Plasma Neurofilament Light Chain Is Associated with Cognitive Functions but Not Patient-Reported Outcomes in Multiple Sclerosis
Abstract
1. Introduction
2. Methods
2.1. Study Design and Population
2.2. Demographics and Clinical Variables
2.3. NfL Measurement
2.4. Cognitive Variables
2.5. PROs
2.6. Statistical Analyses
Power Calculation
3. Results
3.1. Study Population
3.2. Demographic and Clinical Correlates
3.3. Cognitive and PRO Correlates
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dineen, R.A.; Vilisaar, J.; Hlinka, J.; Bradshaw, C.M.; Morgan, P.S.; Constantinescu, C.S.; Auer, D.P. Disconnection as a mechanism for cognitive dysfunction in multiple sclerosis. Brain 2009, 132, 239–249. [Google Scholar] [CrossRef]
- Benedict, R.H.B.; Amato, M.P.; DeLuca, J.; Geurts, J.J.G. Cognitive impairment in multiple sclerosis: Clinical management, MRI, and therapeutic avenues. Lancet Neurol. 2020, 19, 860–871. [Google Scholar] [CrossRef]
- Zaratin, P.; Vermersch, P.; Amato, M.P.; Brichetto, G.; Coetzee, T.; Cutter, G.; Edan, G.; Giovannoni, G.; Gray, E.; Hartung, H.P.; et al. The agenda of the global patient reported outcomes for multiple sclerosis (PROMS) initiative: Progresses and open questions. Mult. Scler. Relat. Disord. 2022, 61, 103757. [Google Scholar] [CrossRef]
- Abdelhak, A.; Antweiler, K.; Kowarik, M.C.; Senel, M.; Havla, J.; Zettl, U.K.; Hoshi, M.M.; Skripuletz, T.; Haarmann, A.; Sthmann, A.; et al. Patient-reported outcome parameters and disability worsening in progressive multiple sclerosis. Mult. Scler. Relat. Disord. 2024, 81, 105139. [Google Scholar] [CrossRef]
- Cruz Rivera, S.; Buxhoeveden, S.; Aiyegbusi, O.L.; Bozinov, N.; Kamudoni, P.; McBurney, R.; Calvert, M. The importance of patient-reported outcomes: A call for their integration in the routine care of patients with multiple sclerosis. Mult. Scler. 2025, 13524585251349354. [Google Scholar] [CrossRef]
- Moccia, M.; Fontana, L.; Palladino, R.; Falco, F.; Finiello, F.; Fedele, M.; Lanzillo, R.; Reppuccia, L.; Triassi, M.; Brascia Morra, V.; et al. Determinants of early working impairments in multiple sclerosis. Front. Neurol. 2022, 13, 1062847. [Google Scholar] [CrossRef]
- Tang, S.; Liu, R.; Ren, J.; Song, L.; Dong, L.; Quin, Y.; Zhao, M.; Wang, Y.; Dong, Y.; Zhao, T.; et al. Association of objective sleep duration with cognition and brain aging biomarkers in older adults. Brain Commun. 2024, 6, fcae144. [Google Scholar] [CrossRef] [PubMed]
