Sulbactam: A β–Lactam Compound with Neuroprotective Effects in Epilepsy
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
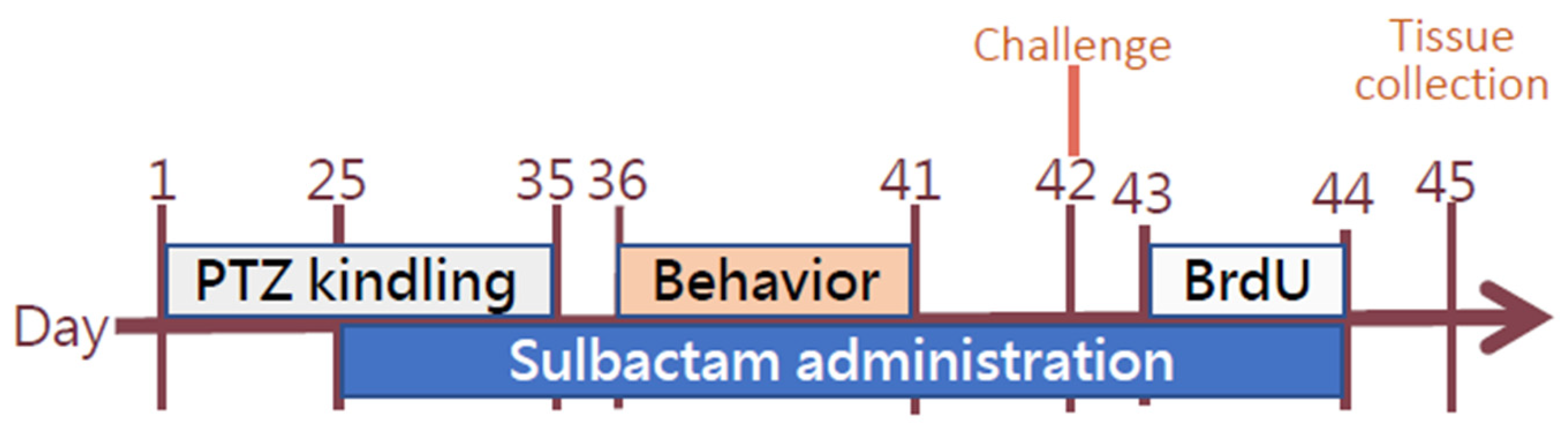
2.2. General Procedures
2.3. Kindling
2.4. Behavioral Tests
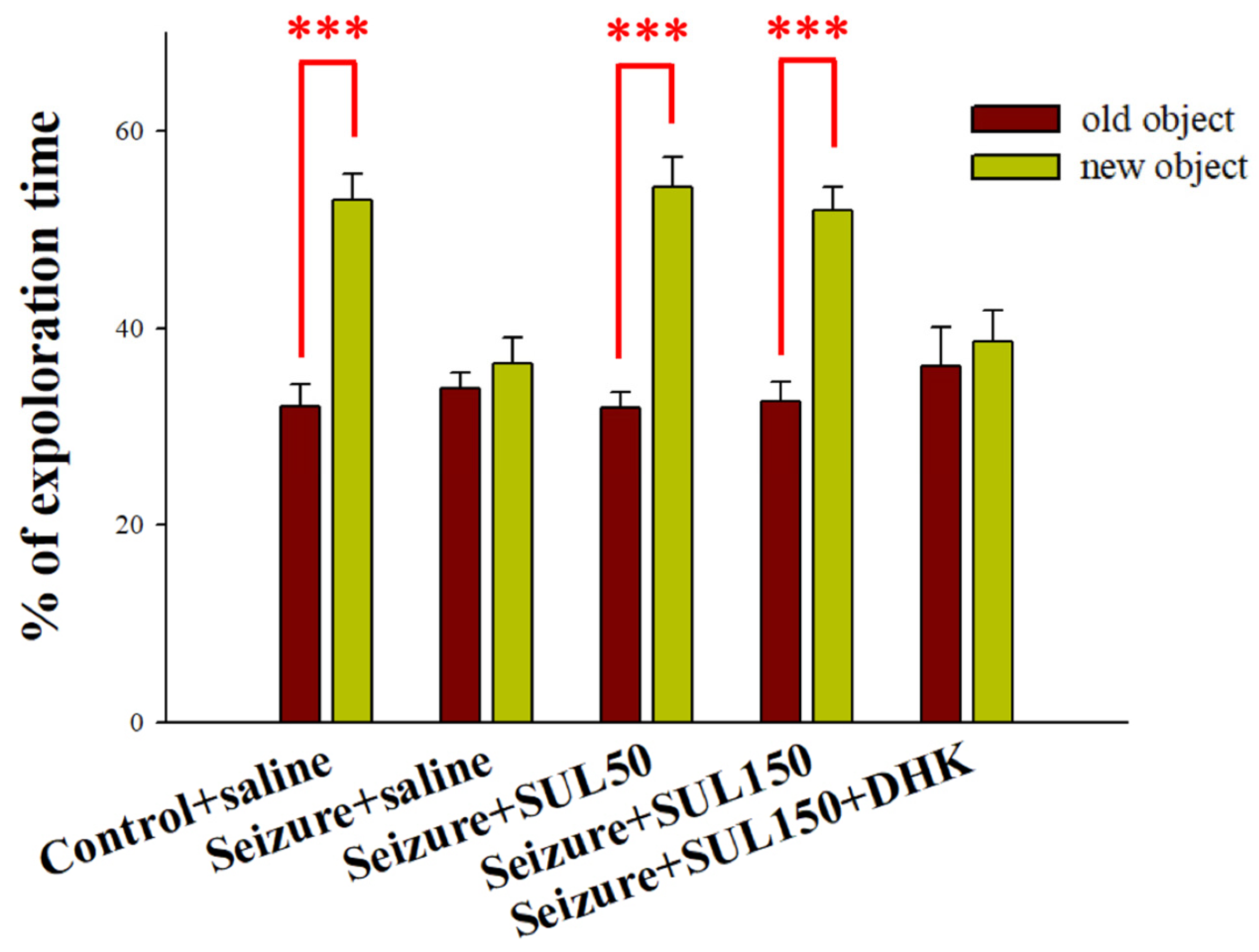
2.4.1. Object Recognition Test
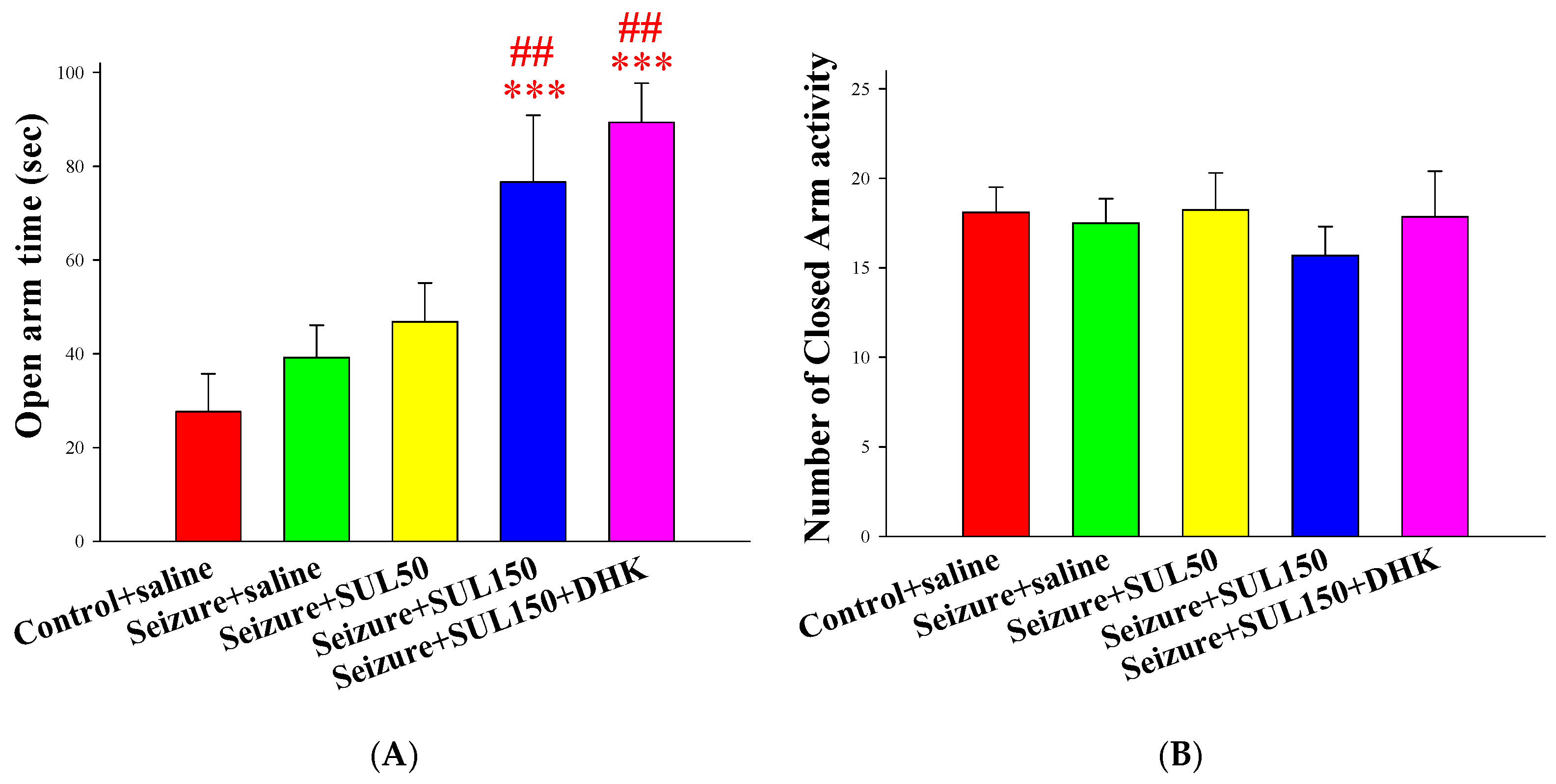
2.4.2. Elevated Plus-Maze Test (EPM)
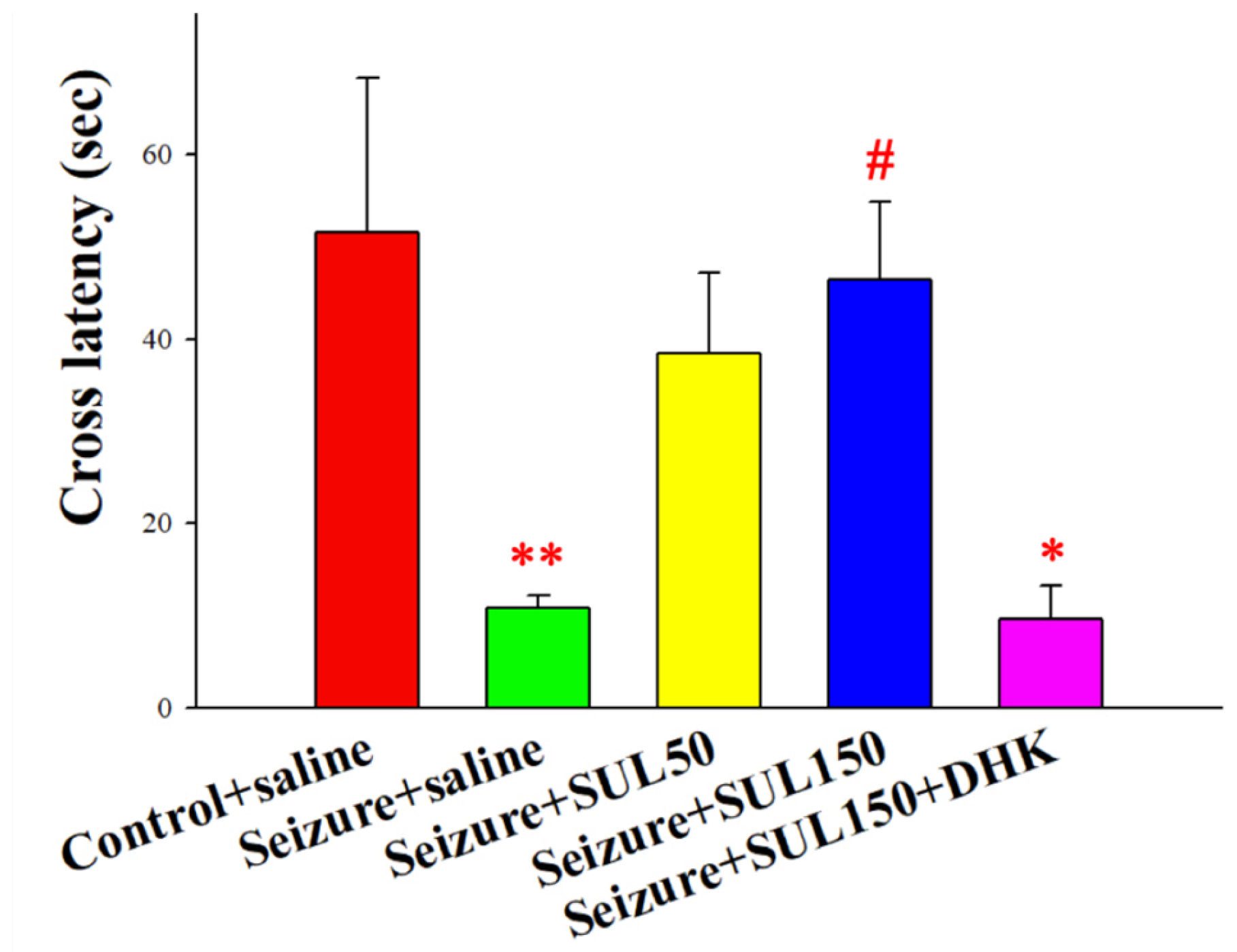
2.4.3. Passive Avoidance Test (PAT)
2.4.4. Histological Assessment
2.5. Data Analysis
3. Results
3.1. Seizure Severity Score
3.2. Object Recognition Test
3.3. PAT
3.4. EPM Test
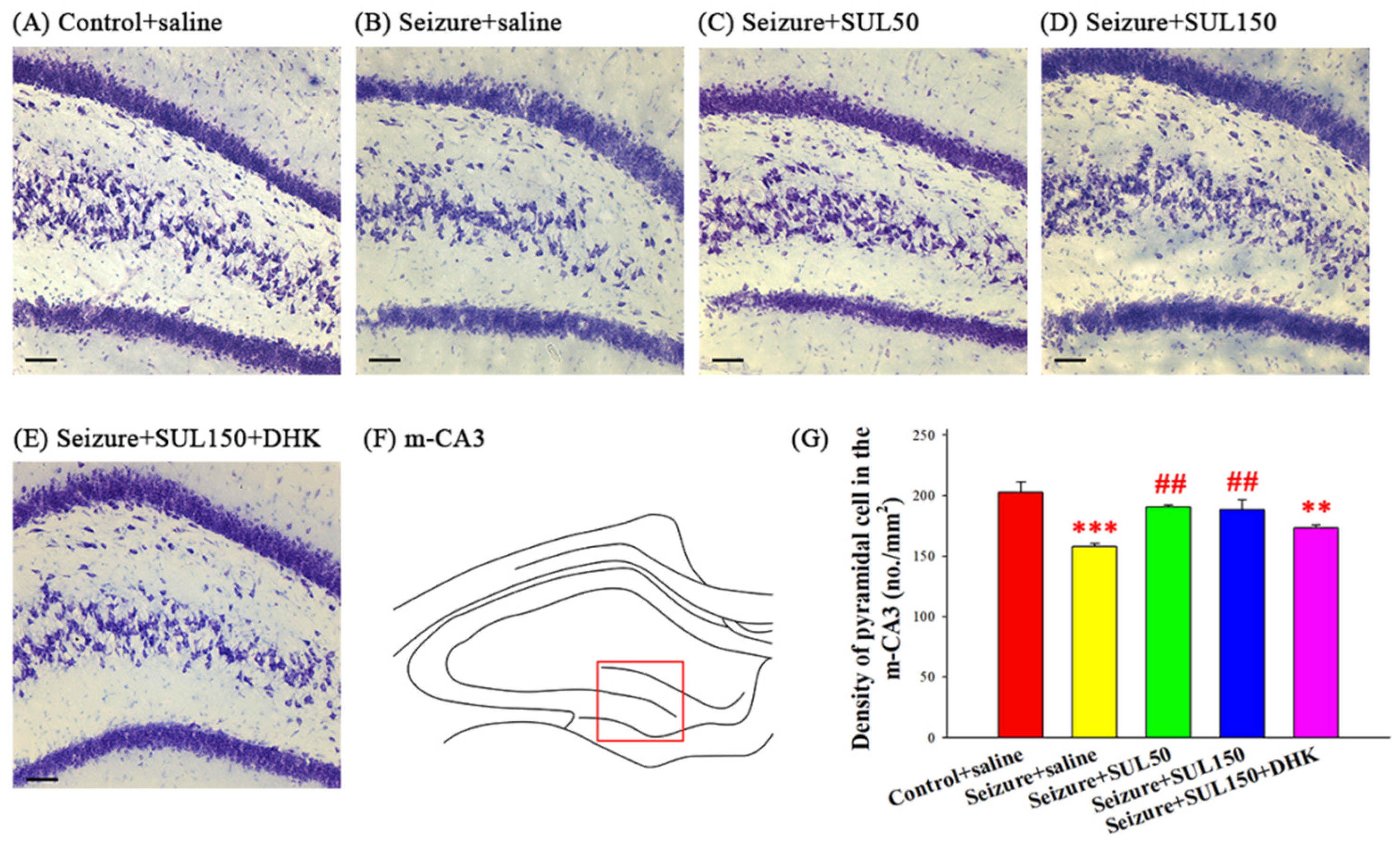
3.5. Neuronal Density in the Hippocampus
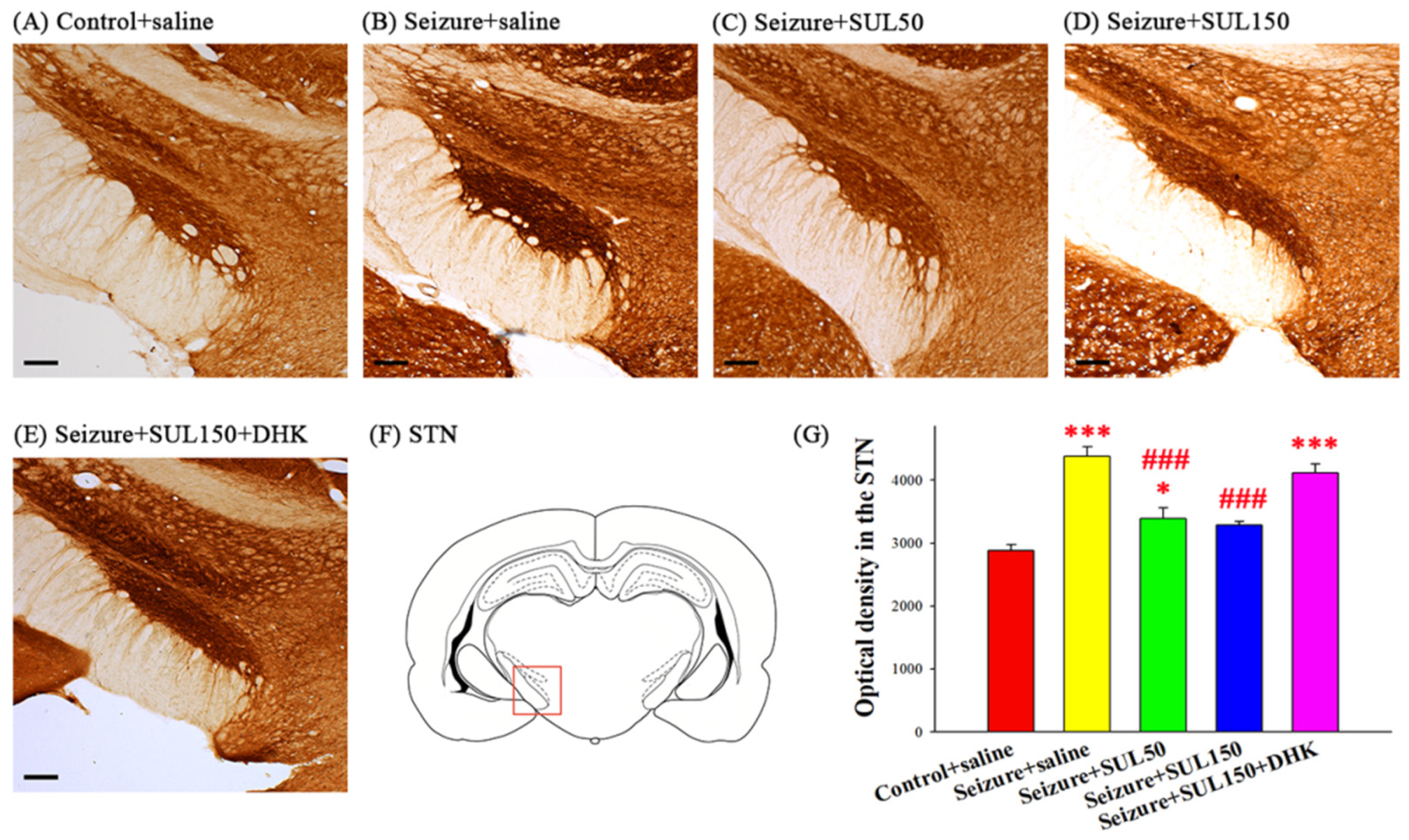
3.6. Cytochrome C Oxidase in the STN
3.7. BrdU-Positive Cells
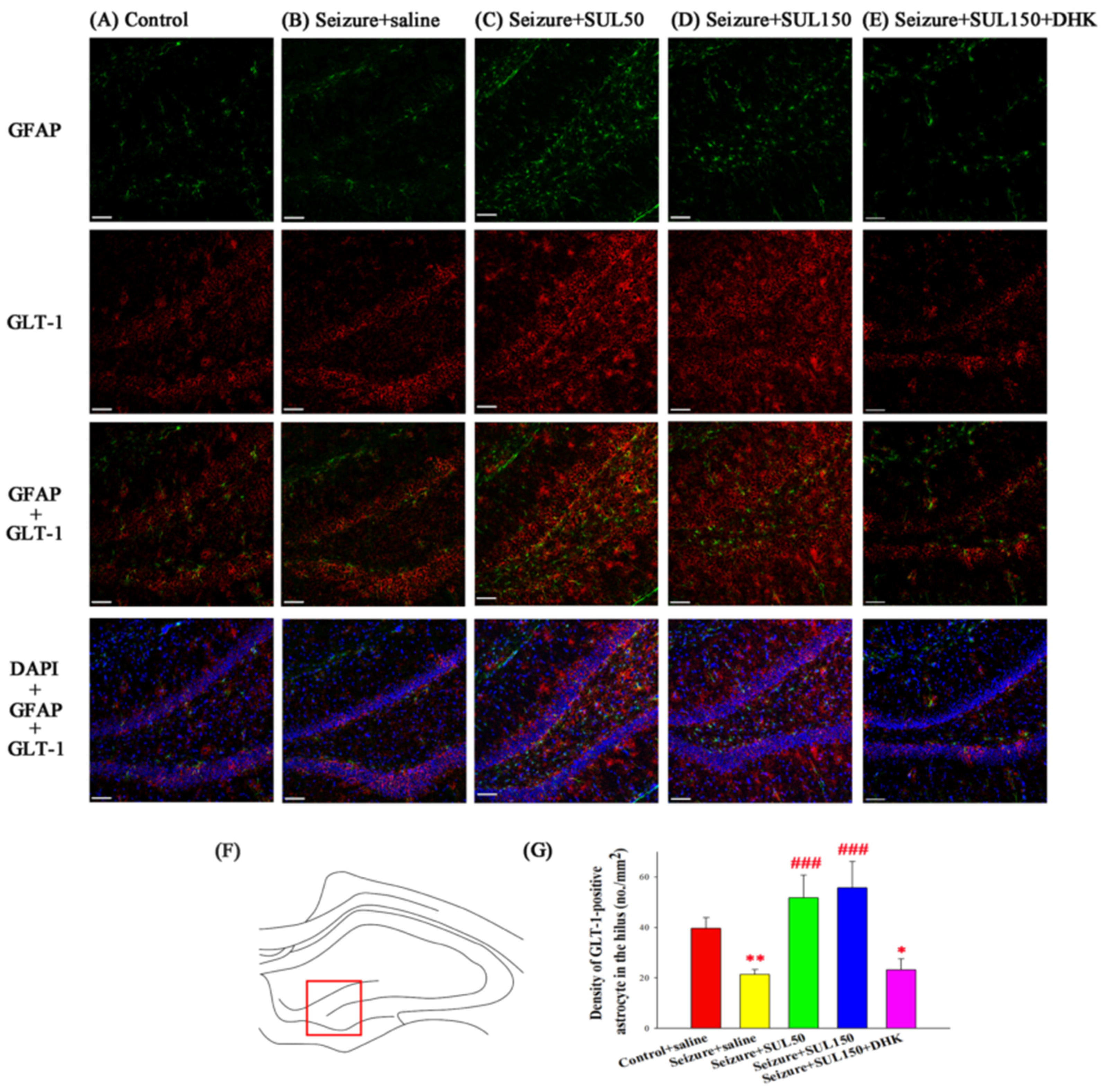
3.8. GLT–1 Expression in the Hippocampus
4. Discussion
4.1. Kindling Epilepsy Rat Model
4.2. Behavior
4.3. Histology and Behavior
4.4. Metabolic Neuronal Activity in the STN
4.5. Mechanism of SUL
4.6. SUL Effects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Piazzini, A.; Canger, R. Depression and Anxiety in Patients with Epilepsy. Epilepsia 2001, 2, 481–489. [Google Scholar] [CrossRef]
- Holmes, G.L. Cognitive impairment in epilepsy: The role of network abnormalities. Epileptic Disord. 2015, 17, 101–116. [Google Scholar] [CrossRef]
- Kwon, O.Y.; Park, S.P. Depression and anxiety in people with epilepsy. J. Clin. Neurol. 2014, 10, 175–188. [Google Scholar] [CrossRef]
- de Lanerolle, N.C.; Kim, J.H.; Robbins, R.; Spencer, D. Hippocampal interneuron loss and plasticity in human temporal lobe epilepsy. Brain Res. 1989, 495, 387–395. [Google Scholar] [CrossRef]
- Bernasconi, N.; Bernasconi, A.; Caramanos, Z.; Antel, S.B.; Andermann, F.; Arnold, D.L. Mesial temporal damage in temporal lobe epilepsy: A volumetric MRI study of the hippocampus, amygdala and parahippocampal region. Brain 2003, 126, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Fisher, R.; Salanova, V.; Witt, T.; Worth, R.; Henry, T.; Gross, R.; Oommen, K.; Osorio, I.; Nazzaro, J.; Labar, D.; et al. Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia 2010, 51, 899–908. [Google Scholar] [CrossRef] [PubMed]
- Okun, M.S. Deep-brain stimulation—Entering the era of human neural-network modulation. N. Engl. J. Med. 2014, 371, 1369–1373. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Du, J.; Wang, D.; Xu, C.; Ren, Z.; Wang, Y.; Li, Y.; Yu, T.; Ren, L. Long-term outcome of unilateral deep brain stimulation of the subthalamic nucleus for a patient with drug-resistant focal myoclonic seizure. Ann. Transl. Med. 2020, 8, 18. [Google Scholar] [CrossRef]
- Bozzi, Y.; Provenzano, G.; Casarosa, S. Neurobiological bases of autism–epilepsy comorbidity: A focus on excitation/inhibition imbalance. Eur. J. Neurosci. 2017, 47, 534–548. [Google Scholar] [CrossRef]
- Koshal, P.; Jamwal, S.; Kumar, P. Glucagon-like Peptide-1 (GLP-1) and neurotransmitters signaling in epilepsy: An insight review. Neuropharmacology 2018, 136, 271–279. [Google Scholar] [CrossRef]
