The Effects of Co-Culturing ND7/23 Sensory Neuron-like Cells and IFRS1 Schwann Cells on Myelination: A Single-Arm Nonrandomized Study
Abstract
1. Introduction
2. Materials and Methods
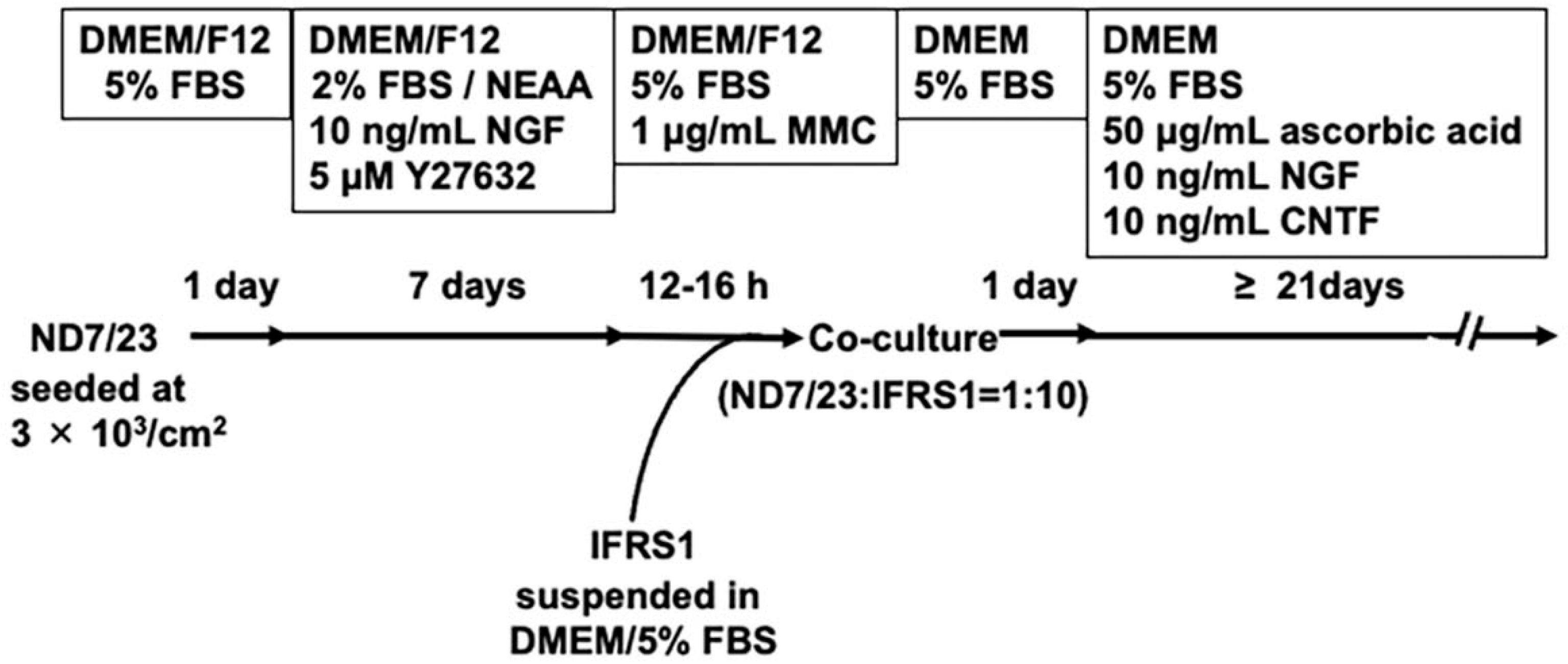
2.1. Culture of ND7/23 and IFRS1 Cells
2.2. Co-Culture of ND7/23 and IFRS1 Cells
2.3. Immunofluorescence
- (1)
- (2)
- Rabbit anti-peripheral myelin protein 22 (PMP22) polyclonal antibody (1:1000, Sigma) [30] and mouse anti-βIII tubulin monoclonal antibody.