- Kos, D.; Kerckhofs, E.; Nagels, G.; D’hooghe, M.B.; Ilsbroukx, S. Origin of fatigue in multiple sclerosis: Review of the literature. Neurorehabilit. Neural Repair 2008, 22, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, I.; Nicolella, V.; Fiorenza, M.; Novarella, F.; Carotenuto, A.; Lanzillo, R.; Mauriello, L.; Scalia, G.; Castaldo, G.; Terracciano, D.; et al. The ocrelizumab wearing-off phenomenon is associated with reduced immunomodulatory response and increased neuroaxonal damage in multiple sclerosis. J. Neurol. 2024, 271, 5012–5024. [Google Scholar] [CrossRef] [PubMed]
- Esposito, A.; Falco, F.; Scalia, G.; Gentile, L.; Spiezia, A.L.; Corsini, G.; Manganiello, R.; Eliano, M.; Lamagna, F.; Moccia, M.; et al. Association between CD20 + T lymphocytes and neuropsychological findings in multiple sclerosis. Eur. J. Neurol. 2025, 32, e16536. [Google Scholar] [CrossRef]
- Capone, F.; Collorone, S.; Cortese, R.; Di Lazzaro, V.; Moccia, M. Fatigue in multiple sclerosis: The role of thalamus. Mult. Scler. 2020, 26, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Khalil, M.; Teunissen, C.E.; Lehmann, S.; Otto, M.; Piehl, F.; Ziemssen, T.; Bittner, S.; Sormani, M.P.; Gattringer, T.; Abu-Rumeileh, S.; et al. Neurofilaments as biomarkers in neurological disorders—Towards clinical application. Nat. Rev. Neurol. 2024, 20, 269–287. [Google Scholar] [CrossRef] [PubMed]
- Abdelhak, A.; Benkert, P.; Schaedelin, S.; Boscardin, W.J.; Cordano, C.; Oechtering, J.; Ananth, K.; Granziera, C.; Melie-Garcia, L.; Montes, S.C.; et al. Neurofilament Light Chain Elevation and Disability Progression in Multiple Sclerosis. JAMA Neurol. 2023, 80, 1317–1325. [Google Scholar] [CrossRef]
- Kuhle, J.; Kropshofer, H.; Haering, D.A.; Kundu, U.; Meinert, R.; Barro, C.; Dahlke, F.; Tomic, D.; Leppert, D.; Kappos, L.; et al. Blood neurofilament light chain as a biomarker of MS disease activity and treatment response. Neurology 2019, 92, E1007–E1015. [Google Scholar] [CrossRef] [PubMed]
- Gaetani, L.; Salvadori, N.; Lisetti, V.; Eusebi, P.; Mancini, A.; Gentili, L.; Borrelli, A.; Portaccio, E.; Sarchielli, P.; Blennow, K.; et al. Cerebrospinal fluid neurofilament light chain tracks cognitive impairment in multiple sclerosis. J. Neurol. 2019, 266, 2157–2163. [Google Scholar] [CrossRef]
- Jakimovski, D.; Zivadinov, R.; Ramanthan, M.; Hagemeier, J.; Weinstock-Guttman, B.; Tomic, D.; Kropshofer, H.; Fuchs, T.A.; Barro, C.; Leppert, D.; et al. Serum neurofilament light chain level associations with clinical and cognitive performance in multiple sclerosis: A longitudinal retrospective 5-year study. Mult. Scler. J. 2020, 26, 1670–1681. [Google Scholar] [CrossRef]
