Safety and Effectiveness of Unilateral Transcranial Magnetic Resonance-Guided Focused Ultrasound in Essential Tremor: One-Year Single-Center Real-World Results
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Study Outcomes
2.3. Adverse Events
2.4. TcMRgFUS Thalamotomy Procedure
2.5. Statistical Analyses
3. Results
3.1. tcMRgFUS Procedure
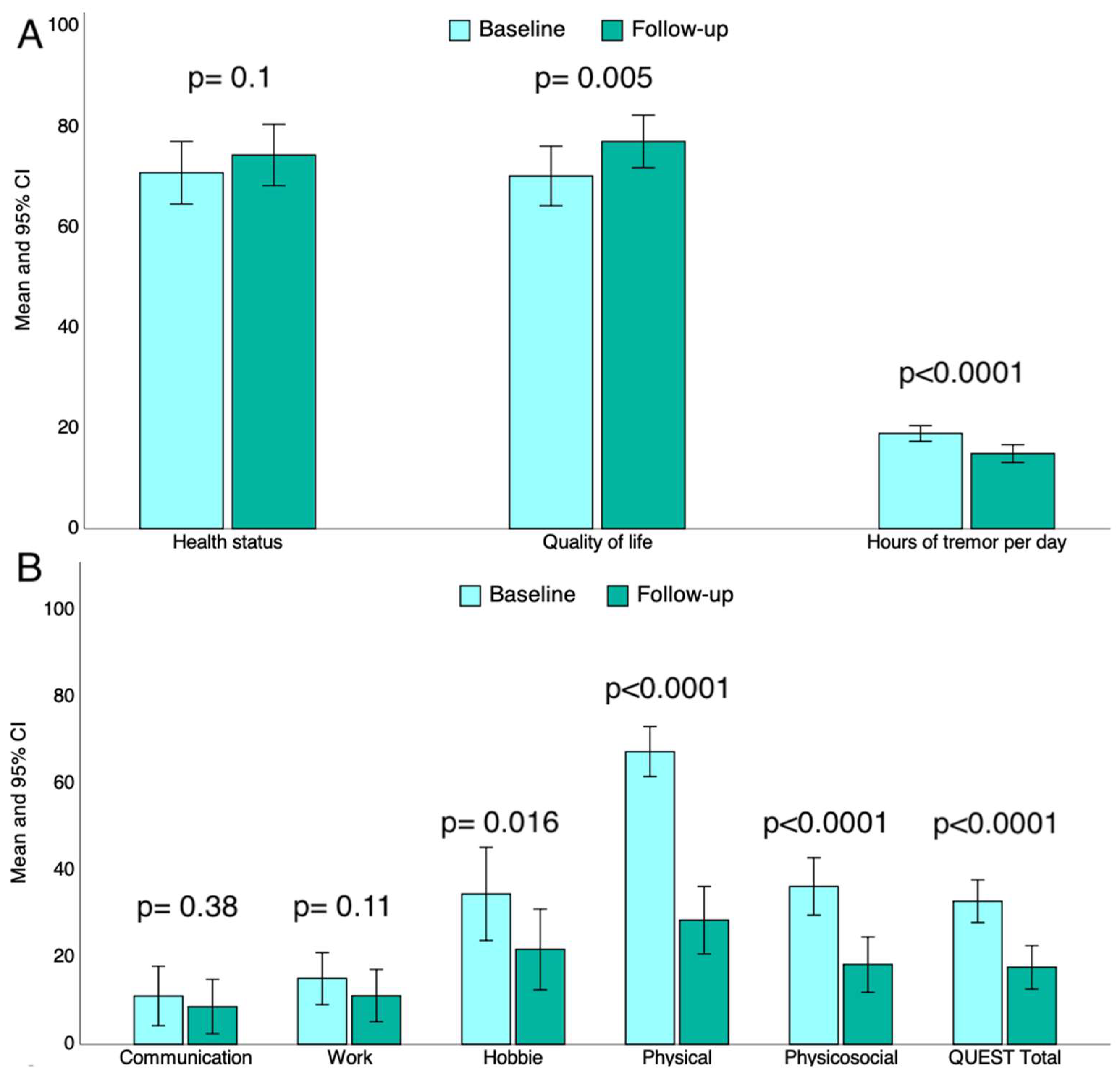
3.2. Impact of tcMRgFUS Thalamotomy on Tremor-Related, Self-Perceived Interference with Quality of Life
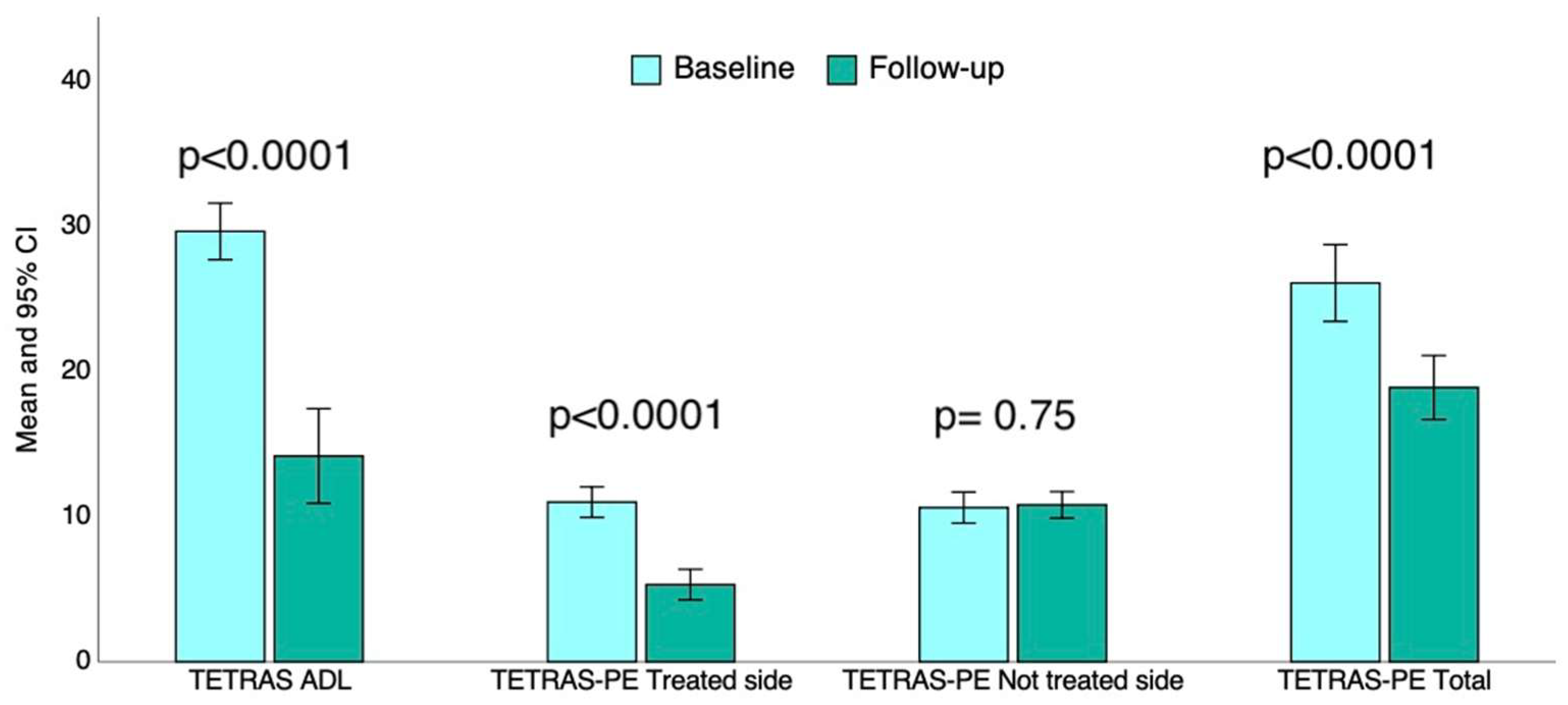
3.3. Impact of tcMRgFUS Thalamotomy on Tremor-Related Disability in ADL and Tremor Severity
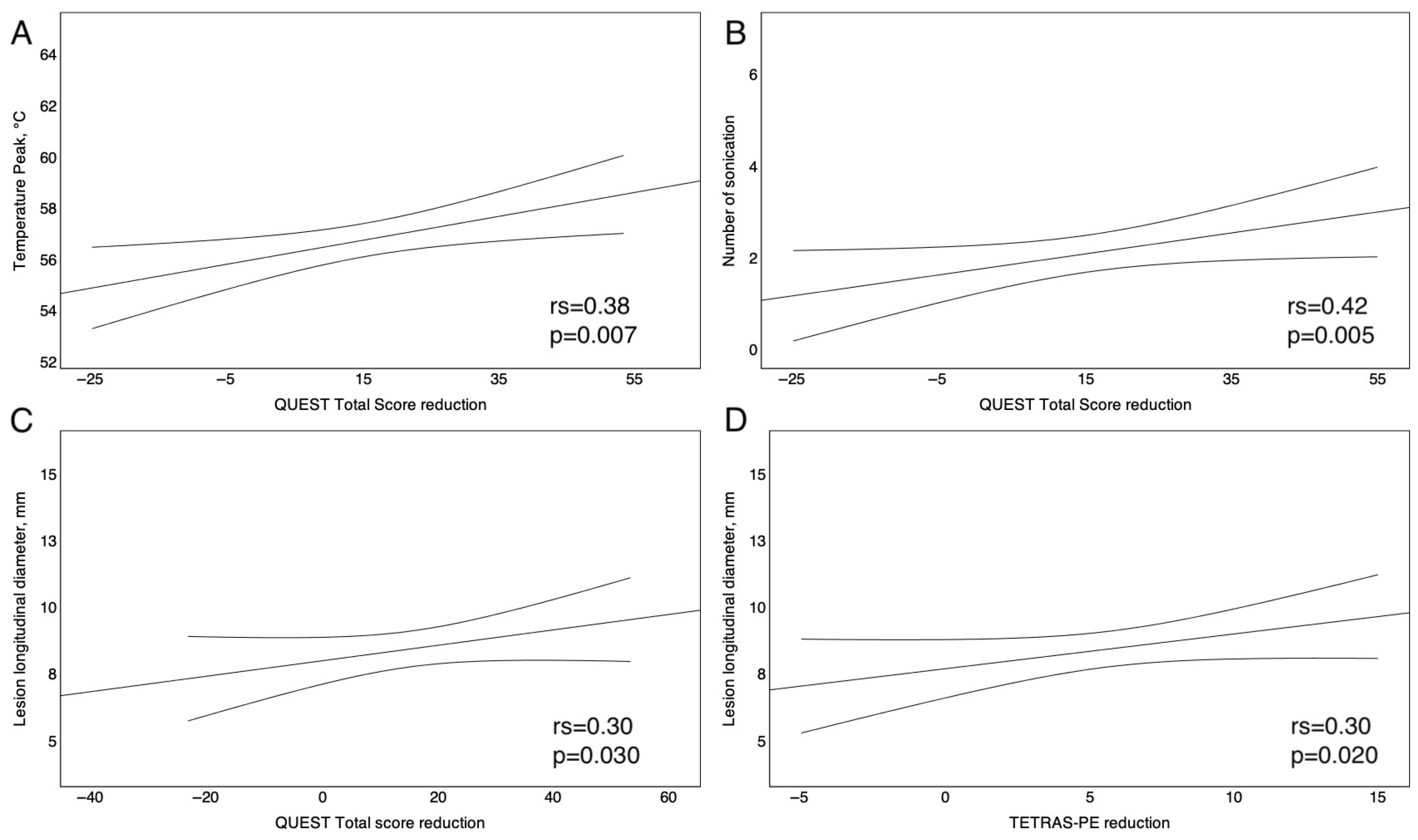
3.4. Correlation Between tcMRgFUS Operating Parameters and Clinical Outcomes
3.5. Predictors of tcMRgFUS Thalamotomy Effectiveness
3.6. Safety Analyses
4. Discussion
5. Strengths and Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Okelberry, T.; Lyons, K.E.; Pahwa, R. Updates in essential tremor. Park. Relat. Disord. 2024, 122, 106086. Available online: https://pubmed.ncbi.nlm.nih.gov/38538475/ (accessed on 16 July 2024). [CrossRef] [PubMed]
- Bhatia, K.P.; Bain, P.; Bajaj, N.; Elble, R.J.; Hallett, M.; Louis, E.D.; Raethjen, J.; Stamelou, M.; Testa, C.M.; Deuschl, G.; et al. Consensus Statement on the classification of tremors. from the task force on tremor of the International Parkinson and Movement Disorder Society. Mov. Disord. 2018, 33, 75–87. [Google Scholar] [CrossRef]
- Gupta, H.V.; Pahwa, R.; Dowell, P.; Khosla, S.; Lyons, K.E. Exploring essential tremor: Results from a large online survey. Clin. Park. Relat. Disord. 2021, 5, 100101. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Wu, S.; Jankovic, J. Essential tremor: Genetic update. Expert Rev. Mol. Med. 2019, 21, e8. Available online: https://pubmed.ncbi.nlm.nih.gov/31818346/ (accessed on 16 July 2024). [CrossRef]
- Poston, K.L.; Wagle Shukla, A. Diagnosis and Treatment of Essential Tremor. Contin. Lifelong Learn. Neurol. 2022, 28, 1333–1349. Available online: https://journals.lww.com/continuum/fulltext/2022/10000/diagnosis_and_treatment_of_essential_tremor.8.aspx (accessed on 16 July 2024). [CrossRef]
