Experimental Primary Brain Calcification Model and Its Application to Pathogenesis Mechanism Analysis and Therapeutic Research
Abstract
1. Introduction
2. Genes Causing Familial PBC
2.1. SLC20A2
2.2. PDGFB
2.3. PDGFRB
2.4. XPR1
2.5. MYORG
2.6. JAM2
2.7. CMPK2
2.8. NAA60
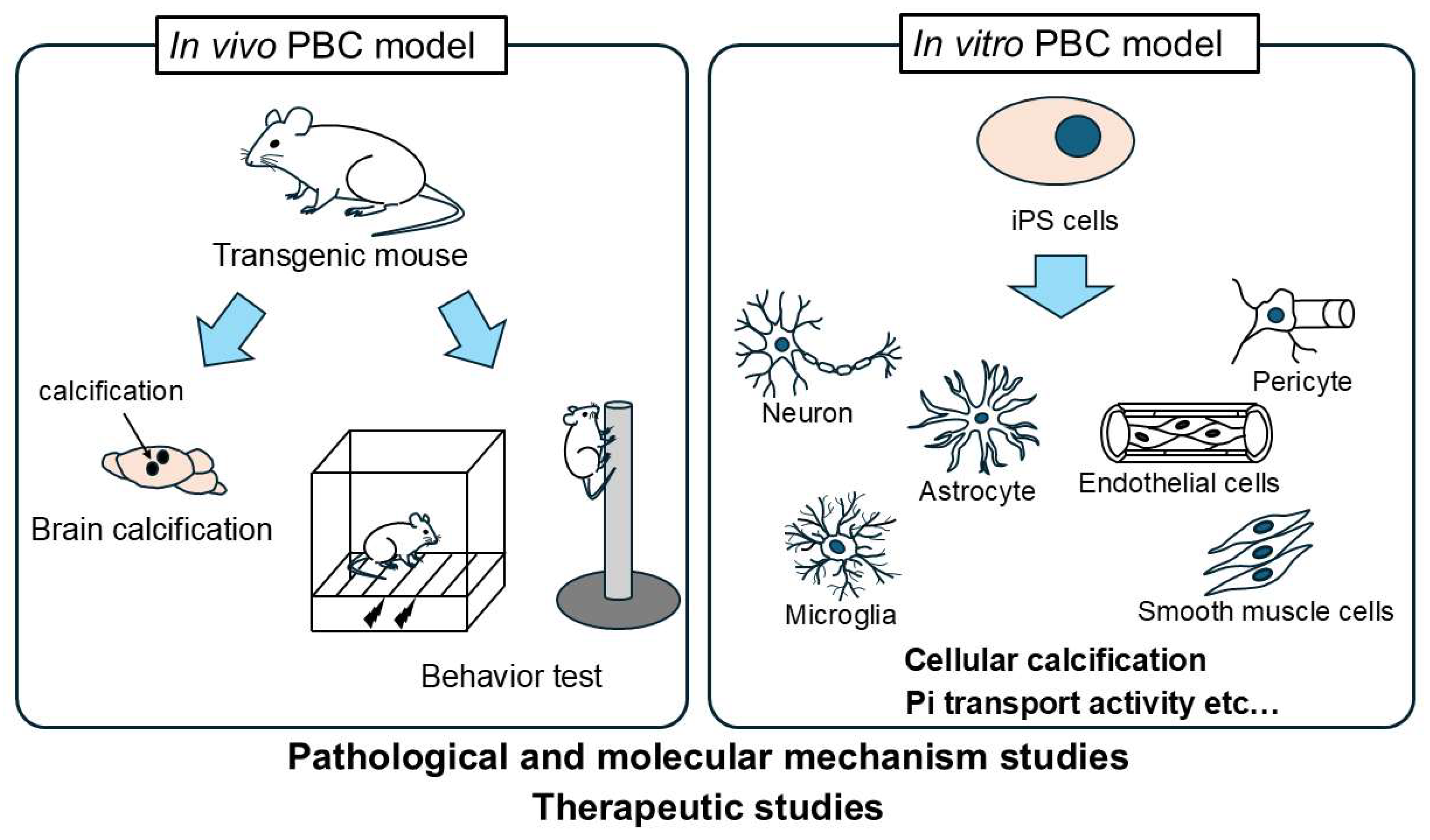
3. PBC Models
3.1. In Vivo Models
| Resourse Name | Target Gene | Information of Gene Modification or Variant | Brain Calcification | Other Phenotypes | Reference |
|---|---|---|---|---|---|
| C57BL/6NTac-Slc20a2tm1a-(EUCOMM)Wtsi/Ieg mice (EM: 05549) | Slc20a2 | Slc20a2 gene knockout | Calcifications were found in basal ganglia, cortex, and thalamus in homozygous 19-week-old mice (Jensen et al., J Mol Neurosci, 2013 [14]). Calcifications were found in the brains of heterozygous and homozygous 1-year-old mice (Wallingford et al., Brain Pathol, 2017 [36]) Calcifications were found in the hypothalamus, midbrain, thalamus and pons in homozygous 20-week-old mice (Jensen et al., Am J Pathol, 2018 [15]). Calcifications were found in the brains of homozygous 3-, 6-, and 12-month-old mice (Nahar et al., Brain Pathol, 2020 [37]) Calcifications were found in midbrain and hypothalamus in homozygous 80-day-old mice, and ventral striatum, basal forebrain, hypothalamus, thalamus, midbrain, and pons in 10-month-old homozygous mice (Ren et al., Front Genet, 2021 [38]). | Pi concentration of CSF was increased in homozygous 3-week-old mice (Jensen, Autzen, and Pedersen, Neurogenetics, 2016 [21]). Placental calcifications were found in heterozygous dam (Wallingford, Gammill, and Giachelli. Reprod Biol, 2016 [35]). Fetal growth retardation was found in hetrozygous and homozygous embryos at embryonic day 17.5 (Wallingford, Gammill, and Giachelli. Reprod Biol, 2016 [35]). Pi concentration of CSF was increased in homozygous 1-year-old mice (Wallingford et al., Brain Pathol, 2017 [36]). Body weight was reduced in homozygous 6-week-old mice (Wallingford et al., Brain Pathol, 2017 [36]). Hydrocephalus and premature death were observed in homozygous mice (Wallingford et al., Brain Pathol, 2017 [36]). Both microphthalmia and cataracts were observed in homozygous 6-week-old mice (Wallingford et al., Brain Pathol, 2017 [34]). Body weight was reduced in homozygous mice (Jensen et al., Am J Pathol, 2018 [14]). Congenital and global developmental delay, lean body mass, skeletal malformation, and a high proportion of unilateral or bilateral eye defects were found in homozygous mice (Ren et al., Front Genet, 2021 [36]) Spatial learning memory impairments and sensorimotor gating deficits were observed in homozygous 8-month-old mice (Ren et al., Front Genet, 2021 [36]). Impairment of blood–brain barrier (BBB) permeability was observed in homozygous mice (Zhang et al., Front Mol Neurosci, 2023 [18]). | Jensen et al., J Mol Neurosci, 2013 [14] Jensen, Autzen, and Pedersen, Neurogenetics, 2016 [21] Wallingford, Gammill, and Giachelli. Reprod Biol, 2016 [35] Wallingford et al., Brain Pathol, 2017 [36] Jensen et al., Am J Pathol, 2018 [15] Nahar et al., Brain Pathol, 2020 [37] Ren et al., Front Genet, 2021 [38] Zhang et al., Front Mol Neurosci, 2023 [18] |