- Traynelis, S.F.; Wollmuth, L.P.; McBain, C.J.; Menniti, F.S.; Vance, K.M.; Ogden, K.K.; Hansen, K.B.; Yuan, H.; Myers, S.J.; Dingledine, R. Glutamate Receptor Ion Channels: Structure, Regulation, and Function. Pharmacol. Rev. 2010, 62, 405–496. [Google Scholar] [CrossRef]
- Stelzer, A.; Slater, N.T.; Bruggencate, G.T. Activation of NMDA receptors blocks GABAergic inhibition in an in vitro model of epilepsy. Nature 1987, 326, 698–701. [Google Scholar] [CrossRef]
- Sayin, Ü.; Rutecki, P.; Sutula, T. NMDA-dependent currents in granule cells of the dentate gyrus contribute to induction but not permanence of kindling. J. Neurophysiol. 1999, 81, 564–574. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Watase, K.; Manabe, T.; Yamada, K.; Watanabe, M.; Takahashi, K.; Iwama, H.; Nishikawa, T.; Ichihara, N.; Kikuchi, T.; et al. Epilepsy and Exacerbation of Brain Injury in Mice Lacking the Glutamate Transporter GLT-1. Science 1997, 276, 1699–1702. [Google Scholar] [CrossRef]
- Coulter, D.A.; Eid, T. Astrocytic regulation of glutamate homeostasis in epilepsy. Glia 2012, 60, 1215–1226. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Gong, S.; Zhang, M.; Zhang, L.; Hu, Y.; Liu, Y.; Li, W. Cerebral ischemic preconditioning reduces glutamate excitotoxicity by up-regulating the uptake activity of GLT-1 in rats. Amino. Acids. 2014, 46, 1537–1545. [Google Scholar] [CrossRef]
- Hänninen, P.; Rossi, T. Penetration of Sulbactam into Cerebrospinal Fluid of Patients with Viral Meningitis or without Meningitis. Clin. Infect. Dis. 1986, 8, S609–S611. [Google Scholar] [CrossRef] [PubMed]
- Foulds, G.; McBride, T.J.; Knirsch, A.K.; Rodriguez, W.J.; Khan, W.N. Penetration of sulbactam and ampicillin into cerebrospinal fluid of infants and young children with meningitis. Antimicrob. Agents Chemother. 1987, 31, 1703–1705. [Google Scholar] [CrossRef]
- Qi, J.; Xian, X.-H.; Li, L.; Zhang, M.; Hu, Y.-Y.; Zhang, J.-G.; Li, W.-B. Sulbactam Protects Hippocampal Neurons Against Oxygen-Glucose Deprivation by Up-Regulating Astrocytic GLT-1 via p38 MAPK Signal Pathway. Front. Mol. Neurosci. 2018, 11, 281. [Google Scholar] [CrossRef]
- Hammad, A.M.; Sari, Y. Effects of Cocaine Exposure on Astrocytic Glutamate Transporters and Relapse-Like Ethanol-Drinking Behavior in Male. Alcohol Alcohol. 2020, 55, 254–263. [Google Scholar] [CrossRef]
- Corda, M.G.; Orlandi, M.; Lecca, D.; Carboni, G.; Frau, V.; Giorgi, O. Pentylenetetrazol-induced kindling in rats: Effect of GABA function inhibitors. Pharmacol. Biochem. Behav. 1991, 40, 329–333. [Google Scholar] [CrossRef]
- Huang, R.Q.; Bell-Horner, C.L.; Dibas, M.I.; Covey, D.F.; Drewe, J.A.; Dillon, G.H. Pentylenetetrazole-induced inhibition of recombinant gamma-aminobutyric acid type A (GABA(A)) receptors: Mechanism and site of action. J. Pharmacol. Exp. Ther. 2001, 298, 986–995. [Google Scholar] [CrossRef]
- Ho, Y.J.; Shen, M.-S.; Tai, C.-H.; Li, H.-H.; Chen, J.-H.; Liao, W.-C.; Chiu, P.-Y.; Lee, I.-Y.; Lin, C.-L.; Hung, C.-S. Use of Ceftriaxone in Treating Cognitive and Neuronal Deficits Associated with Dementia with Lewy Bodies. Front. Neurosci. 2019, 13, 507. [Google Scholar] [CrossRef]