- Helmich, R.C.; Toni, I.; Deuschl, G.; Bloem, B.R. The pathophysiology of essential tremor and Parkinson’s tremor. Curr. Neurol. Neurosci. Rep. 2013, 13, 378. Available online: https://pubmed.ncbi.nlm.nih.gov/23893097/ (accessed on 16 July 2024). [CrossRef]
- Chandran, V.; Pal, P.K. Quality of life and its determinants in essential tremor. Park. Relat. Disord. 2013, 19, 62–65. Available online: https://pubmed.ncbi.nlm.nih.gov/22771281/ (accessed on 16 July 2024). [CrossRef]
- Louis, E.D.; Barnes, L.; Albert, S.M.; Cote, L.; Schneier, F.R.; Pullman, S.L.; Yu, Q. Correlates of functional disability in essential tremor. Mov. Disord. 2001, 16, 914–920. Available online: https://pubmed.ncbi.nlm.nih.gov/11746622/ (accessed on 16 July 2024). [CrossRef]
- Vetterick, C.; Lyons, K.E.; Matthews, L.G.; Pendal, R.; Ravina, B. The Hidden Burden of Disease and Treatment Experiences of Patients with Essential Tremor: A Retrospective Claims Data Analysis. Adv. Ther. 2022, 39, 5546–5567. Available online: https://pubmed.ncbi.nlm.nih.gov/36239902/ (accessed on 16 July 2024). [CrossRef] [PubMed]
- Ferreira, J.J.; Mestre, T.A.; Lyons, K.E.; Benito-León, J.; Tan, E.; Abbruzzese, G.; Hallett, M.; Haubenberger, D.; Elble, R.; Deuschl, G.; et al. MDS evidence-based review of treatments for essential tremor. Mov. Disord. 2019, 34, 950–958. Available online: https://pubmed.ncbi.nlm.nih.gov/31046186/ (accessed on 16 July 2024). [CrossRef]
- Stoycheva, T.; Jameel, A.; Bain, P.; Nandi, D.; Jones, B.; Honeyfield, L.; Gedroyc, W.; Moore, J. “Am I fixed, am I better now?”: Undergoing MR-guided focused ultrasound for essential tremor: An interpretative phenomenological analysis. Front. Neurol. 2024, 15, 1352581. Available online: https://pubmed.ncbi.nlm.nih.gov/38390595/ (accessed on 17 July 2024). [CrossRef] [PubMed]
- Chang, W.S.; Jung, H.H.; Kweon, E.J.; Zadicario, E.; Rachmilevitch, I.; Chang, J.W. Unilateral magnetic resonance guided focused ultrasound thalamotomy for essential tremor: Practices and clinicoradiological outcomes. J. Neurol. Neurosurg. Psychiatry 2015, 86, 257–264. Available online: https://pubmed.ncbi.nlm.nih.gov/24876191/ (accessed on 17 July 2024). [CrossRef] [PubMed]