- Gaetani, L.; Schoonheim, M.M. Serum neurofilament light chain predicts cognitive worsening in secondary progressive multiple sclerosis better than brain MRI measures. Mult. Scler. 2022, 28, 1831–1833. [Google Scholar] [CrossRef]
- Williams, T.; Tur, C.; Eshaghi, A.; Doshi, A.; Chan, D.; Binks, S.; Wellington, H.; Heslegrave, A.; Zetterberg, H.; Chataway, J. Serum neurofilament light and MRI predictors of cognitive decline in patients with secondary progressive multiple sclerosis: Analysis from the MS-STAT randomised controlled trial. Mult. Scler. J. 2022, 28, 1913–1926. [Google Scholar] [CrossRef]
- Barro, C.; Healy, B.C.; Saxena, S.; Glanz, B.I.; Paul, A.; Polgar-Turcsanyi, M.; Guttmann, C.R.; Bakshi, R.; Weiner, H.L.; Chitnis, T. Serum NfL but not GFAP predicts cognitive decline in active progressive multiple sclerosis patients. Mult. Scler. J. 2023, 29, 206–211. [Google Scholar] [CrossRef]
- Aktas, O.; Renner, A.; Huss, A.; Filser, M.; Baetge, S.; Stute, N.; Gasis, M.; Lepka, K.; Goebels, N.; Senel, M.; et al. Serum neurofilament light chain: No clear relation to cognition and neuropsychiatric symptoms in stable MS. Neurol. Neuroimmunol. Neuroinflammation 2020, 7, e885. [Google Scholar] [CrossRef]
- Håkansson, I.; Johansson, L.; Dahle, C.; Vrethem, M.; Ernerudh, J. Fatigue scores correlate with other self-assessment data, but not with clinical and biomarker parameters, in CIS and RRMS. Mult. Scler. Relat. Disord. 2019, 36, 101424. [Google Scholar] [CrossRef]
- Thebault, S.; Tessier, D.R.; Lee, H.; Bowman, M.; Bar-Or, A.; Arnold, D.L.; Atkins, H.L.; Tabard-Cossa, V.; Freedman, M.S. High serum neurofilament light chain normalizes after hematopoietic stem cell transplantation for MS. Neurol. Neuroimmunol. Neuroinflammation 2019, 6, e598. [Google Scholar] [CrossRef]
- Galetta, K.; Deshpande, C.; Healy, B.C.; Glanz, B.; Ziehn, M.; Saleh, F.; Paul, A.; Saleh, F.; Collins, M.; Gaitan-Walsh, P.; et al. Serum neurofilament levels and patient-reported outcomes in multiple sclerosis. Ann. Clin. Transl. Neurol. 2021, 8, 631–638. [Google Scholar] [CrossRef]
- Simren, J.; Andreasson, U.; Gobom, J.; Calvet, M.S.; Borroni, B.; Gillberg, C.; Nyberg, L.; Ghidoni, R.; Fernell, E.; Johnson, M.; et al. Establishment of reference values for plasma neurofilament light based on healthy individuals aged 5–90 years. Brain Commun. 2022, 4, fcac174. [Google Scholar] [CrossRef] [PubMed]
- Novarella, F.; Nicolella, V.; Fiorenza, M.; Falco, F.; Monteiro, I.; Corsini, G.; Ranucci, D.; Carotenuto, A.; Petracca, M.; Lanzillo, R.; et al. Neurofilament light chain and Alzheimer pathology biomarkers in elderly people with multiple sclerosis. J. Neurol. Sci. 2025, 475, 123562. [Google Scholar] [CrossRef]
