Validation of the Charlotte Large Artery Occlusion Endovascular Therapy Outcome Score in a Modern Cohort of Thrombectomy Patients
Abstract
1. Introduction
2. Materials and Methods
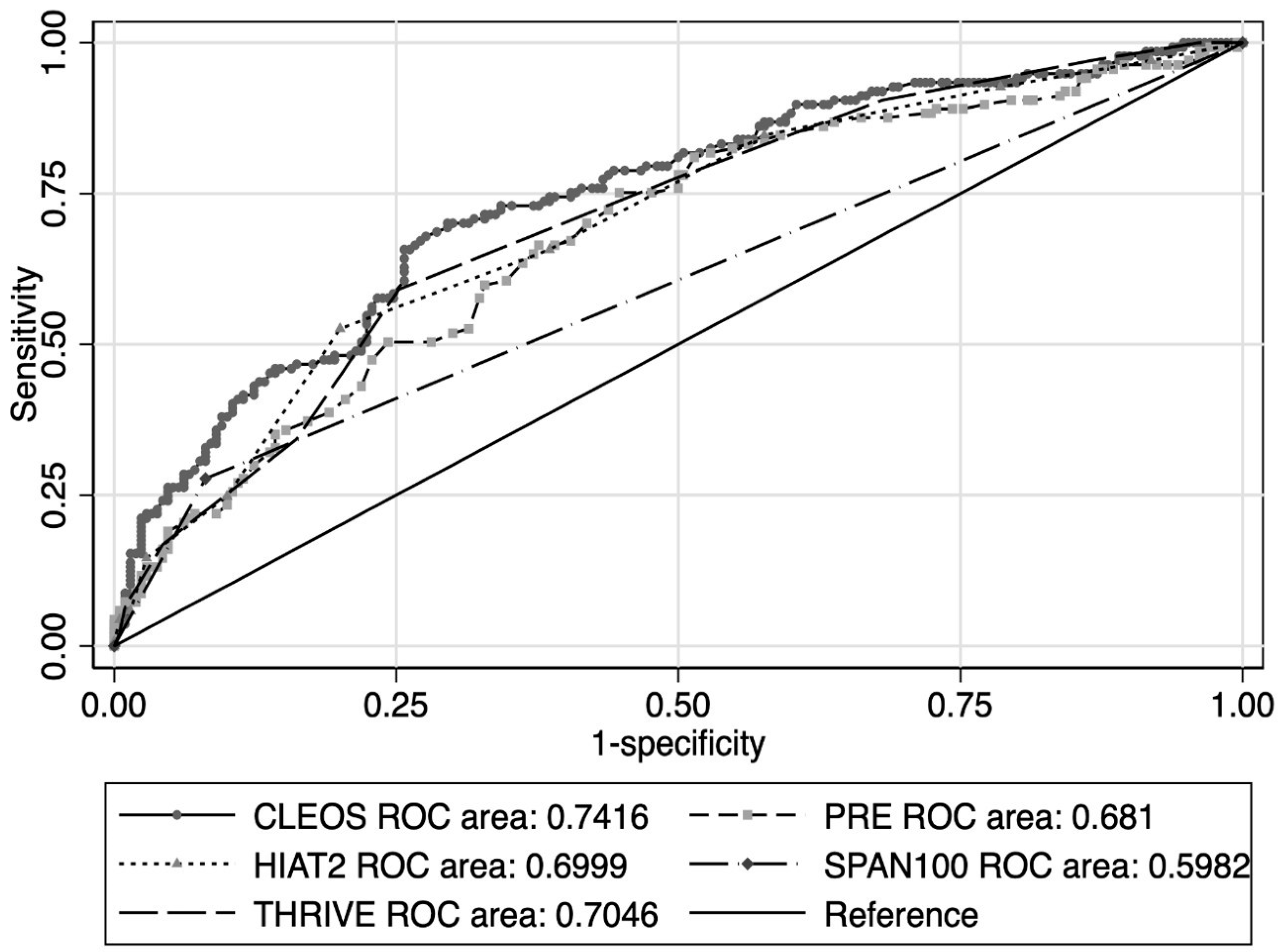
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CLEOS | Charlotte Large artery occlusion Endovascular therapy Outcome Score |
| EVT | endovascular thrombectomy |
| NIHSS | National Institutes of Health Stroke Scale |
| CBV | cerebral blood volume |
| CTP | computed tomography perfusion |
| mRS | modified Rankin Scale |
| CT | computed tomography |
| ASPECTS | Alberta Stroke Program Early Computed Tomography Score |
| SD | standard deviation |
| IQR | interquartile range |
| ROC | receiver operator characteristics |
| AUC | area under the curve |
| THRIVE | Totaled Health Risks in Vascular Events |
| HIAT-2 | Houston Intra-Arterial Therapy-2 |
| PRE | Pittsburgh Response to Endovascular therapy |
| SPAN-100 | Stroke Prognostication using Age and NIHSS |
| LKW | last known well |
| ml | milliliters |
| OR | odds ratio |
References
- Karamchandani, R.R.; Prasad, T.; Strong, D.; Rhoten, J.B.; Asimos, A.W. A Tool to Improve Stroke Outcome Prediction: The Charlotte Large Artery Occlusion Endovascular Therapy Outcome Score. J. Stroke Cerebrovasc. Dis. 2022, 31, 106393. [Google Scholar] [CrossRef] [PubMed]
- Karamchandani, R.R.; Satyanarayana, S.; Yang, H.; Rhoten, J.B.; Strong, D.; Singh, S.; Clemente, J.D.; Defilipp, G.; Hazim, M.; Patel, N.M.; et al. The Charlotte Large Artery Occlusion Endovascular Therapy Outcome Score Compares Favorably to the Critical Area Perfusion Score for Prognostication Before Basilar Thrombectomy. J. Stroke Cerebrovasc. Dis. 2023, 32, 107147. [Google Scholar] [CrossRef] [PubMed]
- Bala, F.; Singh, N.; Buck, B.; Ademola, A.; Coutts, S.B.; Deschaintre, Y.; Khosravani, H.; Appireddy, R.; Moreau, F.; Phillips, S.; et al. Safety and Efficacy of Tenecteplase Compared with Alteplase in Patients with Large Vessel Occlusion Stroke: A Prespecified Secondary Analysis of the ACT Randomized Clinical Trial. JAMA Neurol. 2023, 80, 824–832. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Colasurdo, M. Endovascular Thrombectomy for Large Ischemic Strokes: Meta-Analysis of Six Multicenter Randomized Controlled Trials. J. Neurointerv. Surg. 2025, 17, 580–585. [Google Scholar] [CrossRef] [PubMed]