- Ferré, P.; Núñez, J.F.; García, E.; Tobeña, A.; Escorihuela, R.M.; Fernández-Teruel, A. Postnatal handling reduces anxiety as measured by emotionality rating and hyponeophagia tests in female rats. Pharmacol. Biochem. Behav. 1995, 51, 199–203. [Google Scholar] [CrossRef]
- Samokhina, E.; Samokhin, A. Neuropathological profile of the pentylenetetrazol (PTZ) kindling model. Int. J. Neurosci. 2018, 128, 1086–1096. [Google Scholar] [CrossRef]
- Alasmari, F.; Alhaddad, H.; Wong, W.; Bell, R.L.; Sari, Y. Ampicillin/Sulbactam Treatment Modulates NMDA Receptor NR2B Subunit and Attenuates Neuroinflammation and Alcohol Intake in Male High Alcohol Drinking Rats. Biomolecules 2020, 10, 1030. [Google Scholar] [CrossRef] [PubMed]
- Hammad, A.M.; Alasmari, F.; Althobaiti, Y.S.; Sari, Y. Modulatory effects of Ampicillin/Sulbactam on glial glutamate transporters and metabotropic glutamate receptor 1 as well as reinstatement to cocaine-seeking behavior. Behav. Brain Res. 2017, 332, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Uziel, A.; Gelfand, A.; Amsalem, K.; Berman, P.; Lewitus, G.M.; Meiri, D.; Lewitus, D.Y. Full-Spectrum Cannabis Extract Microdepots Support Controlled Release of Multiple Phytocannabinoids for Extended Therapeutic Effect. ACS. Appl. Mater. Interfaces 2020, 12, 23707–23716. [Google Scholar] [CrossRef] [PubMed]
- Tanapat, P.; Hastings, N.B.; Reeves, A.J.; Gould, E. Estrogen Stimulates a Transient Increase in the Number of New Neurons in the Dentate Gyrus of the Adult Female Rat. J. Neurosci. 1999, 19, 5792–5801. [Google Scholar] [CrossRef]
- Dhir, A. Pentylenetetrazol (PTZ) Kindling Model of Epilepsy. Curr. Protoc. Neurosci. 2012, 58, 9.37.1–9.37.12. [Google Scholar] [CrossRef]
- Gupta, Y.K.; Veerendra Kumar, M.H.; Srivastava, A.K. Effect of Centella asiatica on pentylenetetrazole-induced kindling, cognition and oxidative stress in rats. Pharmacol. Biochem. Behav. 2003, 74, 579–585. [Google Scholar] [CrossRef]
- Hussein, A.M.; Ghalwash, M.; Magdy, K.; Abulseoud, O.A. Beta Lactams Antibiotic Ceftriaxone Modulates Seizures, Oxidative Stress and Connexin 43 Expression in Hippocampus of Pentylenetetrazole Kindled Rats. J. Epilepsy Res. 2016, 6, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Li, H.H.; Li, H.-H.; Lin, P.-J.; Wang, W.-H.; Tseng, L.-H.; Liu, W.-Y.; Lin, C.-L.; Liu, C.-H.; Liao, W.-C.; Hung, C.-S.; et al. Treatment effects of the combination of ceftriaxone and valproic acid on neuronal and behavioural functions in a rat model of epilepsy. Exp. Physiol. 2021, 106, 1814–1828. [Google Scholar] [CrossRef]
- Leger, M.; Quiedeville, A.; Bouet, V.; Haelewyn, B.; Boulouard, M.; Schumann-Bard, P.; Freret, T. Object recognition test in mice. Nat. Protoc. 2013, 8, 2531–2537. [Google Scholar] [CrossRef] [PubMed]
- Walf, A.A.; Frye, C.A. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat. Protoc. 2007, 2, 322–328. [Google Scholar] [CrossRef]