- Nicolella, V.; Fiorenza, M.; Monteiro, I.; Novarella, F.; Sirica, R.; D’Angelo, M.; Carbone, G.; La Civita, E.; Esposito, A.; Criscuolo, V.; et al. Clinical utility of the Lumipulse™ immunoassay for plasma neurofilament light chain in multiple sclerosis. J. Neurol. Sci. 2024, 463, 123115. [Google Scholar] [CrossRef]
- Saccà, F.; Costabile, T.; Carotenuto, A.; Lanzillo, R.; Moccia, M.; Pane, C.; Russo, C.V.; Barbarulo, A.M.; Casertano, S.; Rossi, F.; et al. The EDSS integration with the Brief International Cognitive Assessment for Multiple Sclerosis and orientation tests. Mult. Scler. J. 2017, 23, 1289–1296. [Google Scholar] [CrossRef]
- Goretti, B.; Niccolai, C.; Hakiki, B.; Sturchio, A.; Falautano, M.; Minacapelli, E.; Martinelli, V.; Incerti, C.; Nocentini, U.; Murgia, M.; et al. The Brief International Cognitive Assessment for Multiple Sclerosis (BICAMS): Normative values with gender, age and education corrections in the Italian population. BMC Neurol. 2014, 14, 171. [Google Scholar] [CrossRef] [PubMed]
- Flachenecker, P.; Kümpfel, T.; Kallmann, B.; Gottschalk, M.; Grauer, O.; Rieckmann, P.; Trenkwalder, C.; Toyka, K.V. Fatigue in multiple sclerosis: A comparison of different rating scales and correlation to clinical parameters. Mult. Scler. J. 2002, 8, 523–526. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.T.; Sterr, R.A.; Brown, G. Beck Depression Inventory–II (BDI-II); APA PsycTests: Washington, DC, USA, 1996. [Google Scholar]
- Beck, A.T.; Epstein, N.; Brown, G.; Steer, R.A. An inventory for measuring clinical anxiety: Psychometric properties. J. Consult. Clin. Psychol. 1988, 56, 893–897. [Google Scholar] [CrossRef]
- Buysse, D.J.; Reynolds, C.F.; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Moccia, M.; Lanzillo, R.; Palladino, R.; Chang, K.C.M.; Costabile, T.; Russo, C.; De Rosa, A.; Carotenuto, A.; Saccà, F.; Maniscalco, G.T.; et al. Cognitive impairment at diagnosis predicts 10-year multiple sclerosis progression. Mult. Scler. 2016, 22, 659–667. [Google Scholar]
- Bhan, A.; Jacobsen, C.; Dalen, I.; Alves, G.; Bergsland, N.; Myhr, K.M.; Zetterberg, H.; Zivadinov, R.; Farbu, E. Neurofilament and Brain Atrophy and Their Association with Cognition in Multiple Sclerosis: A 10-Year Follow-Up Study. Acta Neurol. Scand. 2023, 2023, 7136599. [Google Scholar] [CrossRef]
- Butler Pagnotti, R.; Hua, L.H.; Miller, J.B. Cognition and disease characteristics in adult onset versus late onset multiple sclerosis. Mult. Scler. 2022, 28, 933–941. [Google Scholar] [CrossRef]
