Gastroparesis, Thymoma, and Asymptomatic Myasthenia: A Rare Clinical Scenario
Abstract
1. Introduction
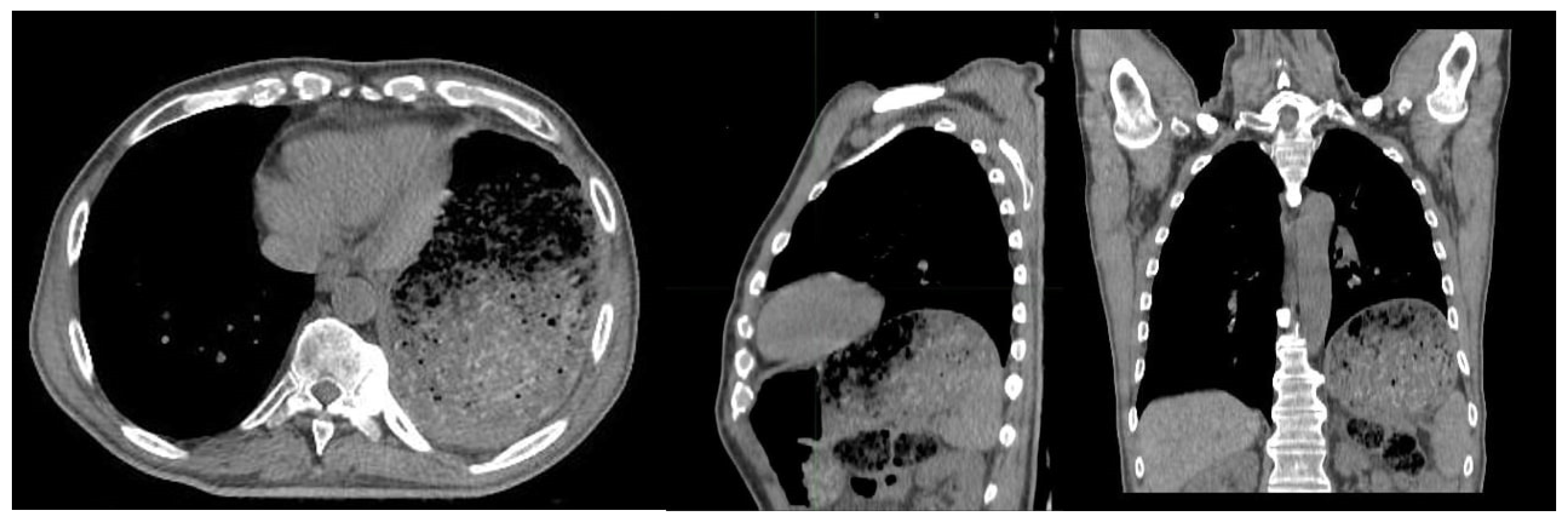
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Topuzova, M.P.; Bisaga, G.N.; Alekseeva, T.M.; Isabekova, P.S.; Chaykovskaya, A.D.; Panina, E.B.; Pavlova, T.A.; Ternovykh, I.K. Transverse myelitis syndrom as a result of neuromyelitis optica spectrum disorders, systemic lupus erythematosus and myasthenia gravis combination. Zhurnal Nevrol. I Psikhiatrii Im. S.S. Korsakova 2020, 120, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Tabbaa, M.A.; Leshner, R.T.; Campbell, W.W. Malignant Thymoma With Dysautonomia and Disordered Neuromuscular Transmission. Arch. Neurol. 1986, 43, 955–957. [Google Scholar] [CrossRef]
- Tan, C.K.; Ng, H.S.; Ho, J.S.; Theobald, D.M.; Lim, Y.C. Acute intestinal pseudo-obstruction due to malignant thymoma. Singap. Med. J. 1993, 34, 8266165. [Google Scholar]
- Anderson, N.E.; Hutchinson, D.O.; Nicholson, G.I.; Aitcheson, F.; Nixon, J.M. Intestinal pseudo-obstruction, myasthenia gravis, and thymoma. Neurology 1996, 47, 985–987. [Google Scholar] [CrossRef] [PubMed]
- Vernino, S.; Cheshire, W.; Lennon, V.A. Myasthenia gravis with autoimmune autonomic neuropathy. Auton. Neurosci. 2001, 88, 187–192. [Google Scholar] [CrossRef]
- Sindoni, A.; Minutoli, F.; Pontoriero, A.; Iatì, G.; Baldari, S.; Pergolizzi, S. Usefulness of four dimensional (4D) PET/CT imaging in the evaluation of thoracic lesions and in radiotherapy planning: Review of the literature. Lung Cancer 2016, 96, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Ramella, S.; Maranzano, E.; Frata, P.; Mantovani, C.; Lazzari, G.; Menichelli, C.; Navarria, P.; Pergolizzi, S.; Salvi, F. Radiotherapy in Italy for non-small cell lung cancer: Patterns of care survey. Tumori J. 2012, 98, 66–78. [Google Scholar] [CrossRef]
- Ferini, G.; Tripoli, A.; Molino, L.; Cacciola, A.; Lillo, S.; Parisi, S.; Umina, V.; Illari, S.I.; Marchese, V.A.; Cravagno, I.R.; et al. How Much Daily Image-guided Volumetric Modulated Arc Therapy Is Useful for Proctitis Prevention With Respect to Static Intensity Modulated Radiotherapy Supported by Topical Medications Among Localized Prostate Cancer Patients? Anticancer Res. 2021, 41, 2101–2110. [Google Scholar] [CrossRef] [PubMed]
- Spatola, C.; Militello, C.; Tocco, A.; Salamone, V.; Raffaele, L.; Migliore, M.; Pagana, A.; Milazzotto, R.; Chillura, I.; Pergolizzi, S.; et al. Intensity-modulated radiotherapy for relapsed malignant pleural mesothelioma. Futur. Oncol. 2016, 12, 67–71. [Google Scholar] [CrossRef]
- Sindoni, A.; Minutoli, F.; Baldari, S.; Pergolizzi, S. Importance of Respiratory-gated PET/CT in Thoracic Tumors. Radiology 2016, 281, 321–323. [Google Scholar] [CrossRef][Green Version]
- Seki, S.; Koyama, H.; Ohno, Y.; Nishio, M.; Takenaka, D.; Maniwa, Y.; Itoh, T.; Nishimura, Y.; Sugimura, K. Diffusion-weighted MR imaging vs. multi-detector row CT: Direct comparison of capability for assessment of management needs for anterior mediastinal solitary tumors. Eur. J. Radiol. 2014, 83, 835–842. [Google Scholar] [CrossRef] [PubMed]