- Chang, J.W.; Park, C.K.; Lipsman, N.; Schwartz, M.L.; Ghanouni, P.; Henderson, J.M.; Gwinn, R.; Witt, J.; Tierney, T.S.; Cosgrove, G.R.; et al. A prospective trial of magnetic resonance-guided focused ultrasound thalamotomy for essential tremor: Results at the 2-year follow-up. Ann. Neurol. 2018, 83, 107–114. Available online: https://pubmed.ncbi.nlm.nih.gov/29265546/ (accessed on 17 July 2024). [CrossRef]
- Ondo, W.G.; Shukla, A.W.; Ondo, C. TETRAS Spirals and Handwriting Samples: Determination of Optimal Scoring Examples. Tremor Other Hyperkinetic Mov. 2021, 11, 50. Available online: https://pubmed.ncbi.nlm.nih.gov/34820149/ (accessed on 3 October 2024). [CrossRef]
- Elble, R.; Comella, C.; Fahn, S.; Hallett, M.; Jankovic, J.; Juncos, J.L.; LeWitt, P.; Lyons, K.; Ondo, W.; Pahwa, R.; et al. Reliability of a new scale for essential tremor. Mov. Disord. 2012, 27, 1567–1569. Available online: https://pubmed.ncbi.nlm.nih.gov/23032792/ (accessed on 3 October 2024). [CrossRef]
- Tröster, A.I.; Pahwa, R.; Fields, J.A.; Tanner, C.M.; Lyons, K.E. Quality of life in Essential Tremor Questionnaire (QUEST): Development and initial validation. Parkinsonism Park. Relat. Disord. 2005, 11, 367–373. [Google Scholar] [CrossRef]
- Cancer Institute, N. Common Terminology Criteria for Adverse Events (CTCAE) Common Terminology Criteria for Adverse Events (CTCAE) v5.0. 2017. Available online: https://www.meddra.org/ (accessed on 5 March 2025).
- Gagliardo, C.; Cannella, R.; D’angelo, C.; Toia, P.; Salvaggio, G.; Feraco, P.; Marrale, M.; Iacopino, D.G.; D’amelio, M.; La Tona, G.; et al. Transcranial magnetic resonance imaging-guided focused ultrasound with a 1.5 tesla scanner: A prospective intraindividual comparison study of intraoperative imaging. Brain Sci. 2021, 11, 46. Available online: https://pubmed.ncbi.nlm.nih.gov/33406708/ (accessed on 20 May 2025). [CrossRef]