- Ho, Y.J.; Ho, S.-C.; Pawlak, C.R.; Yeh, K.-Y. Effects of d-cycloserine on MPTP-induced behavioral and neurological changes: Potential for treatment of Parkinson’s disease dementia. Behav. Brain Res. 2011, 219, 280–290. [Google Scholar] [CrossRef]
- Liu, C.H.; Liao, W.; Li, H.; Tseng, L.; Wang, W.; Tung, H.; Lin, P.; Jao, H.; Liu, W.; Hung, C.; et al. Treatment with the combination of clavulanic acid and valproic acid led to recovery of neuronal and behavioral deficits in an epilepsy rat model. Fundam. Clin. Pharmacol. 2021, 35, 1032–1044. [Google Scholar] [CrossRef] [PubMed]
- Ögren, S.O.; Stiedl, O. Passive avoidance. In Encyclopedia of Psychopharmacology; Springer: Berlin, Germany, 2010; Volume 2, pp. 960–967. [Google Scholar]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 6th ed.; Elsevier Academic Press: Amsterdam, The Netherlands; London, UK, 2007. [Google Scholar]
- Tikhonova, M.A.; Amstislavskaya, T.G.; Ho, Y.-J.; Akopyan, A.A.; Tenditnik, M.V.; Ovsyukova, M.V.; Bashirzade, A.A.; Dubrovina, N.I.; Aftanas, L.I. Neuroprotective Effects of Ceftriaxone Involve the Reduction of Aβ Burden and Neuroinflammatory Response in a Mouse Model of Alzheimer’s Disease. Front. Neurosci. 2021, 15, 736786. [Google Scholar] [CrossRef]
- Hsu, C.Y.; Hung, C.-S.; Chang, H.-M.; Liao, W.-C.; Ho, S.-C.; Ho, Y.-J. Ceftriaxone prevents and reverses behavioral and neuronal deficits in an MPTP-induced animal model of Parkinson’s disease dementia. Neuropharmacology 2015, 91, 43–56. [Google Scholar] [CrossRef]
- Weng, J.C.; Tikhonova, M.A.; Chen, J.-H.; Shen, M.-S.; Meng, W.-Y.; Chang, Y.-T.; Chen, K.-H.; Liang, K.-C.; Hung, C.-S.; Amstislavskaya, T.G.; et al. Ceftriaxone prevents the neurodegeneration and decreased neurogenesis seen in a Parkinson’s disease rat model: An immunohistochemical and MRI study. Behav. Brain Res. 2016, 305, 126–139. [Google Scholar] [CrossRef]
- Kronenberg, G.; Reuter, K.; Steiner, B.; Brandt, M.D.; Jessberger, S.; Yamaguchi, M.; Kempermann, G. Subpopulations of proliferating cells of the adult hippocampus respond differently to physiologic neurogenic stimuli. J. Comp. Neurol. 2003, 467, 455–463. [Google Scholar] [CrossRef]
- Kaplan, J.S.; Stella, N.; Catterall, W.A.; Westenbroek, R.E. Cannabidiol attenuates seizures and social deficits in a mouse model of Dravet syndrome. Proc. Natl. Acad. Sci. USA 2017, 114, 11229–11234. [Google Scholar] [CrossRef]
- Soni, N.; Koushal, P.; Reddy, B.V.K.; Deshmukh, R.; Kumar, P. Effect of GLT-1 modulator and P2X7 antagonists alone and in combination in the kindling model of epilepsy in rats. Epilepsy Behav. 2015, 48, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Franke, H.; Kittner, H. Morphological alterations of neurons and astrocytes and changes in emotional behavior in pentylenetetrazol-kindled rats. Pharmacol. Biochem. Behav. 2001, 70, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.J.; Wang, F.; Li, C.K. Resveratrol is Neuroprotective and Improves Cognition in Pentylenetetrazole-kindling Model of Epilepsy in Rats. Indian J. Pharm. Sci. 2014, 76, 125–131. [Google Scholar] [PubMed]
- Shimada, T.; Yamagata, K. Pentylenetetrazole-Induced Kindling Mouse Model. J. Vis. Exp. 2018, 136, 56573. [Google Scholar] [CrossRef]