- Siesling, S.; van der Zwan, J.M.; Izarzugaza, I.; Jaal, J.; Treasure, T.; Foschi, R.; Ricardi, U.; Groen, H.; Tavilla, A.; Ardanaz, E.; et al. Rare thoracic cancers, including peritoneum mesothelioma. Eur. J. Cancer 2012, 48, 949–960. [Google Scholar] [CrossRef] [PubMed]
- Scorsetti, M.; Leo, F.; Trama, A.; D’Angelillo, R.; Serpico, D.; Macerelli, M.; Zucali, P.; Gatta, G.; Garassino, M.C. Thymoma and thymic carcinomas. Crit. Rev. Oncol. Hematol. 2016, 99, 332–350. [Google Scholar] [CrossRef]
- Marx, A.; Ströbel, P.; Badve, S.; Chalabreysse, L.; Chan, J.K.; Chen, G.; de Leval, L.; Detterbeck, F.; Girard, N.; Huang, J.; et al. ITMIG Consensus Statement on the Use of the WHO Histological Classification of Thymoma and Thymic Carcinoma: Refined Definitions, Histological Criteria, and Reporting. J. Thorac. Oncol. 2014, 9, 596–611. [Google Scholar] [CrossRef]
- Buckley, C.; Douek, D.; Newsom-Davis, J.; Vincent, A.; Willcox, N. Mature, long-lived CD4+ and CD8+ T cells are generated by the thymoma in myasthenia gravis. Ann. Neurol. 2001, 50, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Posner, J.B. Immunology of Paraneoplastic Syndromes. Ann. N. Y. Acad. Sci. 2003, 998, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Conti-Fine, B.M.; Milani, M.; Kaminski, H.J. Myasthenia gravis: Past, present, and future. J. Clin. Investig. 2006, 116, 2843–2854. [Google Scholar] [CrossRef]
- Keesey, J.C. Clinical evaluation and management of myasthenia gravis. Muscle Nerve 2004, 29, 484–505. [Google Scholar] [CrossRef]
- Evoli, A.; Minicuci, G.M.; Vitaliani, R.; Battaglia, A.; Della Marca, G.; Lauriola, L.; Fattorossi, A. Paraneoplastic diseases associated with thymoma. J. Neurol. 2007, 254, 756–762. [Google Scholar] [CrossRef]
- Gronseth, G.S.; Barohn, R.J. Practice parameter: Thymectomy for autoimmune myasthenia gravis (an evidence-based review): Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2000, 55, 7–15. [Google Scholar] [CrossRef]
- Kim, H.K.; Park, M.S.; Choi, Y.S.; Kim, K.; Shim, Y.M.; Han, J.; Kim, B.J.; Kim, J. Neurologic outcomes of thymectomy in myasthenia gravis: Comparative analysis of the effect of thymoma. J. Thorac. Cardiovasc. Surg. 2007, 134, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, E.D.; Portela, A.R.; De Oliveira, M.V.B.; Guimarães, J.R.G.; Ramos, S.B.; Pena, T.B. Gastric pseudo-obstruction as an initial manifestation of thymoma. J. Bras. Pneumol. 2019, 45. [Google Scholar] [CrossRef]