- Goyal, M.; Ospel, J.M.; Ganesh, A.; Dowlatshahi, D.; Volders, D.; Möhlenbruch, M.A.; Jumaa, M.A.; Nimjee, S.M.; Booth, T.C.; Buck, B.H.; et al. Endovascular Treatment of Stroke due to Medium-Vessel Occlusion. N. Engl. J. Med. 2025, 392, 1385–1395. [Google Scholar] [CrossRef] [PubMed]
- Psychogios, M.; Brehm, A.; Ribo, M.; Rizzo, F.; Strbian, D.; Räty, S.; Arenillas, J.F.; Martínez-Galdámez, M.; Hajdu, S.D.; Michel, P.; et al. Endovascular Treatment for Stroke due to Occlusion of Medium or Distal Vessels. N. Engl. J. Med. 2025, 392, 1374–1384. [Google Scholar] [CrossRef] [PubMed]
- Flint, A.C.; Cullen, S.P.; Faigeles, B.S.; Rao, V.A. Predicting Long-Term Outcome after Endovascular Stroke Treatment: The Totaled Health Risks in Vascular Events Score. Amer. J. Neuroradiol. 2010, 31, 1192–1196. [Google Scholar] [CrossRef] [PubMed]
- Sarraj, A.; Albright, K.; Barreto, A.D.; Boehme, A.K.; Sitton, C.W.; Choi, J.; Lutzker, S.L.; Sun, C.-H.J.; Bibars, W.; Nguyen, C.B.; et al. Optimizing Prediction Scores for Poor Outcome after Intra-Arterial Therapy in Anterior Circulation Acute Ischemic Stroke. Stroke 2013, 44, 3324–3330. [Google Scholar] [CrossRef] [PubMed]
- Rangaraju, S.; Aghaebrahim, A.; Streib, C.; Sun, C.-H.; Ribo, M.; Muchada, M.; Nogueira, R.; Frankel, M.; Gupta, R.; Jadhav, A.; et al. Pittsburgh Response to Endovascular Therapy (PRE) Score: Optimizing Patient Selection for Endovascular Therapy for Large Vessel Occlusion Strokes. J. Neurointerv. Surg. 2015, 7, 783–788. [Google Scholar] [CrossRef] [PubMed]
- Saposnik, G.; Guzik, A.K.; Reeves, M.; Ovbiagele, B.; Johnston, S.C. Stroke Prognostication Using Age and NIH Stroke Scale: SPAN-100. Neurology 2013, 80, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Kremers, F.; Venema, E.; Duvekot, M.; Yo, L.; Bokkers, R.; Lycklama, À.; Nijeholt, G.; van Es, A.; van der Lugt, A.; Majoie, C.; et al. Outcome Prediction Models for Endovascular Treatment of Ischemic Stroke: Systematic Review and External Validation. Stroke 2022, 53, 825–836. [Google Scholar] [CrossRef] [PubMed]