- Singh, N.; Vijayanti, S.; Saha, L.; Bhatia, A.; Banerjee, D.; Chakrabarti, A. Neuroprotective effect of Nrf2 activator dimethyl fumarate, on the hippocampal neurons in chemical kindling model in rat. Epilepsy Res. 2018, 143, 98–104. [Google Scholar] [CrossRef]
- Fabisiak, J.P.; Schwark, W. Aspects of the pentylenetetrazol kindling model of epileptogenesis in the rat. Exp. Neurol. 1982, 78, 7–14. [Google Scholar] [CrossRef]
- Akyüz, E. A New View of The Racine Scoring System in The Pentylenetetrazol Epilepsy Model. J. Harran Univ. Med. Fac. 2020, 17, 306–310. [Google Scholar] [CrossRef]
- Doi, T.; Ueda, Y.; Nagatomo, K.; Willmore, L.J. Role of Glutamate and GABA Transporters in Development of Pentylenetetrazol-Kindling. Neurochem. Res. 2009, 34, 1324–1331. [Google Scholar] [CrossRef]
- Zhu, X.; Dong, J.; Shen, K.; Bai, Y.; Zhang, Y.; Lv, X.; Chao, J.; Yao, H. NMDA receptor NR2B subunits contribute to PTZ-kindling-induced hippocampal astrocytosis and oxidative stress. Brain Res. Bull. 2015, 114, 70–78. [Google Scholar] [CrossRef]
- Akyuz, E.; Polat, K.; Ates, S.; Unalmis, D.; Tokpinar, A.; Yilmaz, S.; Kaymak, E.; Doganyigit, Z.; Villa, C. Investigating Cardiac Morphological Alterations in a Pentylenetetrazol-Kindling Model of Epilepsy. Diagnostics 2020, 10, 388. [Google Scholar] [CrossRef]
- Patra, P.H.; Barker-Haliski, M.; White, H.S.; Whalley, B.J.; Glyn, S.; Sandhu, H.; Jones, N.; Bazelot, M.; Williams, C.M.; McNeish, A.J. Cannabidiol reduces seizures and associated behavioral comorbidities in a range of animal seizure and epilepsy models. Epilepsia 2018, 60, 303–314. [Google Scholar] [CrossRef]
- Elger, C.E.; Helmstaedter, C.; Kurthen, M. Chronic epilepsy and cognition. Lancet. Neurol. 2004, 3, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, S.; Heydari, A.; Alinaghipour, A.; Salami, M. Effect of probiotic supplementation on seizure activity and cognitive performance in PTZ-induced chemical kindling. Epilepsy Behav. 2019, 95, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Aniol, V.A.; Ivanova-Dyatlova, A.Y.; Keren, O.; Guekht, A.B.; Sarne, Y.; Gulyaeva, N.V. A single pentylenetetrazole-induced clonic-tonic seizure episode is accompanied by a slowly developing cognitive decline in rats. Epilepsy Behav. 2013, 26, 196–202. [Google Scholar] [CrossRef]
- Cendes, F.; Andermann, F.; Gloor, P.; Gambardella, A.; Lopes-Cendes, I.; Watson, C.; Evans, A.; Carpenter, S.; Olivier, A. Relationship between atrophy of the amygdala and ictal fear in temporal lobe epilepsy. Brain 1994, 117, 739–746. [Google Scholar] [CrossRef]
- Monti, G.; Meletti, S. Emotion recognition in temporal lobe epilepsy: A systematic review. Neurosci. Biobehav. Rev. 2015, 55, 280–293. [Google Scholar] [CrossRef]
- Kanner, A.M. Epilepsy and mood disorders. Epilepsia 2007, 48, 20–22. [Google Scholar] [CrossRef]
- Tracy, J.I.; Dechant, V.; Sperling, M.R.; Cho, R.; Glosser, D. The association of mood with quality of life ratings in epilepsy. Neurology 2007, 68, 1101–1107. [Google Scholar] [CrossRef]