- Meng, X.; D’Arcy, C. Education and Dementia in the Context of the Cognitive Reserve Hypothesis: A Systematic Review with Meta-Analyses and Qualitative Analyses. PLoS ONE 2012, 7, e38268. [Google Scholar]
- Mowry, E.M.; Beheshtian, A.; Waubant, E.; Goodin, D.S.; Cree, B.A.; Qualley, P.; Lincoln, R.; George, M.F.; Gomez, R.; Hauser, S.L.; et al. Quality of life in multiple sclerosis is associated with lesion burden and brain volume measures. Neurology 2009, 72, 1760–1765. [Google Scholar] [CrossRef]
- Kim, R.E.; Lee, M.; Kang, D.W.; Wang, S.M.; Kim, D.; Lim, H.K. Effects of education mediated by brain size on regional brain volume in adults. Psychiatry Res. Neuroimaging 2023, 330, 111600. [Google Scholar] [CrossRef] [PubMed]
- Sumowski, J.F.; Rocca, M.A.; Leavitt, V.M.; Dackovic, J.; Mesaros, S.; Drulovic, J.; DeLuca, J.; Filippi, M. Brain reserve and cognitive reserve protect against cognitive decline over 4.5 years in MS. Neurology 2014, 82, 1776–1783. [Google Scholar] [CrossRef] [PubMed]
- Petracca, M.; Ruggieri, S.; Nistri, R.; Tomasso, I.; Barbuti, E.; Pozzilli, V.; Haggiag, S.; Tortorella, C.; Gasperini, C.; Pozzilli, C.; et al. Brain reserve and timing of clinical onset in multiple sclerosis. Mult. Scler. J. 2024, 30, 1290–1295. [Google Scholar] [CrossRef]
- Dobson, R.; Rice, D.R.; D’hooghe, M.; Horne, R.; Learmonth, Y.; Mateen, F.J.; Marck, C.H.; Reyes, S.; Williams, M.J.; Giovannoni, G.; et al. Social determinants of health in multiple sclerosis. Nat. Rev. Neurol. 2022, 18, 723–734. [Google Scholar] [CrossRef]
- Manjaly, Z.M.; Harrison, N.A.; Critchley, H.D.; Do, C.T.; Stefanics, G.; Wenderoth, N.; Lutterotti, A.; Müller, A.; Stephan, K.E. Pathophysiological and cognitive mechanisms of fatigue in multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 2019, 90, 642–651. [Google Scholar] [CrossRef]
- Tur, C.; Moccia, M.; Barkhof, F.; Chataway, J.; Sastre-Garriga, J.; Thompson, A.J.; Ciccarelli, O. Assessing treatment outcomes in multiple sclerosis trials and in the clinical setting. Nat. Rev. Neurol. 2018, 14, 75–93. [Google Scholar] [CrossRef]
- Larson, R.D. Psychometric properties of the modified fatigue impact scale. Int. J. MS Care 2013, 15, 15–20. [Google Scholar] [CrossRef]
- Wang, Y.P.; Gorenstein, C. Psychometric properties of the Beck Depression Inventory-II: A comprehensive review. Braz. J. Psychiatry 2013, 35, 416–431. [Google Scholar] [CrossRef] [PubMed]
- Jerković, A.; Mikac, U.; Matijaca, M.; Košta, V.; Ćurković Katić, A.; Dolić, K. Psychometric Properties of the Pittsburgh Sleep Quality Index (PSQI) in Patients with Multiple Sclerosis: Factor Structure, Reliability, Correlates, and Discrimination. J. Clin. Med. 2022, 11, 2037. [Google Scholar] [CrossRef] [PubMed]