- Gagliardo, C.; Cannella, R.; Quarrella, C.; D’aMelio, M.; Napoli, A.; Bartolotta, T.V.; Catalano, C.; Midiri, M.; Lagalla, R. Intraoperative imaging findings in transcranial MR imaging-guided focused ultrasound treatment at 1.5T may accurately detect typical lesional findings correlated with sonication parameters. Eur. Radiol. 2020, 30, 5059–5070. [Google Scholar] [CrossRef]
- Iacopino, D.G.; Gagliardo, C.; Giugno, A.; Giammalva, G.R.; Napoli, A.; Maugeri, R.; Graziano, F.; Valentino, F.; Cosentino, G.; D’Amelio, M.; et al. Preliminary experience with a transcranial magnetic resonance-guided focused ultrasound surgery system integrated with a 1.5-T MRI unit in a series of patients with essential tremor and Parkinson’s disease. Neurosurg. Focus 2018, 44, E7. Available online: https://pubmed.ncbi.nlm.nih.gov/29385927/ (accessed on 20 May 2025). [CrossRef]
- Gagliardo, C.; Marrale, M.; D’Angelo, C.; Cannella, R.; Collura, G.; Iacopino, G.; D’Amelio, M.; Napoli, A.; Bartolotta, T.V.; Catalano, C.; et al. Transcranial Magnetic Resonance Imaging-Guided Focused Ultrasound Treatment at 1.5 T: A Retrospective Study on Treatment- and Patient-Related Parameters Obtained From 52 Procedures. Front. Phys. 2020, 7, 491248. [Google Scholar] [CrossRef]
- Tian, Q.; Wintermark, M.; Elias, W.J.; Ghanouni, P.; Halpern, C.H.; Henderson, J.M.; Huss, D.S.; Goubran, M.; Thaler, C.; Airan, R.; et al. Diffusion MRI tractography for improved transcranial MRI-guided focused ultrasound thalamotomy targeting for essential tremor. NeuroImage Clin. 2018, 19, 572–580. Available online: https://pubmed.ncbi.nlm.nih.gov/29984165/ (accessed on 20 May 2025). [CrossRef]
- Tsolaki, E.; Downes, A.; Speier, W.; Elias, W.J.; Pouratian, N. The potential value of probabilistic tractography-based for MR-guided focused ultrasound thalamotomy for essential tremor. NeuroImage Clin. 2018, 17, 1019–1027. Available online: https://pubmed.ncbi.nlm.nih.gov/29527503/ (accessed on 20 May 2025). [CrossRef]
- Rosset, A.; Spadola, L.; Ratib, O. OsiriX: An open-source software for navigating in multidimensional DICOM images. J. Digit. Imaging 2004, 17, 205–216. Available online: https://pubmed.ncbi.nlm.nih.gov/15534753/ (accessed on 20 May 2025). [CrossRef]