| Slc20a2 knockout mice | Slc20a2 | Slc20a2 gene knockout | Calcifications were found in the thalamus, hypothalamus, midbrain, pons, and cerebral cortex in homozygous 11-month-old mice. | Body weight and survival rate were decreased in homozygous mice. | Kurita et al., Mol Brain, 2025 [17] |
| Aldh1l1-CreERT2:Pit2f/f mice | Slc20a2 | Astrocyte-specific Slc20a2 knockout | Calcifications were found in the basal forebrain and hypothalamus in 12-month-old Aldh1l1-CreERT2:Pit2f/f mice. | Pi concentration of CSF was increased in Aldh1l1-CreERT2:Pit2f/f mice. | Cheng et al., Neuron, 2024 [19] |
| A humanized SLC20A2 intron mice (SLC20A2-KI) | Slc20a2 | knocked in with the entire human intron 2 sequence (carrying the SLC20A2 c.289+1007 C>G variant) | Homozygous SLC20A2-KI mice began to show brain calcification in the hypothalamus and basal forebrain at the age of 5 months. Homozygous SLC20A2-KI mice exhibited calcification deposits widely distributed in the basal forebrain, thalamus, hypothalamus, midbrain, and pons at the age of 7 months. | Heterozygous and homozygous SLC20A2-KI mice exhibited significantly increased CSF Pi levels. | Zhao et al., Neuron, 2024 [39] |
| Disease-specific iPS cells for PBC | SLC20A2 | SLC20A2 (c.1848C>G (p.Trp616Ter)) | Not applicable | Decrease in Pi transport activity in endothelial cells derived from iPSC (Sekine et al., Biochem Biophys Res Commun, 2019 [42]) | Sekine et al., Stem Cell Res, 2017 [43] Sekine et al., Biochem Biophys Res Commun, 2019 [42] |
| Disease-specific iPS cells for PBC | SLC20A2 | SLC20A2 (c.613G>A (p.Val205Met)) | Not applicable | Golgi damage (Sun et al., Biochem Biophys Res Commun, 2023 [44]) | Zhang et al., Stem Cell Res, 2019 [45] Sun et al., Biochem Biophys Res Commun, 2023 [44] |
| Disease-specific iPS cells for PBC | SLC20A2 | SLC20A2 (del exon10) | Not applicable | Golgi damage (Sun et al., Biochem Biophys Res Commun, 2023 [44]) | Sun et al., Biochem Biophys Res Commun, 2023 [44] |
| Disease-specific iPS cells for PBC | SLC20A2 | SLC20A2 (c.687dupT (p.Val230CysfsTer28)) | Not applicable | No data | Begentas et al., Stem Cell Res, 2023 [46] |
| Pdgfbret/ret mice | Pdgfb | hypomorphic Pdgfb alleles | Calcifications were found in the basal forebrain, thalamus, midbrain and pons of homozygous 1-year-old mice (Keller et al., Nat Genet, 2013 [23]). Calcifications were found in the brains of homozygous 3-, 6-, and 12-month-old mice (Nahar et al., Brain Pathol, 2020 [37]). | Impairment of blood–brain barrier (BBB) permeability was observed in homozygous mice (Armulik et al., Nature, 2010 [25]; Nahar et al., Brain Pathol, 2020 [37]). | Keller et al., Nat Genet, 2013 [23] Armulik et al., Nature, 2010 [25] Nahar et al., Brain Pathol, 2020 [37] |
| Pdgfb−/−; R26P+/0 mice | Pdgfb | Pdgfb-null mice rescued to adulthood by transgenic re-expression of PDGF-B in the endothelium | Calcifications were found in the thalamus in 1-year-old Pdgfb−/−; R26P+/0 mice (Keller et al., Nat Genet, 2013 [23]). | Impairment of blood–brain barrier (BBB) permeability was observed in Pdgfb−/−; R26P+/0 mice (Armulik et al., Nature, 2010 [25]). | Keller et al., Nat Genet, 2013 [23] Armulik et al., Nature, 2010 [25] |
| Disease-specific iPS cells for PBC | PDGFB | PDGFB (c.160+2T>A) | Not applicable | Decrease in PDGFB level in the culture media from endothelial cells derived from iPSC (Sekine et al., Sci Rep, 2019 [47]) | Sekine et al., Sci Rep, 2019 [47] |