- Bark, L.; Larsson, I.M.; Wallin, E.; Simrén, J.; Zetterberg, H.; Lipcsey, M.; Frithiof, R.; Rostami, E.; Hultström, M. Central nervous system biomarkers GFAp and NfL associate with post-acute cognitive impairment and fatigue following critical COVID-19. Sci. Rep. 2023, 13, 13144. [Google Scholar] [CrossRef]
- Husseini, L.; Jung, J.; Boess, N.; Kruse, N.; Nessler, S.; Stadelmann, C.; Metz, I.; Haupts, M.; Weber, M.S. Neurofilament Light Chain Serum Levels Mirror Age and Disability in Secondary Progressive Multiple Sclerosis. Neurol. Neuroimmunol. Neuroinflammation 2024, 11, e200279. [Google Scholar] [CrossRef]
- Benkert, P.; Meier, S.; Schaedelin, S.; Manouchehrinia, A.; Yaldizli, Ö.; Maceski, A.; Oechtering, J.; Achtnichts, L.; Conen, D.; Derfuss, T.; et al. NfL Reference Database in the Swiss Multiple Sclerosis Cohort Study Group. Serum neurofilament light chain for individual prognostication of disease activity in people with multiple sclerosis: A retrospective modelling and validation study. Lancet Neurol. 2022, 21, 246–257. [Google Scholar]
- Nicolella, V.; Varelli, M.; Fasano, S.; Sirica, R.; Polito, C.; Saviano, A.; Fiorenza, M.; Novarella, F.; Ranucci, D.; Carotenuto, A.; et al. Clinical application of age-derived cut-offs for plasma neurofilament light chain in multiple sclerosis. J. Neurol. 2025, 272, 495. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, A.; Charest, K.; Brando, E.; Roger, E.; Duquette, P.; Rouleau, I. Cognitive reserve as a moderating factor between EDSS and cognition in multiple sclerosis. Mult. Scler. Relat. Disord. 2023, 70, 104482. [Google Scholar] [CrossRef]
- Barch, D.M.; Luby, J.L. Understanding Social Determinants of Brain Health During Development. Am. J. Psychiatry 2023, 180, 108–110. [Google Scholar] [CrossRef] [PubMed]
- Carotenuto, A.; Valsasina, P.; Preziosa, P.; Mistri, D.; Filippi, M.; Rocca, M.A. Monoaminergic network abnormalities: A marker for multiple sclerosis-related fatigue and depression. J. Neurol. Neurosurg. Psychiatry 2023, 94, 94–101. [Google Scholar] [CrossRef] [PubMed]
| n = 211 | |
|---|---|
| Age, years | 44.7 ± 12.2 |
| Age group 18–50, n (%) 50–60, n (%) 60–70, n (%) 70+, n (%) | 132 (62.56%) 58 (27.49%) 20 (9.48%) 1 (0.47%) |
| Sex, females (%) | 138 (65.4%) |
| Education, years Middle School (%) High School (%) University (%) | 15.53 ± 9.10 47 (22.27%) 102 (48.34%) 62 (29.38%) |
| Cardiovascular comorbidity (%) | 47 (22.27%) |
| Ever Smoking (%) | 30 (14.22%) |
| BMI (n = 139) | 24.60 ± 4.7 |
| Disease duration, years | 13.13 ± 3.58 |