- Wintermark, M.; Druzgal, J.; Huss, D.S.; Khaled, M.A.; Monteith, S.; Raghavan, P.; Huerta, T.; Schweickert, L.C.; Burkholder, B.; Loomba, J.J.; et al. Imaging findings in mr imaging-guided focused ultrasound treatment for patients with essential tremor. Am. J. Neuroradiol. 2014, 35, 891–896. Available online: https://pubmed.ncbi.nlm.nih.gov/24371027/ (accessed on 20 May 2025). [CrossRef] [PubMed]
- Abe, K.; Horisawa, S.; Yamaguchi, T.; Hori, H.; Yamada, K.; Kondo, K.; Furukawa, H.; Kamada, H.; Kishima, H.; Oshino, S.; et al. Focused Ultrasound Thalamotomy for Refractory Essential Tremor: A Japanese Multicenter Single-Arm Study. Neurosurgery 2021, 88, 751–757. Available online: https://pubmed.ncbi.nlm.nih.gov/33469648/ (accessed on 18 September 2024). [CrossRef] [PubMed]
- Elias, W.J.; Lipsman, N.; Ondo, W.G.; Ghanouni, P.; Kim, Y.G.; Lee, W.; Schwartz, M.; Hynynen, K.; Lozano, A.M.; Shah, B.B.; et al. A Randomized Trial of Focused Ultrasound Thalamotomy for Essential Tremor. N. Engl. J. Med. 2016, 375, 730–739. Available online: https://pubmed.ncbi.nlm.nih.gov/27557301/ (accessed on 3 October 2024). [CrossRef] [PubMed]
- Gerbasi, M.E.; Elble, R.J.; Jones, E.; Gillespie, A.; Jarvis, J.; Chertavian, E.; Smith, Z.; Nejati, M.; Shih, L.C. Associations Among Tremor Amplitude, Activities of Daily Living, and Quality of Life in Patients with Essential Tremor. Tremor Other Hyperkinetic Mov. 2024, 14, 22. Available online: https://pubmed.ncbi.nlm.nih.gov/38708124/ (accessed on 3 October 2024). [CrossRef]
- Ondo, W.; Hashem, V.; LeWitt, P.A.; Pahwa, R.; Shih, L.; Tarsy, D.; Zesiewicz, T.; Elble, R. Comparison of the Fahn-Tolosa-Marin Clinical Rating Scale and the Essential Tremor Rating Assessment Scale. Mov. Disord. Clin. Pract. 2017, 5, 60–65. Available online: https://pubmed.ncbi.nlm.nih.gov/30363460/ (accessed on 3 October 2024). [CrossRef]