- Jackson, M.J.; Turkington, D. Depression and anxiety in epilepsy. J. Neurol. Neurosurg. Psychiatry 2005, 76, i45–i47. [Google Scholar] [CrossRef] [PubMed]
- Pitkänen, A.; Tuunanen, J.; Kälviäinen, R.; Partanen, K.; Salmenperä, T. Amygdala damage in experimental and human temporal lobe epilepsy. Epilepsy Res. 1998, 32, 233–253. [Google Scholar] [CrossRef] [PubMed]
- Lamberty, Y.; Klitgaard, H. Consequences of Pentylenetetrazole Kindling on Spatial Memory and Emotional Responding in the Rat. Epilepsy Behav. 2000, 1, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Adamec, R.E. Amygdala kindling and anxiety in the rat. Neuroreport 1990, 1, 255–258. [Google Scholar] [CrossRef]
- Inostroza, M.; Cid, E.; Brotons-Mas, J.; Gal, B.; Aivar, P.; Uzcategui, Y.G.; Sandi, C.; de la Prida, L.M.; Avoli, M. Hippocampal-Dependent Spatial Memory in the Water Maze is Preserved in an Experimental Model of Temporal Lobe Epilepsy in Rats. PLoS ONE 2011, 6, e22372. [Google Scholar] [CrossRef]
- Szyndler, J.; Rok, P.; Maciejak, P.; Walkowiak, J.; Członkowska, A.I.; Sienkiewicz-Jarosz, H.; Wisłowska, A.; Zienowicz, M.; Lehner, M.; Bidziński, A.; et al. Effects of pentylenetetrazol-induced kindling of seizures on rat emotional behavior and brain monoaminergic systems. Pharmacol. Biochem. Behav. 2002, 73, 851–861. [Google Scholar] [CrossRef]
- Kalynchuk, L. Long-term amygdala kindling in rats as a model for the study of interictal emotionality in temporal lobe epilepsy. Neurosci. Biobehav. Rev. 2000, 24, 691–704. [Google Scholar] [CrossRef]
- Jones, N.C.; Salzberg, M.R.; Kumar, G.; Couper, A.; Morris, M.J.; O’BRien, T.J. Elevated anxiety and depressive-like behavior in a rat model of genetic generalized epilepsy suggesting common causation. Exp. Neurol. 2008, 209, 254–260. [Google Scholar] [CrossRef]
- Buckman, S.G.; Hodgson, S.R.; Hofford, R.S.; Eitan, S. Increased elevated plus maze open-arm time in mice during spontaneous morphine withdrawal. Behav. Brain Res. 2009, 197, 454–456. [Google Scholar] [CrossRef]
- Knierim, J.J. The hippocampus. Curr. Biol. 2015, 25, R1116–R1121. [Google Scholar] [CrossRef]
- Mathern, G.W.; Adelson, P.D.; Cahan, L.D.; Leite, J.P. Hippocampal neuron damage in human epilepsy: Meyer’s hypothesis revisited. Prog. Brain Res. 2002, 135, 237–251. [Google Scholar] [CrossRef]
- Malmgren, K.; Thom, M. Hippocampal sclerosis-Origins and imaging. Epilepsia 2012, 53, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Thom, M. Review: Hippocampal sclerosis in epilepsy: A neuropathology review. Neuropathol. Appl. Neurobiol. 2014, 40, 520–543. [Google Scholar] [CrossRef] [PubMed]
- Hammen, T.; Hildebrandt, M.; Stadlbauer, A.; Doelken, M.; Engelhorn, T.; Kerling, F.; Kasper, B.; Romstoeck, J.; Ganslandt, O.; Nimsky, C.; et al. Non-invasive detection of hippocampal sclerosis: Correlation between metabolite alterations detected by (1)H-MRS and neuropathology. NMR Biomed. 2008, 21, 545–552. [Google Scholar] [CrossRef]
- Tai, X.Y.; Bernhardt, B.; Thom, M.; Thompson, P.; Baxendale, S.; Koepp, M.; Bernasconi, N. Review: Neurodegenerative processes in temporal lobe epilepsy with hippocampal sclerosis: Clinical, pathological and neuroimaging evidence. Neuropathol. Appl. Neurobiol. 2017, 44, 70–90. [Google Scholar] [CrossRef]