| EDSS, median (range) | 2.5 (1.0–7.5) |
| Descriptor of disease progression Relapsing Progressive | 170 (80.53%) 41 (19.47%) |
| Current DMT duration(years) DMT group No DMT Oral DMTs Monoclonal antibody DMTs Injective DMTs | 4.01 ± 4.20 5 (2.37%) 137 (64.97%) 53 (25.12%) 16 (7.58%) |
| SDMT Impaired SDMT (%) | 43.53 ± 12.24 55 (26.07%) |
| CVLT Impaired CVLT (%) | 42.76 ± 13.42 65 (30.81%) |
| BVMT Impaired BVMT (%) | 42.30 ± 11.39 60 (28.44%) |
| MFIS cognitive MFIS physical MFIS psychosocial MFIS total Impaired MFIS (%) | 8.78 ± 9.76 10.09 ± 10.39 1.65 ± 3.31 20.53 ± 21.08 46 (21.80%) |
| BDI Impaired BDI (%) | 7.79 ± 10.23 49 (23.22%) |
| BAI Impaired BAI (%) | 6.97 ± 13.31 38 (24.84%) |
| PSQI Impaired PSQI (%) | 3.32 ± 4.33 27 (17.65%) |
| NfL pNfL (pg/mL) pNfL above normality (%) | 12.32 ± 11.35 71 (33.75%) |
| NfL Cut-Offs from Simrén and Colleagues [24] | ||||||
|---|---|---|---|---|---|---|
| 95% CI | ||||||
| Normal n = 140 | Higher than Normal n = 71 | Lower | Upper | p Value | ||
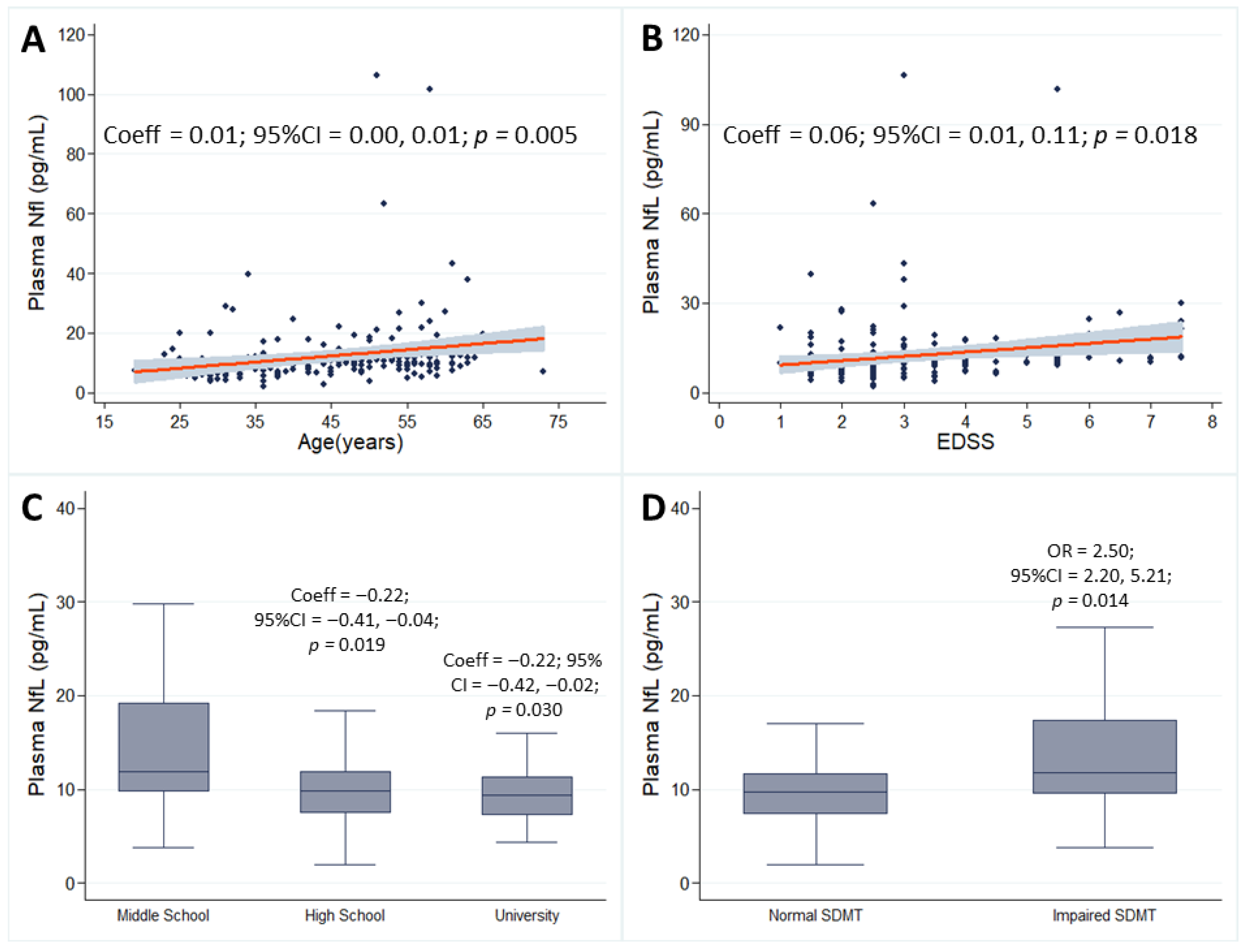
| Age | 45.34 ± 13.19 | 43.31 ± 9.78 | Coeff 0.01 OR 0.97 | 0.00 0.94 | 0.01 0.99 | 0.005 0.04 |
| Sex females vs. males | Males 44 (60.27%) | Males 29 (39.73%) | Coeff 0.00 OR 1.25 | −0.14 0.66 | 0.14 2.36 | 0.95 0.49 |
| Education class middle school (reference) high school University | 27 (19.29%) 70 (50.00%) 43 (30.71%) | 20 (28.17%) 32 (45.07%) 19 (26.76%) | reference Coeff−0.22 OR 0.64 Coeff−0.22 OR 0.78 | −0.41 0.27 −0.42 0.31 | −0.04 1.53 −0.02 2.00 | 0.019 0.319 0.030 0.611 |
| Cardiovascular comorbidity | 34 (24.29%) | 13 (18.31%) | Coeff 0.10 OR 0.84 | −0.08 0.37 | 0.27 1.90 | 0.270 0.686 |
| Smoking | 15 (10.71%) | 15 (21.13%) | Coeff 0.03 OR 2.19 | −0.15 0.95 | 0.22 5.03 | 0.710 0.065 |
| EDSS | 2.5 (1–7.0) | 3 (1–7.5) | Coeff 0.06 OR 1.56 | 0.01 1.23 | 0.11 1.98 | 0.018 <0.001 |
| Disease duration | 15.31 ± 9.48 | 15.96 ± 8.35 | Coeff 0.00 OR 1.02 | −0.01 0.98 | 0.01 1.07 | 0.590 0.382 |
| Relapsing vs. progressive | Progressive 24 (17.27%) | Progressive 17 (23.94%) | Coeff 3.19 OR 0.56 | −8.02 0.20 | 1.64 1.53 | 0.194 0.259 |
| NfL Cut-Offs from Simrén and Colleagues [24] | ||||||
|---|---|---|---|---|---|---|
| 95% CI | ||||||
| Normal n = 140 | Impaired n = 71 | Lower | Upper | p Value | ||
| SDMT Impaired vs. Normal | Impaired 28 (20.00%) | Impaired 27 (38.03%) | Coeff 0.29 OR 2.50 | 0.11 1.20 | 0.45 5.21 | 0.289 0.014 |
| CVLT Impaired vs. Normal | Impaired 42 (30.00%) | Impaired 23 (32.39%) | Coeff −0.06 OR 0.87 | −0.21 0.42 | 0.1 1.77 | 0.476 0.697 |
| BVMT Impaired vs. Normal | Impaired 38 (27.14%) | Impaired 22 (30.00%) | Coeff −0.33 OR 0.94 | −0.19 0.45 | 0.13 1.94 | 0.682 0.858 |