- Rakocevic, G.; Barohn, R.; McVey, A.L.; Damjanov, I.; Morte, P.D.; Vernino, S.; Lennon, V. Myasthenia Gravis, Thymoma, and Intestinal Pseudo-Obstruction. J. Clin. Neuromuscul. Dis. 2003, 5, 93–95. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Bhatnagar, V.; Ding, L.; Atay, S.M.; David, E.A.; McFadden, P.M.; Stamnes, S.; Lechtholz-Zey, E.; Wightman, S.C.; Detterbeck, F.C.; et al. A systematic review of paraneoplastic syndromes associated with thymoma: Treatment modalities, recurrence, and outcomes in resected cases. J. Thorac. Cardiovasc. Surg. 2020, 160, 306–314.e14. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M.; Dubois, D.; Coulie, B.; Jones, M.; Kahrilas, P.J.; Rentz, A.M.; Sonnenberg, A.; Stanghellini, V.; Stewart, W.F.; Tack, J.; et al. Prevalence and Socioeconomic Impact of Upper Gastrointestinal Disorders in the United States: Results of the USA Upper Gastrointestinal Study. Clin. Gastroenterol. Hepatol. 2005, 3, 543–552. [Google Scholar] [CrossRef]
- Camilleri, M.; Parkman, H.P.; Shafi, M.A.; Abell, T.L.; Gerson, L.; American College of Gastroenterology. Clinical Guideline: Management of Gastroparesis. Am. J. Gastroenterol. 2013, 108, 18–37. [Google Scholar] [CrossRef] [PubMed]
- Maranzano, E.; De Angelis, V.; Pergolizzi, S.; Lupattelli, M.; Frata, P.; Spagnesi, S.; Frisio, M.L.; Mandoliti, G.; Malinverni, G.; Trippa, F.; et al. A prospective observational trial on emesis in radiotherapy: Analysis of 1020 patients recruited in 45 Italian radiation oncology centres. Radiother. Oncol. 2010, 94, 36–41. [Google Scholar] [CrossRef]
- Pergolizzi, S.; Maranzano, E.; De Angelis, V.; Lupattelli, M.; Frata, P.; Spagnesi, S.; Frisio, M.L.; Mandoliti, G.; Delia, P.; Malinverni, G.; et al. Diarrhoea in irradiated patients: A prospective multicentre observational study. Dig. Liver Dis. 2013, 45, 933–937. [Google Scholar] [CrossRef]
- Pergolizzi, S.; Maranzano, E.; Santacaterina, A. Diarrhoea and Pelvic Irradiation: A Neglected Issue. Clin. Oncol. 2014, 26, 669. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamburella, C.; Parisi, S.; Lillo, S.; Ferrantelli, G.; Critelli, P.; Viola, A.; Platania, A.; Santoro, M.; Cacciola, A.; Santacaterina, A.; et al. Gastroparesis, Thymoma, and Asymptomatic Myasthenia: A Rare Clinical Scenario. Gastroenterol. Insights 2022, 13, 27-32. https://doi.org/10.3390/gastroent13010004
Tamburella C, Parisi S, Lillo S, Ferrantelli G, Critelli P, Viola A, Platania A, Santoro M, Cacciola A, Santacaterina A, et al. Gastroparesis, Thymoma, and Asymptomatic Myasthenia: A Rare Clinical Scenario. Gastroenterology Insights. 2022; 13(1):27-32. https://doi.org/10.3390/gastroent13010004
Chicago/Turabian StyleTamburella, Consuelo, Silvana Parisi, Sara Lillo, Giacomo Ferrantelli, Paola Critelli, Anna Viola, Angelo Platania, Maria Santoro, Alberto Cacciola, Anna Santacaterina, and et al. 2022. "Gastroparesis, Thymoma, and Asymptomatic Myasthenia: A Rare Clinical Scenario" Gastroenterology Insights 13, no. 1: 27-32. https://doi.org/10.3390/gastroent13010004
APA StyleTamburella, C., Parisi, S., Lillo, S., Ferrantelli, G., Critelli, P., Viola, A., Platania, A., Santoro, M., Cacciola, A., Santacaterina, A., & Ferini, G. (2022). Gastroparesis, Thymoma, and Asymptomatic Myasthenia: A Rare Clinical Scenario. Gastroenterology Insights, 13(1), 27-32. https://doi.org/10.3390/gastroent13010004