- Raza, S.A.; Rangaraju, S. A Review of Pre-Intervention Prognostic Scores for Early Prognostication and Patient Selection in Endovascular Management of Large Vessel Occlusion Stroke. Interv. Neurol. 2018, 7, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Bendszus, M.; Fiehler, J.; Subtil, F.; Bonekamp, S.; Aamodt, A.H.; Fuentes, B.; Gizewski, E.R.; Hill, M.D.; Krajina, A.; Pierot, L.; et al. Endovascular Thrombectomy for Acute Ischaemic Stroke with Established Large Infarct: Multicentre, Open-Label, Randomised Trial. Lancet 2023, 402, 1753–1763. [Google Scholar] [CrossRef] [PubMed]
- Costalat, V.; Jovin, T.G.; Albucher, J.F.; Cognard, C.; Henon, H.; Nouri, N.; Gory, B.; Richard, S.; Marnat, G.; Sibon, I.; et al. Trial of Thrombectomy for Stroke with a Large Infarct of Unrestricted Size. N. Engl. J. Med. 2024, 390, 1677–1689. [Google Scholar] [CrossRef] [PubMed]
- Sarraj, A.; Hassan, A.E.; Abraham, M.G.; Ortega-Gutierrez, S.; Kasner, S.E.; Hussain, M.S.; Chen, M.; Blackburn, S.; Sitton, C.W.; Churilov, L.; et al. Trial of Endovascular Thrombectomy for Large Ischemic Strokes. N. Engl. J. Med. 2023, 388, 1259–1271. [Google Scholar] [CrossRef] [PubMed]
- Karamchandani, R.R.; Satyanarayana, S.; Yang, H.; Rhoten, J.B.; Strong, D.; Patel, N.M.; Clemente, J.D.; Defilipp, G.; Bernard, J.D.; Stetler, W.R.; et al. The Charlotte Large Artery Occlusion Endovascular Therapy Outcome Score Predicts Poor Outcomes 1 Year After Endovascular Thrombectomy. World Neurosurg. 2023, 173, e415–e421. [Google Scholar] [CrossRef] [PubMed]

| Derivation, n = 453 | Validation, n = 347 | p-Value b | |
|---|---|---|---|
| Age, years, mean ± SD | 66.3 (15.4) | 67.6 (14.9) | 0.23 |
| Sex, male, n (%) | 230 (50.8%) | 180 (51.9%) | 0.76 |
| Hypertension, n (%) | 326 (72.0%) | 245 (70.6%) | 0.67 |
| Hyperlipidemia, n (%) | 199 (43.9%) | 154 (44.4%) | 0.90 |
| Diabetes mellitus, n (%) | 117 (25.9%) | 85 (24.5%) | 0.65 |
| Coronary artery disease, n (%) | 43 (9.5%) | 65 (18.7%) | <0.05 |
| Atrial Fibrillation, n (%) | 138 (30.5%) | 93 (26.8%) | 0.26 |
| Smoking, n (%) | 170 (37.5%) | 146 (42.1%) | 0.19 |
| Site of Occlusion, n (%) | 0.37 | ||
| Internal Carotid Artery | 110 (24.3%) | 83 (23.9%) | |
| Middle Cerebral Artery—M1 | 253 (55.8%) | 178 (51.3%) | |
| Middle Cerebral Artery—M2 | 87 (19.2%) | 84 (24.2%) | |
| Middle Cerebral Artery—M3 | 3 (0.7%) | 2 (0.6%) | |
| Initial NIHSS, median (IQR) | 16.0 (11.0–21.0) | 15.0 (10.0–20.0) | <0.05 |