- Hashida, M.; Maesawa, S.; Kato, S.; Nakatsubo, D.; Tsugawa, T.; Torii, J.; Tanei, T.; Ishizaki, T.; Mutoh, M.; Ito, Y.; et al. Outcomes and Prognostic Factors of Magnetic Resonance-guided Focused Ultrasound Thalamotomy for Essential Tremor at 2-year Follow-up. Neurol. Med. Chir. 2024, 64, 137–146. Available online: https://pubmed.ncbi.nlm.nih.gov/38355128/ (accessed on 3 October 2024). [CrossRef]
- Torii, J.; Maesawa, S.; Nakatsubo, D.; Tsugawa, T.; Kato, S.; Ishizaki, T.; Takai, S.; Shibata, M.; Wakabayashi, T.; Tsuboi, T.; et al. Cutoff values for the best management strategy for magnetic resonance-guided focused ultrasound ablation for essential tremor. J. Neurosurg. 2022, 138, 38–49. Available online: https://pubmed.ncbi.nlm.nih.gov/35993838/ (accessed on 3 October 2024). [CrossRef]
- Park, Y.S.; Jung, N.Y.; Na, Y.C.; Chang, J.W. Four-year follow-up results of magnetic resonance-guided focused ultrasound thalamotomy for essential tremor. Mov. Disord. 2019, 34, 727–734. Available online: https://pubmed.ncbi.nlm.nih.gov/30759322/ (accessed on 3 October 2024). [CrossRef] [PubMed]
- Cosgrove, G.R.; Lipsman, N.; Lozano, A.M.; Chang, J.W.; Halpern, C.; Ghanouni, P.; Eisenberg, H.; Fishman, P.; Taira, T.; Schwartz, M.L.; et al. Magnetic resonance imaging-guided focused ultrasound thalamotomy for essential tremor: 5-year follow-up results. J. Neurosurg. 2022, 138, 1028–1033. Available online: https://pubmed.ncbi.nlm.nih.gov/35932269/ (accessed on 4 October 2024). [CrossRef] [PubMed]
- Stieglitz, L.H.; Oertel, M.F.; Accolla, E.A.; Bally, J.; Bauer, R.; Baumann, C.R.; Benninger, D.; Bohlhalter, S.; Büchele, F.; Hägele-Link, S.; et al. Consensus Statement on High-Intensity Focused Ultrasound for Functional Neurosurgery in Switzerland. Front. Neurol. 2021, 12, 722762. [Google Scholar] [CrossRef] [PubMed]
- Welton, T.; Cardoso, F.; Carr, J.A.; Chan, L.L.; Deuschl, G.; Jankovic, J.; Tan, E.K. Essential tremor. Nat. Rev. Dis. Primers 2021, 7, 83. [Google Scholar] [CrossRef]
- Pouratian, N.; Baltuch, G.; Elias, W.J.; Gross, R. American Society for Stereotactic and Functional Neurosurgery position statement on magnetic resonance-guided focused ultrasound for the management of essential tremor. Neurosurgery 2020, 87, E126–E129. Available online: https://pubmed.ncbi.nlm.nih.gov/31832649/ (accessed on 20 May 2025). [CrossRef]