2.4. Sudan Black B Staining
2.5. Image Presentation
2.6. Electron Microscopy
2.7. Statistical Analysis
3. Results
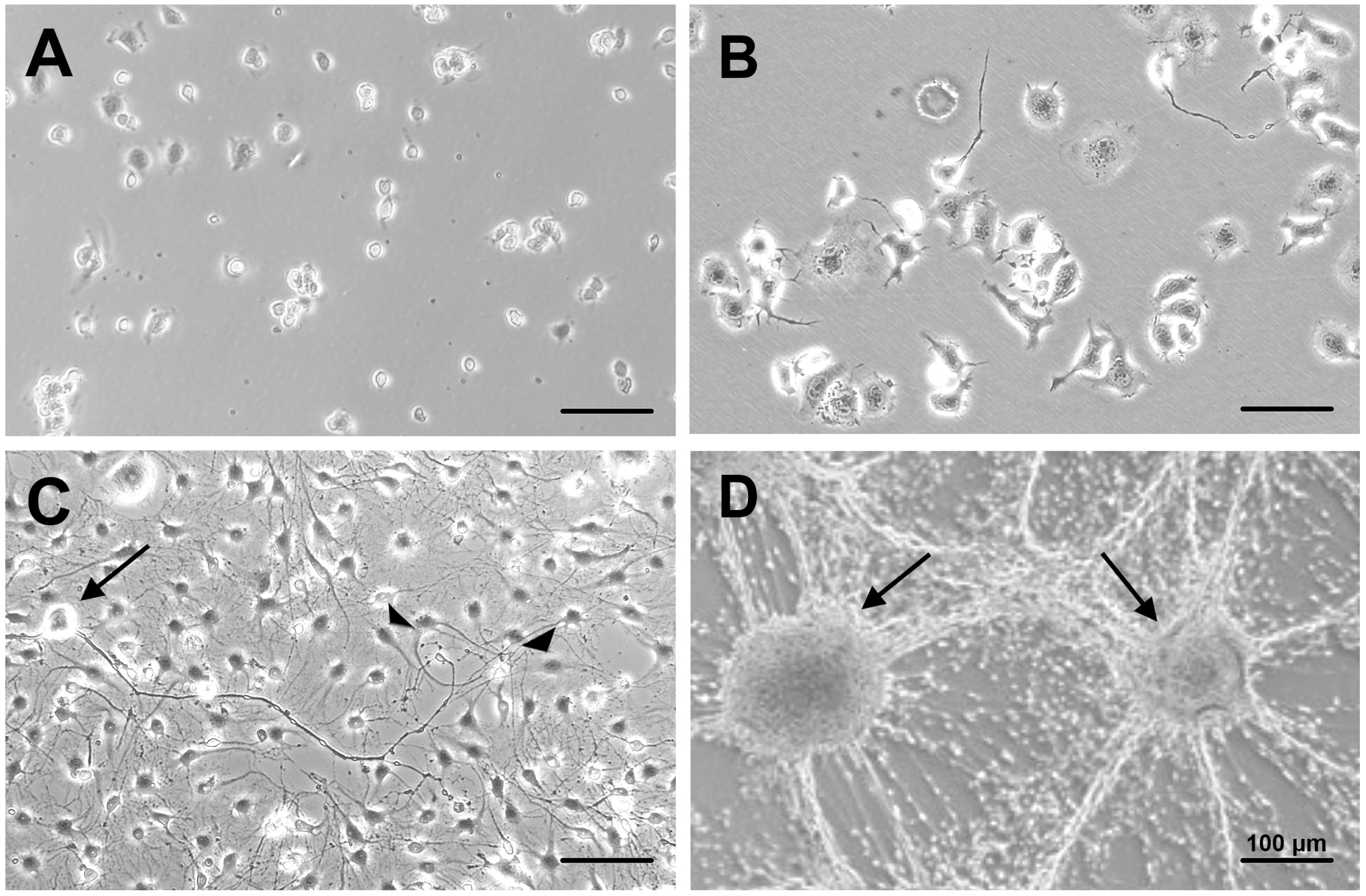
3.1. Differentiation of ND7/23 Cells Prior to Co-Culturing
3.2. Maintenance of Co-Culture with Stable Neuron–SC Interactions
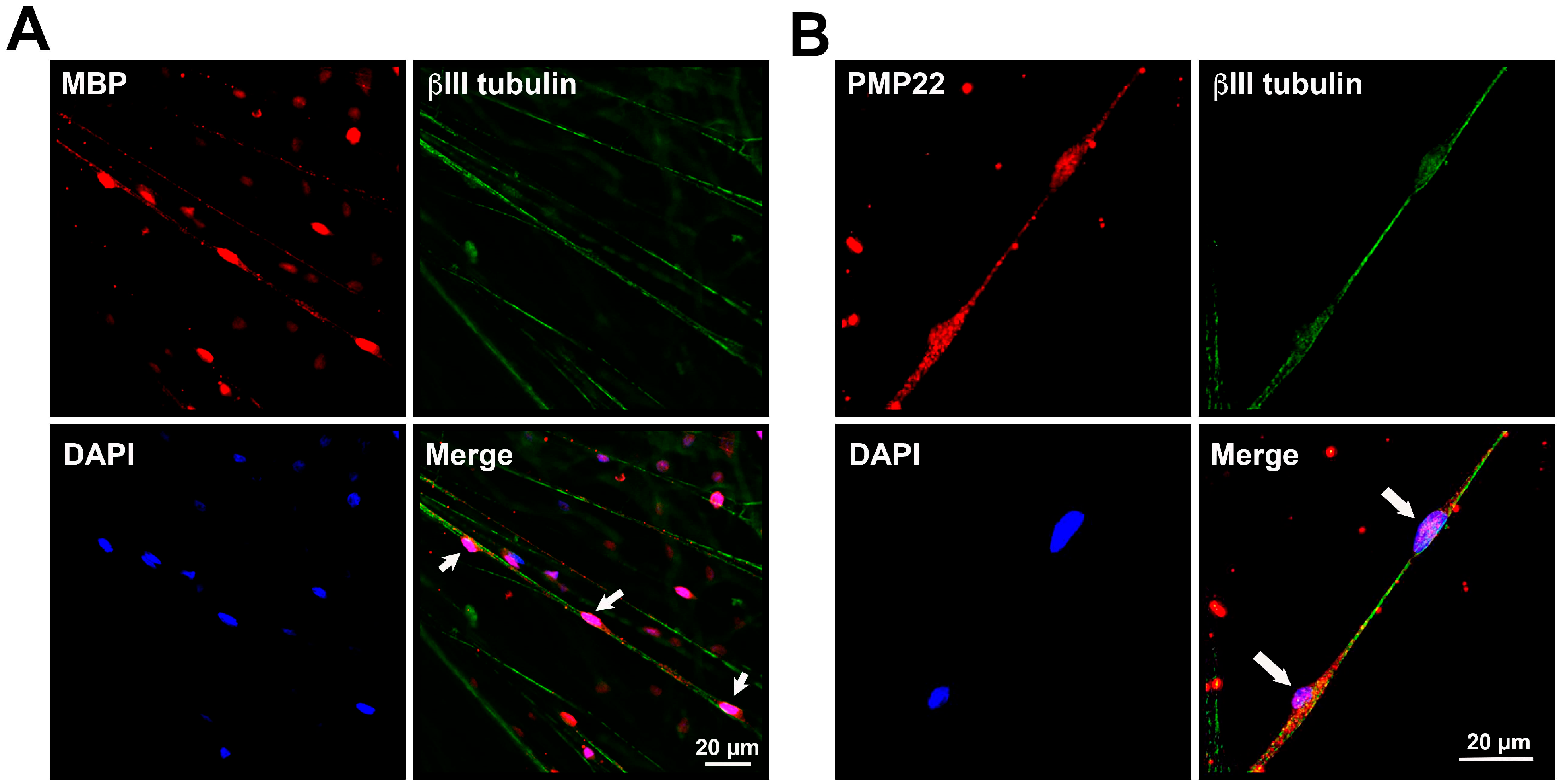
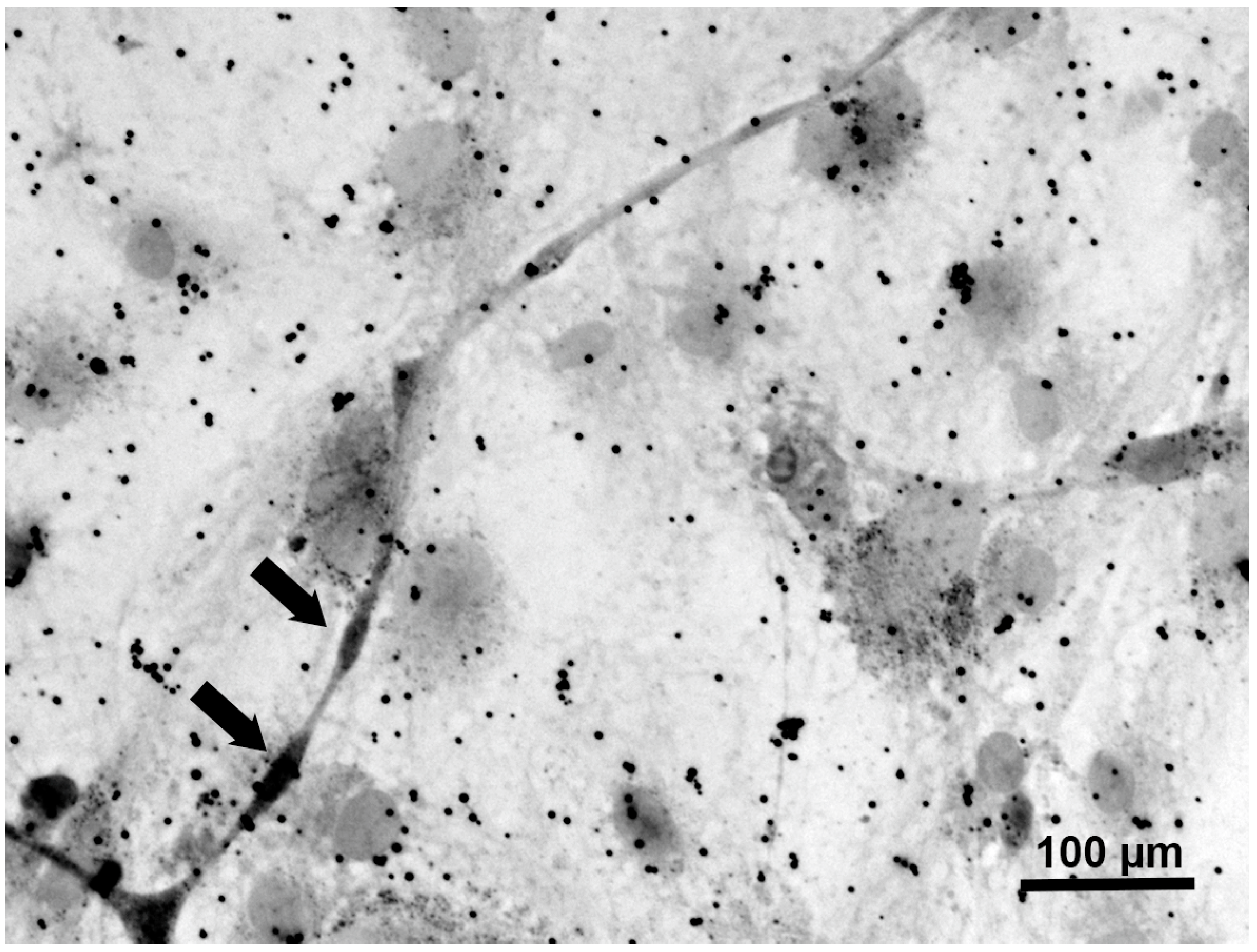
3.3. Myelination in NSC-34–IFRS1 Co-Cultures
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AGEs | Advanced Glycation End Products |
| BDNF | Brain-Derived Neurotrophic Factor |
| CNTF | Ciliary Neurotrophic Factor |
| DMEM | Dulbecco’s Modified Eagle’s Medium |
| DMEM/F12 | Dulbecco’s Modified Eagle’s Medium/Nutrient Mixture F-12 |
| FBS | Fetal Bovine Serum |
| IFRS1 | Immortalized Fischer344 Rat Schwann Cells 1 |
| iPS | Induced Pluripotent Stem (Cells) |
| JAK2 | Janus Kinase 2 |
| MBP | Myelin Basic Protein |
| MMC | Mitomycin C |
| NEAA | Non-Essential Amino Acids |
| NF-κB | Nuclear Factor-Kappa B |
| NGF | Nerve Growth Factor |
| PI3K | Phosphatidyl Inositol-3’-Phosphate-Kinase |
| PMP22 | Peripheral Myelin Protein 22 |
| PNS | Peripheral Nervous System |
| ROCK | Rho-associated Coiled-Coil Forming Kinase |
| SCs | Schwann Cells |
| STAT3 | Signal Transducer and Activator of Transcription 3 |
References
- Xu, H.; Fan, Z. The role and mechanism of Schwann cells in the repair of peripheral nerve injury. Cell Tissue Res. 2025, 400, 81–95. [Google Scholar] [CrossRef]
- Park, H.T.; Kim, J.K.; Tricaud, N. The conceptual introduction of the “demyelinating Schwann cell” in peripheral demyelinating neuropathies. Glia 2019, 67, 571–581. [Google Scholar] [CrossRef] [PubMed]
- Schenone, A.; Massucco, S.; Schenone, C.; Venturi, C.B.; Nozza, P.; Prada, V.; Pomili, T.; Di Patrizi, I.; Capodivento, G.; Nobbio, L.; et al. Basic pathological mechanisms in peripheral nerve diseases. Int. J. Mol. Sci. 2025, 26, 3377. [Google Scholar] [CrossRef] [PubMed]