| Disease-specific iPS cells for PBC | PDGFB | PDGFB (c.457-1G>T) | Not applicable | Decrease in PDGFB level in the culture media from endothelial cells derived from iPSC (Sekine et al., Sci Rep, 2019 [47]) | Sekine et al., Sci Rep, 2019 [47] |
| Disease-specific iPS cells for PBC | PDGFB | PDGFB (c.33_34delCT) | Not applicable | Decrease in PDGFB level in the culture media from endothelial cells derived from iPSC (Sekine et al., Sci Rep, 2019 [47]) | Sekine et al., Sci Rep, 2019 [47] |
| Xpr1 knockout mice | Xpr1 | Xpr1 gene knockout | No data | Homozygous mice died, and showed placental calcification, embryonic calcification and growth restriction. | Xu et al., J Bone Miner Res, 2020 [40] |
| C57BL/6N-Xpr1tm1a(KOMP)Wtsi mice (MGI: 4362650) | Xpr1 | Xpr1 gene knockout | Calcifications were found in the thalamus of heterozygous 7-, 10-, 12-, and 16-month-old mice. | Pi concentration of CSF was decreased in heterozygous mice. Heterozygous mice present with an altered acoustic startle response. | Maheshwari et al., Brain Pathol, 2023 [41] |
| Myorg knockout mice | Myorg | Myorg gene knockout | Calcifications were found in the thalamus in homozygous 9-month-old mice (Yao et al., Neuron, 2018 [5]). | Pi concentration of CSF was increased in homozygous mice (Cheng et al., Neuron, 2024 [19]). | Yao et al., Neuron, 2018 [5] Cheng et al., Neuron, 2024 [19] |
| Jam2 knockout mice | Jam2 | Jam2 gene knockout | No calcifications were found in brain. | Prominent vacuolation in the cerebral cortex, thalamus, and cerebellum and particularly widespread vacuolation in the midbrain were found in homozygous mice. Gait abnormalities were observed in homozygous mice. | Schottlaender ey al., Am J Hum Genet, 2020 [7] |
| Cmpk2 knockout mice | Cmpk2 | Cmpk2 gene knockout | Calcifications were found in the thalamus of homozygous 10-, 12-, and 14-month-old mice. | Disruption of mitochondrial function was observed in homozygous mice. | Zhao et al., Cell Discov, 2022 [31] |
| Cmpk2 knock-in mice | Cmpk2 | knocked in with c.2 T>C mutation | Calcifications were found in the thalamus of homozygous 12-month-old mice. | Disruption of mitochondrial function was observed in homozygous mice. | Zhao et al., Cell Discov, 2022 [31] |
| Disease-specific iPS cells for PBC | Not identified (from sporadic patient) | No data | Not applicable | No data | Yada et al., Stem Cell Res, 2021 [48] |
3.2. In Vitro Models
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, C.; Li, Y.; Shi, L.; Ren, J.; Patti, M.; Wang, T.; de Oliveira, J.R.; Sobrido, M.J.; Quintans, B.; Baquero, M.; et al. Mutations in SLC20A2 link familial idiopathic basal ganglia calcification with phosphate homeostasis. Nat. Genet. 2012, 44, 254–256. [Google Scholar] [CrossRef] [PubMed]