- Scantlebury, N.; Rohringer, C.R.; Rabin, J.S.; Yunusova, Y.; Huang, Y.; Jones, R.M.; Meng, Y.; Hamani, C.; McKinlay, S.; Gopinath, G.; et al. Safety of Bilateral Staged Magnetic Resonance-Guided Focused Ultrasound Thalamotomy for Essential Tremor. Mov. Disord. Clin. Pract. 2023, 10, 1559–1561. [Google Scholar] [CrossRef]
- Kaplitt, M.G.; Krishna, V.; Eisenberg, H.M.; Elias, W.J.; Ghanouni, P.; Baltuch, G.H.; Rezai, A.; Halpern, C.H.; Dalm, B.; Fishman, P.S.; et al. Safety and Efficacy of Staged, Bilateral Focused Ultrasound Thalamotomy in Essential Tremor: An Open-Label Clinical Trial. JAMA Neurol. 2024, 81, 939–946. [Google Scholar] [CrossRef]
| Parameters | |
|---|---|
| Average temperature °C of lesioning sonications, median (IQR) | 55.4 (55–57) |
| Temperature peak °C, median (min and max) | 57 (53–64.4) |
| Median delivered energy J, median (IQR) | 13,821 (10,116–25,274) |
| Energy peak J, median (min and max) | 14,306 (7989–94,694) |
| Number of lesional sonication delivered, median (IQR) | 2 (1–3) |
| Skull density ratio, median (IQR) | 0.51 (0.45–0.57) |
| Lesion longitudinal diameter at 48 h MRI, mean (SD) | 8.5 (2.4) |
| TETRAS-ADL Sub-Items | Baseline Mean (SD) | Follow-Up Mean (SD) | Mean Variation% (95%CI) | p | |
|---|---|---|---|---|---|
| Speaking | 0.7 (1.02) | 0.6 (0.84) | −14.3 | (−14.3 to 48.6) | 0.28 |
| Spoon | 3 (0.9) | 0.9 (1.29) | −70.0 | (−80 to −52) | <0.0001 |
| Drinking | 2.9 (0.87) | 1.1 (1.28) | −62.1 | (−75.9 to 49.3) | <0.0001 |
| Hygiene | 1.7 (1.4) | 0.8 (1.17) | −52.9 | (−74.7 to 29.4) | <0.0001 |
| Dressing | 1.6 (1.1) | 0.9 (1.11) | −43.8 | (−62.5 to −23.1) | <0.0001 |
| Pouring | 2.8 (1.02) | 1.4 (1.33) | −50.0 | (−64.3 to −37.5) | <0.0001 |
| Trays | 3.2 (0.9) | 1.9 (1.48) | −40.6 | (−53.8 to −28.1) | <0.0001 |
| Keys | 2.5 (1.04) | 0.96 (1.21) | −61.6 | (−76 to −48) | <0.0001 |
| Writing | 3.2 (0.86) | 1.4 (1.24) | −56.3 | (−68.8 to −45.3) | <0.0001 |
| Working | 2.6 (0.92) | 1.3 (1.24) | −50.0 | (−68.8 to −37.3) | <0.0001 |
| Social impact | 2 (1.51) | 1.1 (1.3) | −45.0 | (−70 to −31.5) | <0.0001 |
