson, P.A.; Johansson, L.; Kvarnström, M.; Moris, L.; Miliotis, T.; Forsberg, G.B.; Risérus, U.; Lind, L.; et al. Effects of dapagliflozin and n-3 carboxylic acids on non-alcoholic fatty liver disease in people with type 2 diabetes: A double-blind randomised place-bo-controlled study. Diabetologia 2018, 61, 1923–1934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimizu, M.; Suzuki, K.; Kato, K.; Jojima, T.; Iijima, T.; Murohisa, T.; Iijima, M.; Takekawa, H.; Usui, I.; Hiraishi, H.; et al. Evaluation of the effects of dapagliflozin, a sodium-glucose co-transporter-2 inhibitor, on hepatic steatosis and fibrosis using transient elastography in patients with type 2 diabetes and non-alcoholic fatty liver disease. Diabetes Obes. Metab. 2018, 21, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Chrysavgis, L.; Papatheodorididi, A.M.; Chatzigeorgiou, A.; Cholongitas, E. The impact of sodium glucose co-transporter 2 inhibitors on non-alcoholic fatty liver disease. JGH 2021, 36, 893–909. [Google Scholar] [CrossRef] [PubMed]
- Koo, B.K.; Kim, D.; Joo, S.K.; Kim, J.H.; Chang, M.S.; Kim, B.G.; Lee, K.L.; Kim, W. Sarcopenia is an independent risk factor for non-alcoholic steatohepatitis and significant fibrosis. J. Hepatol. 2016, 66, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Umegaki, H. Sarcopenia and frailty in older patients with diabetes mellitus. Geriatr. Gerontol. Int. 2016, 16, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Kim, S.U.; Song, K.; Park, J.Y.; Kim, D.Y.; Ahn, S.H.; Lee, B.W.; Kang, E.S.; Cha, B.S.; Han, K.H. Sarcopenia is associated with significant liver fibrosis inde-pendently of obesity and insulin resistance in nonalcoholic fatty liver disease: Nationwide surveys (KNHANES 2008–2011). Hepatology 2016, 63, 776–786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Komiya, C.; Tsuchiya, K.; Shiba, K.; Miyachi, Y.; Furuke, S.; Shimazu, N.; Yamaguchi, S.; Kanno, K.; Ogawa, Y. Ipragliflozin Improves Hepatic Steatosis in Obese Mice and Liver Dysfunction in Type 2 Diabetic Patients Irrespective of Body Weight Reduction. PLoS ONE 2016, 11, e0151511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bodine, S.C.; Stitt, T.N.; Gonzalez, M.; Kline, W.O.; Stover, G.L.; Bauerlein, R.; Zlotchenko, E.; Scrimgeour, A.; Lawrence, J.C.; Glass, D.J.; et al. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat. Cell Biol. 2001, 3, 1014–1019. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, B.T.; Lee, K.Y.; Klaus, K.; Softic, S.; Krumpoch, M.T.; Fentz, J.; Stanford, K.I.; Robinson, M.M.; Cai, W.; Kleinridders, A.; et al. Insulin and IGF-1 receptors regulate FoxO-mediated signaling in muscle proteostasis. J. Clin. Investig. 2016, 126, 3433–3446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaiswal, N.; Gavin, M.; Loro, E.; Sostre-Colón, J.; Roberson, P.A.; Uehara, K.; Rivera-Fuentes, N.; Neinast, M.; Arany, Z.; Kimball, S.R.; et al. AKT controls protein synthesis and oxidative metabolism via combined mTORC1 and FOXO1 signalling to govern muscle physiology. J. Cachex-Sarcopenia Muscle 2021. [Google Scholar] [CrossRef] [PubMed]
- Sakuma, K.; Yamaguchi, A. Sarcopenic Obesity and Endocrinal Adaptation with Age. Int. J. Endocrinol. 2013, 2013, 204164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).