- Ogata, T.; Iijima, S.; Hoshikawa, S.; Miura, T.; Yamamoto, S.; Oda, H.; Nakamura, K.; Tanaka, S. Opposing extracellular signal-regulated kinase and Akt pathways control Schwann cell myelination. J. Neurosci. 2004, 24, 6724–6732. [Google Scholar] [CrossRef] [PubMed]
- La Marca, R.; Cerri, F.; Horiuchi, K.; Bachi, A.; Feltri, M.L.; Wrabetz, L.; Blobel, C.P.; Quattrini, A.; Salzer, J.L.; Taveggia, C. TACE (ADAM17) inhibits Schwann cell myelination. Nat. Neurosci. 2011, 14, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Nishimoto, S.; Tanaka, H.; Okamoto, M.; Okada, K.; Murase, T.; Yoshikawa, H. Methylcobalamin promotes the differentiation of Schwann cells and remyelination in lysophosphatidylcholine-induced demyelination of the rat sciatic nerve. Front. Cell. Neurosci. 2015, 9, 298. [Google Scholar] [CrossRef]
- El Chemali, L.; Boutary, S.; Liu, S.; Liu, G.J.; Middleton, R.J.; Banati, R.B.; Bahrenberg, G.; Rupprecht, R.; Schumacher, M.; Massaad-Massade, L. GRT-X stimulates dorsal root ganglia axonal growth in culture via TSPO and Kv7.2/3 potassium channel activation. Int. J. Mol. Sci. 2024, 25, 7327. [Google Scholar] [CrossRef] [PubMed]
- Lobsiger, C.S.; Smith, P.M.; Buchstaller, J.; Schweitzer, B.; Franklin, R.J.; Suter, U.; Taylor, V. SpL201: A conditionally immortalized Schwann cell precursor line that generates myelin. Glia 2001, 36, 31–47. [Google Scholar] [CrossRef]
- Saavedra, J.T.; Wolterman, R.A.; Baas, F.; ten Asbroek, A.L. Myelination competent conditionally immortalized mouse Schwann cells. J. Neurosci. Methods 2008, 174, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.J.; Kaller, M.S.; Galino, J.; Willison, H.J.; Rinaldi, S.; Bennett, D.L.H. Co-cultures with stem cell-derived human sensory neurons reveal regulators of peripheral myelination. Brain 2017, 140, 898–913. [Google Scholar] [CrossRef]