- Sayin, U.; Osting, S.; Hagen, J.; Rutecki, P.; Sutula, T. Spontaneous Seizures and Loss of Axo-Axonic and Axo-Somatic Inhibition Induced by Repeated Brief Seizures in Kindled Rats. J. Neurosci. 2003, 23, 2759–2768. [Google Scholar] [CrossRef]
- Fuerst, D.; Shah, J.; Shah, A.; Watson, C. Hippocampal sclerosis is a progressive disorder: A longitudinal volumetric MRI study. Ann. Neurol. 2003, 53, 413–416. [Google Scholar] [CrossRef]
- Altman, J.; Das, G.D. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J. Comp. Neurol. 1965, 124, 319–335. [Google Scholar] [CrossRef]
- Abbott, L.C.; Nigussie, F. Adult neurogenesis in the mammalian dentate gyrus. Anat. Histol. Embryol. 2019, 49, 3–16. [Google Scholar] [CrossRef]
- Gradari, S.; Pérez-Domper, P.; Butler, R.G.; Martínez-Cué, C.; de Polavieja, G.G.; Trejo, J.L. The relationship between behavior acquisition and persistence abilities: Involvement of adult hippocampal neurogenesis. Hippocampus 2016, 26, 857–874. [Google Scholar] [CrossRef]
- Coulter, D.A.; Steinhäuser, C. Role of Astrocytes in Epilepsy. Cold Spring Harb. Perspect. Med. 2015, 5, a022434. [Google Scholar] [CrossRef]
- Eid, T.; Williamson, A.; Lee, T.S.; Petroff, O.A.; de Lanerolle, N.C. Glutamate and astrocytes—Key players in human mesial temporal lobe epilepsy? Epilepsia 2008, 49, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Khurgel, M.; Ivy, G.O. Astrocytes in kindling: Relevance to epileptogenesis. Epilepsy Res. 1996, 26, 163–175. [Google Scholar] [CrossRef] [PubMed]
- de Lanerolle, N.C.; Lee, T.-S.; Spencer, D.D. Astrocytes and Epilepsy. Neurotherapeutics 2010, 7, 424–438. [Google Scholar] [CrossRef]
- Hamani, C.; Saint-Cyr, J.A.; Fraser, J.; Kaplitt, M.; Lozano, A.M. The subthalamic nucleus in the context of movement disorders. Brain 2004, 127, 4–20. [Google Scholar] [CrossRef]
- Rodriguez, M.C.; Obeso, J.A.; Olanow, C.W. Subthalamic nucleus-mediated excitotoxicity in parkinson’s disease: A target for neuroprotection. Ann. Neurol. 1998, 44, S175–S188. [Google Scholar] [CrossRef]
- Benabid, A.L.; Chabardes, S.; Mitrofanis, J.; Pollak, P. Deep brain stimulation of the subthalamic nucleus for the treatment of Parkinson’s disease. Lancet Neurol. 2009, 8, 67–81. [Google Scholar] [CrossRef]
- Handforth, A.; DeSalles, A.A.F.; Krahl, S.E. Deep Brain Stimulation of the Subthalamic Nucleus as Adjunct Treatment for Refractory Epilepsy. Epilepsia 2006, 47, 1239–1241. [Google Scholar] [CrossRef]
- Deransart, C.; Marescaux, C.; Depaulis, A. Involvement of nigral glutamatergic inputs in the control of seizures in a genetic model of absence epilepsy in the rat. Neuroscience 1996, 71, 721–728. [Google Scholar] [CrossRef]
- Broer, S. Not Part of the Temporal Lobe, but Still of Importance? Substantia Nigra and Subthalamic Nucleus in Epilepsy. Front. Syst. Neurosci. 2020, 14, 581826. [Google Scholar] [CrossRef]