- Andrae, J.; Gallini, R.; Betsholtz, C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 2008, 22, 1276–1312. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, G.; Pottier, C.; Maltete, D.; Coutant, S.; Rovelet-Lecrux, A.; Legallic, S.; Rousseau, S.; Vaschalde, Y.; Guyant-Marechal, L.; Augustin, J.; et al. Mutation of the PDGFRB gene as a cause of idiopathic basal ganglia calcification. Neurology 2013, 80, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Legati, A.; Giovannini, D.; Nicolas, G.; Lopez-Sanchez, U.; Quintans, B.; Oliveira, J.R.; Sears, R.L.; Ramos, E.M.; Spiteri, E.; Sobrido, M.J.; et al. Mutations in XPR1 cause primary familial brain calcification associated with altered phosphate export. Nat. Genet. 2015, 47, 579–581. [Google Scholar] [CrossRef]
- Yao, X.P.; Cheng, X.; Wang, C.; Zhao, M.; Guo, X.X.; Su, H.Z.; Lai, L.L.; Zou, X.H.; Chen, X.J.; Zhao, Y.; et al. Biallelic Mutations in MYORG Cause Autosomal Recessive Primary Familial Brain Calcification. Neuron 2018, 98, 1116–1123.e5. [Google Scholar] [CrossRef]
- Meek, R.W.; Brockerman, J.; Fordwour, O.B.; Zandberg, W.F.; Davies, G.J.; Vocadlo, D.J. The primary familial brain calcification-associated protein MYORG is an alpha-galactosidase with restricted substrate specificity. PLoS Biol. 2022, 20, e3001764. [Google Scholar] [CrossRef]
- Schottlaender, L.V.; Abeti, R.; Jaunmuktane, Z.; Macmillan, C.; Chelban, V.; O’Callaghan, B.; McKinley, J.; Maroofian, R.; Efthymiou, S.; Athanasiou-Fragkouli, A.; et al. Bi-allelic JAM2 Variants Lead to Early-Onset Recessive Primary Familial Brain Calcification. Am. J. Hum. Genet. 2020, 106, 412–421. [Google Scholar] [CrossRef]
- Li, J.; Liu, Q.; Yang, J.; Yang, Z.; Zhang, M.; Zhang, J.; Sun, C. Biological functions and clinical implications of the CMPK2 across multisystemic diseases. Cell Biosci. 2025, 15, 122. [Google Scholar] [CrossRef]
- Siggervag, A.; Bekkelund, A.K.; Saraste, J.; Aksnes, H. Understanding brain calcification via N-terminal acetylation at the Golgi apparatus. Brain 2025, 148, 3085–3094. [Google Scholar] [CrossRef]
- Black, A.; Klein, C.; Westenberger, A. Primary Familial Brain Calcification. In GeneReviews; Updated 2025; University of Washington: Seattle, WA, USA, 2004. [Google Scholar]
- Chen, S.Y.; Ho, C.J.; Lu, Y.T.; Lin, C.H.; Lan, M.Y.; Tsai, M.H. The Genetics of Primary Familial Brain Calcification: A Literature Review. Int. J. Mol. Sci. 2023, 24, 10886. [Google Scholar] [CrossRef]
- Da Silva, R.J.; Pereira, I.C.; Oliveira, J.R. Analysis of gene expression pattern and neuroanatomical correlates for SLC20A2 (PiT-2) shows a molecular network with potential impact in idiopathic basal ganglia calcification (“Fahr’s disease”). J. Mol. Neurosci. 2013, 50, 280–283. [Google Scholar] [CrossRef] [PubMed]
- Inden, M.; Iriyama, M.; Zennami, M.; Sekine, S.I.; Hara, A.; Yamada, M.; Hozumi, I. The type III transporters (PiT-1 and PiT-2) are the major sodium-dependent phosphate transporters in the mice and human brains. Brain Res. 2016, 1637, 128–136. [Google Scholar] [CrossRef]
- Jensen, N.; Schroder, H.D.; Hejbol, E.K.; Fuchtbauer, E.M.; de Oliveira, J.R.; Pedersen, L. Loss of function of Slc20a2 associated with familial idiopathic Basal Ganglia calcification in humans causes brain calcifications in mice. J. Mol. Neurosci. 2013, 51, 994–999. [Google Scholar] [CrossRef] [PubMed]