- Sango, K.; Yanagisawa, H.; Kawakami, E.; Takaku, S.; Ajiki, K.; Watabe, K. Spontaneously immortalized Schwann cells from adult Fischer rat as a valuable tool for exploring neuron-Schwann cell interactions. J. Neurosci. Res. 2011, 89, 898–908. [Google Scholar] [CrossRef] [PubMed]
- Sango, K.; Kawakami, E.; Yanagisawa, H.; Takaku, S.; Tsukamoto, M.; Utsunomiya, K.; Watabe, K. Myelination in coculture of established neuronal and Schwann cell lines. Histochem. Cell Biol. 2012, 137, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Ishii, T.; Kawakami, E.; Endo, K.; Misawa, H.; Watabe, K. Myelinating cocultures of rodent stem cell line-derived neurons and immortalized Schwann cells. Neuropathology 2017, 37, 475–481. [Google Scholar] [CrossRef]
- Cashman, N.R.; Durham, H.D.; Blusztajn, J.K.; Oda, K.; Tabira, T.; Shaw, I.T.; Dahrouge, S.; Antel, J.P. Neuroblastoma x spinal cord (NSC) hybrid cell lines resemble developing motor neurons. Dev. Dyn. 1992, 194, 209–221. [Google Scholar] [CrossRef]
- Takaku, S.; Yako, H.; Niimi, N.; Akamine, T.; Kawanami, D.; Utsunomiya, K.; Sango, K. Establishment of a myelinating co-culture system with a motor neuron-like cell line NSC-34 and an adult rat Schwann cell line IFRS1. Histochem. Cell Biol. 2018, 149, 537–543. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, B.; Toku, K.; Maeda, N.; Sakanaka, M.; Tanaka, J. Improvement of the viability of cultured rat neurons by the non-essential amino acids L-serine and glycine that upregulates expression of the anti-apoptotic gene product Bcl-w. Neurosci. Lett. 2000, 295, 97–100. [Google Scholar] [CrossRef]
- Matusica, D.; Fenech, M.P.; Rogers, M.L.; Rush, R.A. Characterization and use of the NSC-34 cell line for study of neurotrophin receptor trafficking. J. Neurosci. Res. 2008, 86, 553–565. [Google Scholar] [CrossRef]