- Kim, K.; Lee, S.; Kegelman, T.P.; Su, Z.; Das, S.K.; Dash, R.; Dasgupta, S.; Barral, P.M.; Hedvat, M.; Diaz, P.; et al. Role of Excitatory Amino Acid Transporter-2 (EAAT2) and glutamate in neurodegeneration: Opportunities for developing novel therapeutics. J. Cell Physiol. 2010, 226, 2484–2493. [Google Scholar] [CrossRef]
- Henshall, D.C.; Simon, R.P. Epilepsy and apoptosis pathways. J. Cereb. Blood Flow. Metab. 2005, 25, 1557–1572. [Google Scholar] [CrossRef]
- Andersen, J.V.; Christensen, S.K.; Westi, E.W.; Diaz-Delcastillo, M.; Tanila, H.; Schousboe, A.; Aldana, B.I.; Waagepetersen, H.S. Deficient astrocyte metabolism impairs glutamine synthesis and neurotransmitter homeostasis in a mouse model of Alzheimer’s disease. Neurobiol. Dis. 2021, 148, 105198. [Google Scholar] [CrossRef]
- Aniol, V.A.; Stepanichev, M.Y.; Yakovlev, A.A.; Lazareva, N.A.; Gulyaeva, N.V. Anxiogenic Effect of Pentylenetetrazole at a Subconvulsive Dose Is Accompanied by Decreased Cellular Proliferation and Neuronal NO-Synthase Expression in the Posterior Part of the Hippocampus. Neurochem. J. 2024, 18, 734–741. [Google Scholar] [CrossRef]
- Jiang, W.; Wolfe, K.; Xiao, L.; Zhang, Z.-J.; Huang, Y.-G.; Zhang, X. Ionotropic glutamate receptor antagonists inhibit the proliferation of granule cell precursors in the adult brain after seizures induced by pentylenetrazol. Brain Res. 2004, 1020, 154–160. [Google Scholar] [CrossRef]
- Lazarov, O.; Mattson, M.P.; Peterson, D.A.; Pimplikar, S.W.; van Praag, H. When neurogenesis encounters aging and disease. Trends. Neurosci. 2010, 33, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Stevens, C.F.; Gage, F.H. Astroglia induce neurogenesis from adult neural stem cells. Nature 2002, 417, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Rawls, S.M.; Zielinski, M.; Patel, H.; Sacavage, S.; Baron, D.A.; Patel, D. Beta-lactam antibiotic reduces morphine analgesic tolerance in rats through GLT-1 transporter activation. Drug Alcohol Depend. 2010, 107, 261–263. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, F.-C.; Liu, C.-H.; Liao, W.-C.; Tzeng, Y.-S.; Tsai, R.-Y.; Tseng, L.-H.; Hung, C.-S.; Wu, S.-L.; Ho, Y.-J. Sulbactam: A β–Lactam Compound with Neuroprotective Effects in Epilepsy. Neurol. Int. 2025, 17, 135. https://doi.org/10.3390/neurolint17090135
Chang F-C, Liu C-H, Liao W-C, Tzeng Y-S, Tsai R-Y, Tseng L-H, Hung C-S, Wu S-L, Ho Y-J. Sulbactam: A β–Lactam Compound with Neuroprotective Effects in Epilepsy. Neurology International. 2025; 17(9):135. https://doi.org/10.3390/neurolint17090135
Chicago/Turabian StyleChang, Fang-Chia, Chiung-Hui Liu, Wen-Chieh Liao, Yu-Shiuan Tzeng, Ru-Yin Tsai, Li-Ho Tseng, Ching-Sui Hung, Shey-Lin Wu, and Ying-Jui Ho. 2025. "Sulbactam: A β–Lactam Compound with Neuroprotective Effects in Epilepsy" Neurology International 17, no. 9: 135. https://doi.org/10.3390/neurolint17090135
APA StyleChang, F.-C., Liu, C.-H., Liao, W.-C., Tzeng, Y.-S., Tsai, R.-Y., Tseng, L.-H., Hung, C.-S., Wu, S.-L., & Ho, Y.-J. (2025). Sulbactam: A β–Lactam Compound with Neuroprotective Effects in Epilepsy. Neurology International, 17(9), 135. https://doi.org/10.3390/neurolint17090135