- Jensen, N.; Schroder, H.D.; Hejbol, E.K.; Thomsen, J.S.; Bruel, A.; Larsen, F.T.; Vinding, M.C.; Orlowski, D.; Fuchtbauer, E.M.; Oliveira, J.R.M.; et al. Mice Knocked Out for the Primary Brain Calcification-Associated Gene Slc20a2 Show Unimpaired Prenatal Survival but Retarded Growth and Nodules in the Brain that Grow and Calcify over Time. Am. J. Pathol. 2018, 188, 1865–1881. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Miura, T.; Aoki, K.; Saito, S.; Hondo, H.; Konno, T.; Uchiyama, A.; Ikeuchi, T.; Takahashi, H.; Kakita, A. Familial idiopathic basal ganglia calcification: Histopathologic features of an autopsied patient with an SLC20A2 mutation. Neuropathology 2016, 36, 365–371. [Google Scholar] [CrossRef]
- Kurita, H.; Kitaura, H.; Nishii, K.; Masaka, T.; Ohuchi, K.; Inden, M.; Kakita, A.; Osawa, M.; Hozumi, I. Generation of a new Slc20a2 knockout mouse line as in vivo model for primary brain calcification. Mol. Brain 2025, 18, 70. [Google Scholar] [CrossRef]
- Zhang, Y.; Ren, Y.; Zhang, Y.; Li, Y.; Xu, C.; Peng, Z.; Jia, Y.; Qiao, S.; Zhang, Z.; Shi, L. T-cell infiltration in the central nervous system and their association with brain calcification in Slc20a2-deficient mice. Front. Mol. Neurosci. 2023, 16, 1073723. [Google Scholar] [CrossRef]
- Cheng, X.; Zhao, M.; Chen, L.; Huang, C.; Xu, Q.; Shao, J.; Wang, H.T.; Zhang, Y.; Li, X.; Xu, X.; et al. Astrocytes modulate brain phosphate homeostasis via polarized distribution of phosphate uptake transporter PiT2 and exporter XPR1. Neuron 2024, 112, 3126–3142.e8. [Google Scholar] [CrossRef]
- Guerreiro, P.M.; Bataille, A.M.; Parker, S.L.; Renfro, J.L. Active removal of inorganic phosphate from cerebrospinal fluid by the choroid plexus. Am. J. Physiol. Renal Physiol. 2014, 306, F1275–F1284. [Google Scholar] [CrossRef]
- Jensen, N.; Autzen, J.K.; Pedersen, L. Slc20a2 is critical for maintaining a physiologic inorganic phosphate level in cerebrospinal fluid. Neurogenetics 2016, 17, 125–130. [Google Scholar] [CrossRef]
- Hozumi, I.; Kurita, H.; Ozawa, K.; Furuta, N.; Inden, M.; Sekine, S.I.; Yamada, M.; Hayashi, Y.; Kimura, A.; Inuzuka, T.; et al. Inorganic phosphorus (Pi) in CSF is a biomarker for SLC20A2-associated idiopathic basal ganglia calcification (IBGC1). J. Neurol. Sci. 2018, 388, 150–154. [Google Scholar] [CrossRef]
- Keller, A.; Westenberger, A.; Sobrido, M.J.; Garcia-Murias, M.; Domingo, A.; Sears, R.L.; Lemos, R.R.; Ordonez-Ugalde, A.; Nicolas, G.; da Cunha, J.E.; et al. Mutations in the gene encoding PDGF-B cause brain calcifications in humans and mice. Nat. Genet. 2013, 45, 1077–1082. [Google Scholar] [CrossRef] [PubMed]
- Irma, J.; Kartasasmita, A.S.; Kartiwa, A.; Irfani, I.; Rizki, S.A.; Onasis, S. From Growth Factors to Structure: PDGF and TGF-beta in Granulation Tissue Formation. A Literature Review. J. Cell Mol. Med. 2025, 29, e70374. [Google Scholar] [CrossRef] [PubMed]
- Armulik, A.; Genove, G.; Mae, M.; Nisancioglu, M.H.; Wallgard, E.; Niaudet, C.; He, L.; Norlin, J.; Lindblom, P.; Strittmatter, K.; et al. Pericytes regulate the blood-brain barrier. Nature 2010, 468, 557–561. [Google Scholar] [CrossRef] [PubMed]
- Kempuraj, D.; Dourvetakis, K.D.; Cohen, J.; Valladares, D.S.; Joshi, R.S.; Kothuru, S.P.; Anderson, T.; Chinnappan, B.; Cheema, A.K.; Klimas, N.G.; et al. Neurovascular unit, neuroinflammation and neurodegeneration markers in brain disorders. Front. Cell Neurosci. 2024, 18, 1491952. [Google Scholar] [CrossRef]