- Acquarone, M.; de Melo, T.M.; Meireles, F.; Brito-Moreira, J.; Oliveira, G.; Ferreira, S.T.; Castro, N.G.; Tovar-Moll, F.; Houzel, J.C.; Rehen, S.K. Mitomycin-treated undifferentiated embryonic stem cells as a safe and effective therapeutic strategy in a mouse model of Parkinson’s disease. Front. Cell. Neurosci. 2015, 9, 97. [Google Scholar] [CrossRef]
- Wood, J.N.; Bevan, S.J.; Coote, P.R.; Dunn, P.M.; Harmar, A.; Hogan, P.; Latchman, D.S.; Morrison, C.; Rougon, G.; Theveniau, M.; et al. Novel cell lines display properties of nociceptive sensory neurons. Proc. Biol. Sci. 1990, 241, 187–194. [Google Scholar]
- Haberberger, R.V.; Barry, C.; Matusica, D. Immortalized dorsal root ganglion neuron cell lines. Front. Cell. Neurosci. 2020, 14, 184. [Google Scholar] [CrossRef] [PubMed]
- Eggett, C.J.; Crosier, S.; Manning, P.; Cookson, M.R.; Menzies, F.M.; McNeil, C.J.; Shaw, P.J. Development and characterisation of a glutamate-sensitive motor neurone cell line. J. Neurochem. 2000, 74, 1895–1902. [Google Scholar] [CrossRef]
- Fournier, A.E.; Takizawa, B.T.; Strittmatter, S.M. Rho kinase inhibition enhances axonal regeneration in the injured CNS. J. Neurosci. 2003, 23, 1416–1423. [Google Scholar] [CrossRef] [PubMed]
- Ulmann, L.; Rodeau, J.L.; Danoux, L.; Contet-Audonneau, J.L.; Pauly, G.; Schlichter, R. Dehydroepiandrosterone and neurotrophins favor axonal growth in a sensory neuron-keratinocyte coculture model. Neuroscience 2009, 159, 514–525. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Huang, J.; May, J.M. Ascorbic acid spares alpha-tocopherol and decreases lipid peroxidation in neuronal cells. Biochem. Biophys. Res. Commun. 2003, 305, 656–661. [Google Scholar] [CrossRef]
- Podratz, J.L.; Rodriguez, E.; Windebank, A.J. Role of the extracellular matrix in myelination of peripheral nerve. Glia 2001, 35, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Thippeswamy, T.; McKay, J.S.; Quinn, J.; Morris, R. Either nitric oxide or nerve growth factor is required for dorsal root ganglion neurons to survive during embryonic and neonatal development. Brain Res. Dev. Brain Res. 2005, 154, 153–164. [Google Scholar] [CrossRef]