| Glucose (mg/dL), mean ± SD | 136.7 (59.3) | 133.8 (50.3) | 0.48 |
| CT ASPECTS, median (IQR) | 10.0 (9.0–10.0) | 10.0 (8.0–10.0) | 0.80 |
| CBF < 30% (mL), median (IQR) | 8.0 (0.0–30.0) | 9.0 (0.0–35.0) | 0.40 |
| Tmax > 6 s (mL), median (IQR) | 125.0 (83.0–176.0) | 117.5 (73.0–181.0) | 0.65 |
| HIR, median (IQR) | 0.5 (0.2–0.6) | 0.5 (0.2–0.6) | 0.49 |
| CBV index, median (IQR) | 0.8 (0.6–0.8) | 0.7 (0.6–0.8) | 0.41 |
| LKW to skin puncture (min), mean ± SD | 389.9 (331.4) | 476.0 (445.0) | <0.05 |
| Intravenous thrombolysis, n (%) | 185 (40.8%) | 109 (31.4%) | <0.05 |
| Symptomatic ICH, n (%) | 10 (2.2%) | 5 (1.4%) | 0.60 |
| Post-treatment mTICI score 2c-3, n (%) | 243 (53.6%) | 175 (50.4%) | 0.37 |
| Poor 90-day mRS (4–6), n (%) | 178 (39.3%) | 137 (42.2%) | 0.42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karamchandani, R.R.; Wang, L.; Yang, H.; Strong, D.; Rhoten, J.B.; Clemente, J.D.; Defilipp, G.; Adelman, E.A.; Stetler, W.R.; Asimos, A.W. Validation of the Charlotte Large Artery Occlusion Endovascular Therapy Outcome Score in a Modern Cohort of Thrombectomy Patients. Neurol. Int. 2025, 17, 130. https://doi.org/10.3390/neurolint17080130
Karamchandani RR, Wang L, Yang H, Strong D, Rhoten JB, Clemente JD, Defilipp G, Adelman EA, Stetler WR, Asimos AW. Validation of the Charlotte Large Artery Occlusion Endovascular Therapy Outcome Score in a Modern Cohort of Thrombectomy Patients. Neurology International. 2025; 17(8):130. https://doi.org/10.3390/neurolint17080130
Chicago/Turabian StyleKaramchandani, Rahul R., Liang Wang, Hongmei Yang, Dale Strong, Jeremy B. Rhoten, Jonathan D. Clemente, Gary Defilipp, Elizabeth A. Adelman, William R. Stetler, and Andrew W. Asimos. 2025. "Validation of the Charlotte Large Artery Occlusion Endovascular Therapy Outcome Score in a Modern Cohort of Thrombectomy Patients" Neurology International 17, no. 8: 130. https://doi.org/10.3390/neurolint17080130
APA StyleKaramchandani, R. R., Wang, L., Yang, H., Strong, D., Rhoten, J. B., Clemente, J. D., Defilipp, G., Adelman, E. A., Stetler, W. R., & Asimos, A. W. (2025). Validation of the Charlotte Large Artery Occlusion Endovascular Therapy Outcome Score in a Modern Cohort of Thrombectomy Patients. Neurology International, 17(8), 130. https://doi.org/10.3390/neurolint17080130