- Betsholtz, C.; Raines, E.W. Platelet-derived growth factor: A key regulator of connective tissue cells in embryogenesis and pathogenesis. Kidney Int. 1997, 51, 1361–1369. [Google Scholar] [CrossRef]
- Quintans, B.; Oliveira, J.; Sobrido, M.J. Primary familial brain calcifications. Handb. Clin. Neurol. 2018, 147, 307–317. [Google Scholar]
- Lopez-Sanchez, U.; Tury, S.; Nicolas, G.; Wilson, M.S.; Jurici, S.; Ayrignac, X.; Courgnaud, V.; Saiardi, A.; Sitbon, M.; Battini, J.L. Interplay between primary familial brain calcification-associated SLC20A2 and XPR1 phosphate transporters requires inositol polyphosphates for control of cellular phosphate homeostasis. J. Biol. Chem. 2020, 295, 9366–9378. [Google Scholar] [CrossRef]
- Cen, Z.; Chen, Y.; Chen, S.; Wang, H.; Yang, D.; Zhang, H.; Wu, H.; Wang, L.; Tang, S.; Ye, J.; et al. Biallelic loss-of-function mutations in JAM2 cause primary familial brain calcification. Brain 2020, 143, 491–502. [Google Scholar] [CrossRef]
- Zhao, M.; Su, H.Z.; Zeng, Y.H.; Sun, Y.; Guo, X.X.; Li, Y.L.; Wang, C.; Zhao, Z.Y.; Huang, X.J.; Lin, K.J.; et al. Loss of function of CMPK2 causes mitochondria deficiency and brain calcification. Cell Discov. 2022, 8, 128. [Google Scholar] [CrossRef]
- Xu, Y.; Johansson, M.; Karlsson, A. Human UMP-CMP kinase 2, a novel nucleoside monophosphate kinase localized in mitochondria. J. Biol. Chem. 2008, 283, 1563–1571. [Google Scholar] [CrossRef] [PubMed]
- Chelban, V.; Aksnes, H.; Maroofian, R.; LaMonica, L.C.; Seabra, L.; Siggervag, A.; Devic, P.; Shamseldin, H.E.; Vandrovcova, J.; Murphy, D.; et al. Biallelic NAA60 variants with impaired n-terminal acetylation capacity cause autosomal recessive primary familial brain calcifications. Nat. Commun. 2024, 15, 2269. [Google Scholar] [CrossRef]
- Aksnes, H.; Ree, R.; Arnesen, T. Co-translational, Post-translational, and Non-catalytic Roles of N-Terminal Acetyltransferases. Mol. Cell 2019, 73, 1097–1114. [Google Scholar] [CrossRef] [PubMed]
- Wallingford, M.C.; Gammill, H.S.; Giachelli, C.M. Slc20a2 deficiency results in fetal growth restriction and placental calcification associated with thickened basement membranes and novel CD13 and lamininalpha1 expressing cells. Reprod. Biol. 2016, 16, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Wallingford, M.C.; Chia, J.J.; Leaf, E.M.; Borgeia, S.; Chavkin, N.W.; Sawangmake, C.; Marro, K.; Cox, T.C.; Speer, M.Y.; Giachelli, C.M. SLC20A2 Deficiency in Mice Leads to Elevated Phosphate Levels in Cerbrospinal Fluid and Glymphatic Pathway-Associated Arteriolar Calcification, and Recapitulates Human Idiopathic Basal Ganglia Calcification. Brain Pathol. 2017, 27, 64–76. [Google Scholar] [CrossRef]
- Nahar, K.; Lebouvier, T.; Andaloussi Mae, M.; Konzer, A.; Bergquist, J.; Zarb, Y.; Johansson, B.; Betsholtz, C.; Vanlandewijck, M. Astrocyte-microglial association and matrix composition are common events in the natural history of primary familial brain calcification. Brain Pathol. 2020, 30, 446–464. [Google Scholar] [CrossRef]
- Ren, Y.; Shen, Y.; Si, N.; Fan, S.; Zhang, Y.; Xu, W.; Shi, L.; Zhang, X. Slc20a2-Deficient Mice Exhibit Multisystem Abnormalities and Impaired Spatial Learning Memory and Sensorimotor Gating but Normal Motor Coordination Abilities. Front. Genet. 2021, 12, 639935. [Google Scholar] [CrossRef]