- Liu, H.; Liu, G.; Bi, Y. CNTF regulates neurite outgrowth and neuronal migration through JAK2/STAT3 and PI3K/Akt signaling pathways of DRG explants with gp120-induced neurotoxicity in vitro. Neurosci. Lett. 2014, 569, 110–115. [Google Scholar] [CrossRef]
- Wang, K.; Zhou, F.; Zhu, X.; Zhang, K.; Huang, B.; Zhu, L.; Zhu, L. Neuroprotective properties of ciliary neurotrophic factor on retinoic acid (RA)-predifferentiated SH-SY5Y neuroblastoma cells. Folia Neuropathol. 2014, 52, 121–127. [Google Scholar] [CrossRef]
- Vent, J.; Wyatt, T.A.; Smith, D.D.; Banerjee, A.; Ludueña, R.F.; Sisson, J.H.; Hallworth, R. Direct involvement of the isotype-specific C-terminus of beta tubulin in ciliary beating. J. Cell Sci. 2005, 118, 4333–4341. [Google Scholar] [CrossRef][Green Version]
- Roa, B.B.; Dyck, P.J.; Marks, H.G.; Chance, P.F.; Lupski, J.R. Dejerine–Sottas syndrome associated with point mutation in the peripheral myelin protein 22 (PMP22) gene. Nat. Genet. 1993, 5, 269–273. [Google Scholar] [CrossRef]
- Niimi, N.; Takaku, S.; Yako, H.; Sango, K. Drug-induced demyelinating neuropathies. Adv. Exp. Med. Biol. 2019, 1190, 357–369. [Google Scholar] [PubMed]
- Devaux, S.; Cizkova, D.; Mallah, K.; Karnoub, M.A.; Laouby, Z.; Kobeissy, F.; Blasko, J.; Nataf, S.; Pays, L.; Mériaux, C.; et al. RhoA inhibitor treatment at acute phase of spinal cord injury may induce neurite outgrowth and synaptogenesis. Mol. Cell. Proteom. 2017, 16, 1394–1415. [Google Scholar] [CrossRef]
- Turner, B.J.; Murray, S.S.; Piccenna, L.G.; Lopes, E.C.; Kilpatrick, T.J.; Cheema, S.S. Effect of p75 neurotrophin receptor antagonist on disease progression in transgenic amyotrophic lateral sclerosis mice. J. Neurosci. Res. 2004, 78, 193–199. [Google Scholar] [CrossRef]