| Adverse Event | Frequency, n (%) |
|---|---|
| MRI- and sonication-related | |
| Pin site pain | 29 (58) |
| Cold sensation (due to degassed and cooled water) | 22 (44) |
| Headache (during high-power sonications) | 11 (22) |
| Neck, back, or shoulder pain | 10 (20) |
| Thalamotomy-related * | |
| Limb or facial paresthesia/numbness | 5 (10) |
| Hemiparesis | 3 (6) |
| Balance disorder | 5 (10) |
| Dysarthria | 2 (4) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iacono, S.; Gagliardo, C.; Iacopino, D.G.; Schirò, G.; Maugeri, R.; Mastrilli, S.; Picciolo, V.; Bruno, E.; Marrale, M.; Midiri, M.; et al. Safety and Effectiveness of Unilateral Transcranial Magnetic Resonance-Guided Focused Ultrasound in Essential Tremor: One-Year Single-Center Real-World Results. Neurol. Int. 2025, 17, 131. https://doi.org/10.3390/neurolint17080131
Iacono S, Gagliardo C, Iacopino DG, Schirò G, Maugeri R, Mastrilli S, Picciolo V, Bruno E, Marrale M, Midiri M, et al. Safety and Effectiveness of Unilateral Transcranial Magnetic Resonance-Guided Focused Ultrasound in Essential Tremor: One-Year Single-Center Real-World Results. Neurology International. 2025; 17(8):131. https://doi.org/10.3390/neurolint17080131
Chicago/Turabian StyleIacono, Salvatore, Cesare Gagliardo, Domenico Gerardo Iacopino, Giuseppe Schirò, Rosario Maugeri, Sergio Mastrilli, Valentina Picciolo, Eleonora Bruno, Maurizio Marrale, Massimo Midiri, and et al. 2025. "Safety and Effectiveness of Unilateral Transcranial Magnetic Resonance-Guided Focused Ultrasound in Essential Tremor: One-Year Single-Center Real-World Results" Neurology International 17, no. 8: 131. https://doi.org/10.3390/neurolint17080131
APA StyleIacono, S., Gagliardo, C., Iacopino, D. G., Schirò, G., Maugeri, R., Mastrilli, S., Picciolo, V., Bruno, E., Marrale, M., Midiri, M., & D’Amelio, M. (2025). Safety and Effectiveness of Unilateral Transcranial Magnetic Resonance-Guided Focused Ultrasound in Essential Tremor: One-Year Single-Center Real-World Results. Neurology International, 17(8), 131. https://doi.org/10.3390/neurolint17080131