- Zhao, M.; Cheng, X.; Chen, L.; Zeng, Y.H.; Lin, K.J.; Li, Y.L.; Zheng, Z.H.; Huang, X.J.; Zuo, D.D.; Guo, X.X.; et al. Antisense oligonucleotides enhance SLC20A2 expression and suppress brain calcification in a humanized mouse model. Neuron 2024, 112, 3278–3294.e7. [Google Scholar] [CrossRef]
- Xu, X.; Li, X.; Sun, H.; Cao, Z.; Gao, R.; Niu, T.; Wang, Y.; Ma, T.; Chen, R.; Wang, C.; et al. Murine Placental-Fetal Phosphate Dyshomeostasis Caused by an Xpr1 Deficiency Accelerates Placental Calcification and Restricts Fetal Growth in Late Gestation. J. Bone Miner. Res. 2020, 35, 116–129. [Google Scholar] [CrossRef]
- Maheshwari, U.; Mateos, J.M.; Weber-Stadlbauer, U.; Ni, R.; Tamatey, V.; Sridhar, S.; Restrepo, A.; de Jong, P.A.; Huang, S.F.; Schaffenrath, J.; et al. Inorganic phosphate exporter heterozygosity in mice leads to brain vascular calcification, microangiopathy, and microgliosis. Brain Pathol. 2023, 33, e13189. [Google Scholar] [CrossRef]
- Sekine, S.I.; Nishii, K.; Masaka, T.; Kurita, H.; Inden, M.; Hozumi, I. SLC20A2 variants cause dysfunctional phosphate transport activity in endothelial cells induced from Idiopathic Basal Ganglia Calcification patients-derived iPSCs. Biochem. Biophys. Res. Commun. 2019, 510, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Sekine, S.I.; Kondo, T.; Murakami, N.; Imamura, K.; Enami, T.; Shibukawa, R.; Tsukita, K.; Funayama, M.; Inden, M.; Kurita, H.; et al. Induced pluripotent stem cells derived from a patient with familial idiopathic basal ganglia calcification (IBGC) caused by a mutation in SLC20A2 gene. Stem Cell Res. 2017, 24, 40–43. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Wang, Z.; Zhang, Q.; Chen, N.; Tang, M.; Yang, Z.; Xu, Y.; Kang, J.; Wang, Y. Golgi damage caused by dysfunction of PiT-2 in primary familial brain calcification. Biochem. Biophys. Res. Commun. 2023, 642, 167–174. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, Z.; Sun, H.; Zhang, S.; Zhang, J.; Wang, Y.; Fang, H.; Xu, Y. Generation of induced pluripotent stem cell line (ZZUi0012-A) from a patient with Fahr’s disease caused by a novel mutation in SLC20A2 gene. Stem Cell Res. 2019, 35, 101395. [Google Scholar] [CrossRef] [PubMed]
- Begentas, O.C.; Koc, D.; Sendur, N.K.; Besarat, P.; Ezgin, S.; Temel, M.; Bora, H.A.T.; Kiris, E. Generation and characterization of human induced pluripotent stem cell line METUi002-A from a patient with primary familial brain calcification (PFBC) carrying a heterozygous mutation (c.687dupT (p.Val230CysfsTer28)) in the SLC20A2 gene. Stem Cell Res. 2023, 72, 103226. [Google Scholar] [CrossRef]
- Sekine, S.I.; Kaneko, M.; Tanaka, M.; Ninomiya, Y.; Kurita, H.; Inden, M.; Yamada, M.; Hayashi, Y.; Inuzuka, T.; Mitsui, J.; et al. Functional evaluation of PDGFB-variants in idiopathic basal ganglia calcification, using patient-derived iPS cells. Sci. Rep. 2019, 9, 5698. [Google Scholar] [CrossRef]
- Yada, Y.; Kondo, T.; Suga, M.; Tsukita, K.; Enami, T.; Shibukawa, R.; Sagara, Y.; Okanishi, Y.; Imamura, K.; Kihara, T.; et al. Human induced pluripotent stem cells generated from a patient with idiopathic basal ganglia calcification. Stem Cell Res. 2021, 53, 102274. [Google Scholar] [CrossRef]