- Ernsberger, U. Role of neurotrophin signalling in the differentiation of neurons from dorsal root ganglia and sympathetic ganglia. Cell Tissue Res. 2009, 336, 349–384. [Google Scholar] [CrossRef]
- Fujita, Y.; Yamashita, T. Axon growth inhibition by RhoA/ROCK in the central nervous system. Front. Neurosci. 2014, 8, 338. [Google Scholar] [CrossRef]
- Tan, D.; Zhang, H.; Deng, J.; Liu, J.; Wen, J.; Li, L.; Wang, X.; Pan, M.; Hu, X.; Guo, J. RhoA-GTPase modulates neurite outgrowth by regulating the expression of spastin and p60-katanin. Cells 2020, 9, 230. [Google Scholar] [CrossRef]
- Saleh, A.; Roy Chowdhury, S.K.; Smith, D.R.; Balakrishnan, S.; Tessler, L.; Martens, C.; Morrow, D.; Schartner, E.; Frizzi, K.E.; Calcutt, N.A.; et al. Ciliary neurotrophic factor activates NF-κB to enhance mitochondrial bioenergetics and prevent neuropathy in sensory neurons of streptozotocin-induced diabetic rodents. Neuropharmacology 2013, 65, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Sango, K.; Yanagisawa, H.; Takaku, S.; Kawakami, E.; Watabe, K. Immortalized adult rodent Schwann cells as in vitro models to study diabetic neuropathy. Exp. Diabetes Res. 2011, 2011, 374943. [Google Scholar] [CrossRef]
- Bellanti, R.; Keh, R.Y.S.; Keddie, S.; Chou, M.K.L.; Misheva, M.; Smyth, D.; Baskozos, G.; Moodley, K.; Hart, M.S.; Davies, A.; et al. Plasma periaxin is a biomarker of peripheral nerve demyelination. Brain 2025, awaf234. [Google Scholar] [CrossRef] [PubMed]
- Koyanagi, M.; Imai, S.; Iwamitsu, Y.; Matsumoto, M.; Saigo, M.; Moriya, A.; Ogihara, T.; Nakazato, Y.; Yonezawa, A.; Nakagawa, S.; et al. Cilostazol is an effective causal therapy for preventing paclitaxel-induced peripheral neuropathy by suppression of Schwann cell dedifferentiation. Neuropharmacology 2021, 188, 108514. [Google Scholar] [CrossRef]