| MFIS Cognitive Fatigue | 8.70 ± 10.11 | 8.93 ± 9.09 | Coeff −0.00 OR 1.00 | −0.01 0.96 | 0.00 1.03 | 0.253 0.797 |
| MFIS Physical fatigue | 10.44 ± 11.01 | 9.41 ± 8.92 | Coeff −0.00 OR 0.98 | −0.12 0.95 | 0.00 1.00 | 0.119 0.171 |
| MFIS Psychological Fatigue | 1.73 ± 2.40 | 1.51 ± 2.12 | Coeff −0.03 OR 0.90 | −0.06 0.78 | 0.00 1.05 | 0.064 0.180 |
| MFIS Total Fatigue Impaired vs. Normal | Impaired 32 (22.86%) | Impaired 14 (19.72%) | Coeff −0.50 OR 0.07 | −0.22 0.30 | 0.12 1.50 | 0.556 0.333 |
| BDI-II Impaired vs. Normal | Impaired 33 (23.57%) | Impaired 16 (22.54%) | Coeff −0.02 OR 0.99 | −0.19 0.46 | 0.14 2.11 | 0.784 0.974 |
| BAI Impaired vs. Normal | Impaired 25 (24.51%) | Impaired 13 (25.49%) | Coeff −0.05 OR 1.12 | −0.23 0.47 | 0.13 2.68 | 0.563 0.802 |
| PSQI Impaired vs. Normal | Impaired 18 (17.65%) | Impaired 9 (17.65%) | Coeff −0.18 OR 1.03 | −0.40 0.30 | 0.41 3.51 | 0.109 0.961 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nicolella, V.; Novarella, F.; Falco, F.; Polito, C.; Sirica, R.; La Civita, E.; Criscuolo, V.; Corsini, G.; Spiezia, A.L.; Castiello, A.; et al. Plasma Neurofilament Light Chain Is Associated with Cognitive Functions but Not Patient-Reported Outcomes in Multiple Sclerosis. Neurol. Int. 2025, 17, 144. https://doi.org/10.3390/neurolint17090144
Nicolella V, Novarella F, Falco F, Polito C, Sirica R, La Civita E, Criscuolo V, Corsini G, Spiezia AL, Castiello A, et al. Plasma Neurofilament Light Chain Is Associated with Cognitive Functions but Not Patient-Reported Outcomes in Multiple Sclerosis. Neurology International. 2025; 17(9):144. https://doi.org/10.3390/neurolint17090144
Chicago/Turabian StyleNicolella, Valerio, Federica Novarella, Fabrizia Falco, Carmela Polito, Rosa Sirica, Evelina La Civita, Vincenzo Criscuolo, Giuseppe Corsini, Antonio Luca Spiezia, Alessia Castiello, and et al. 2025. "Plasma Neurofilament Light Chain Is Associated with Cognitive Functions but Not Patient-Reported Outcomes in Multiple Sclerosis" Neurology International 17, no. 9: 144. https://doi.org/10.3390/neurolint17090144
APA StyleNicolella, V., Novarella, F., Falco, F., Polito, C., Sirica, R., La Civita, E., Criscuolo, V., Corsini, G., Spiezia, A. L., Castiello, A., Carotenuto, A., Petracca, M., Lanzillo, R., Castaldo, G., Brescia Morra, V., Terracciano, D., & Moccia, M. (2025). Plasma Neurofilament Light Chain Is Associated with Cognitive Functions but Not Patient-Reported Outcomes in Multiple Sclerosis. Neurology International, 17(9), 144. https://doi.org/10.3390/neurolint17090144