- Nicolas, G.; Charbonnier, C.; Campion, D.; Veltman, J.A. Estimation of minimal disease prevalence from population genomic data: Application to primary familial brain calcification. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2018, 177, 68–74. [Google Scholar] [CrossRef]
- Chen, S.; Cen, Z.; Fu, F.; Chen, Y.; Chen, X.; Yang, D.; Wang, H.; Wu, H.; Zheng, X.; Xie, F.; et al. Underestimated disease prevalence and severe phenotypes in patients with biallelic variants: A cohort study of primary familial brain calcification from China. Parkinsonism Relat. Disord. 2019, 64, 211–219. [Google Scholar] [CrossRef]
- Luo, W.; Cen, Z.; Koek, H.; Carecchio, M.; Hozumi, I.; Chen, W.J.; Batla, A.; Balck, A.; Magrinelli, F.; Yang, D.; et al. Primary Brain Calcification: An International Consensus on Nomenclature, Diagnosis, Evaluation, and Management. Mov. Disord. 2025. online ahead of print. [Google Scholar] [CrossRef]
| Gene Name | Functions | Mutations Involved [10] | Frequency in Genetic-Confirmed PBC Patients [10] |
|---|---|---|---|
| SLC20A2 | phosphate transporter | Single-nucleotide variants (SNVs) (Most common variants were missense.), structural variants, and in-frame insertions/deletions | 58% |
| PDGFB | formation of BBB | SNVs (Most common variants were missense.), structural variants and small frameshift insertions or deletions | 13% |
| PDGFRB | formation of BBB | SNVs (Only missense) | 4% |
| XPR1 | phosphate transporter | SNVs (Most common variants were missense.) | 6% |
| MYORG | others/unknown | SNVs (Most common variants were missense.), and small insertions or deletions including in-frame deletions | 14% |
| JAM2 | formation of BBB | SNVs (Most common variants were nonsense.), small frameshift insertions/deletions and structural or splice site/region variants | 3% |
| CMPK2 | mitochondrial function | SNVs (Most common variants were missense and start codon loss.) | unknown |
| NAA60 | others/unknown | SNVs (Most common variants were missense.), and a smaller fraction are frameshift deletions, insertions, or splice site region variants. | 2% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Kurita, H.; Murata, J.; Ohuchi, K.; Hayashi, Y.; Inden, M. Experimental Primary Brain Calcification Model and Its Application to Pathogenesis Mechanism Analysis and Therapeutic Research. Neurol. Int. 2026, 18, 4. https://doi.org/10.3390/neurolint18010004
Kurita H, Murata J, Ohuchi K, Hayashi Y, Inden M. Experimental Primary Brain Calcification Model and Its Application to Pathogenesis Mechanism Analysis and Therapeutic Research. Neurology International. 2026; 18(1):4. https://doi.org/10.3390/neurolint18010004
Chicago/Turabian StyleKurita, Hisaka, Junya Murata, Kazuki Ohuchi, Yuichi Hayashi, and Masatoshi Inden. 2026. "Experimental Primary Brain Calcification Model and Its Application to Pathogenesis Mechanism Analysis and Therapeutic Research" Neurology International 18, no. 1: 4. https://doi.org/10.3390/neurolint18010004
APA StyleKurita, H., Murata, J., Ohuchi, K., Hayashi, Y., & Inden, M. (2026). Experimental Primary Brain Calcification Model and Its Application to Pathogenesis Mechanism Analysis and Therapeutic Research. Neurology International, 18(1), 4. https://doi.org/10.3390/neurolint18010004