- Yin, K.; Baillie, G.J.; Vetter, I. Neuronal cell lines as model dorsal root ganglion neurons: A transcriptomic comparison. Mol. Pain 2016, 12, 1744806916646111. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Williams, M.; Hawkins, K.; Gallo, L.; Grillo, M.; Akanda, N.; Guo, X.; Lambert, S.; Hickman, J.J. Establishment of a serum-free human iPSC-derived model of peripheral myelination. ACS Biomater. Sci. Eng. 2024, 10, 7132–7143. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Rouen, S.; Dobrowsky, R.T. Hyperglycemia and downregulation of caveolin-1 enhance neuregulin-induced demyelination. Glia 2008, 56, 877–887. [Google Scholar] [CrossRef]
- Sato, K.; Tatsunami, R.; Yama, K.; Murao, Y.; Tampo, Y. Glycolaldehyde induces endoplasmic reticulum stress and apoptosis in Schwann cells. Toxicol. Rep. 2015, 2, 1454–1462. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takaku, S.; Sango, K. The Effects of Co-Culturing ND7/23 Sensory Neuron-like Cells and IFRS1 Schwann Cells on Myelination: A Single-Arm Nonrandomized Study. Neurol. Int. 2025, 17, 138. https://doi.org/10.3390/neurolint17090138
Takaku S, Sango K. The Effects of Co-Culturing ND7/23 Sensory Neuron-like Cells and IFRS1 Schwann Cells on Myelination: A Single-Arm Nonrandomized Study. Neurology International. 2025; 17(9):138. https://doi.org/10.3390/neurolint17090138
Chicago/Turabian StyleTakaku, Shizuka, and Kazunori Sango. 2025. "The Effects of Co-Culturing ND7/23 Sensory Neuron-like Cells and IFRS1 Schwann Cells on Myelination: A Single-Arm Nonrandomized Study" Neurology International 17, no. 9: 138. https://doi.org/10.3390/neurolint17090138
APA StyleTakaku, S., & Sango, K. (2025). The Effects of Co-Culturing ND7/23 Sensory Neuron-like Cells and IFRS1 Schwann Cells on Myelination: A Single-Arm Nonrandomized Study. Neurology International, 17(9), 138. https://doi.org/10.3390/neurolint17090138





