Exploring Left Atrial Appendage Thrombi in Large Vessel Occlusion Stroke by Cardiac CT: Thrombus Features, LAA Characteristics and the Impact of Direct Oral Anticoagulation
Abstract
1. Introduction
2. Methods
2.1. Patient Selection and Data Collection
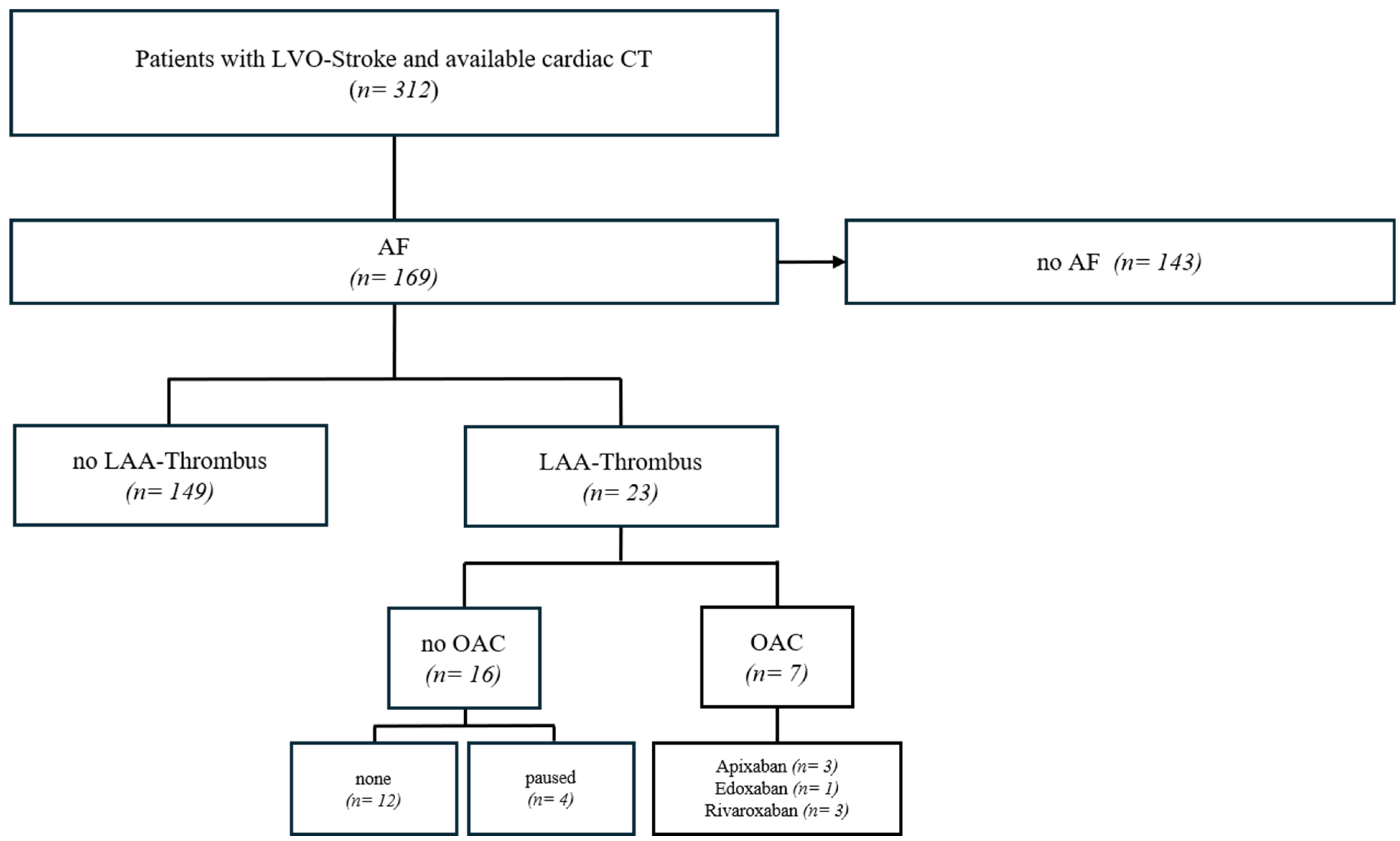
2.2. Assessment of Anticoagulation Status
2.3. Cardiac CT Imaging Acquisition
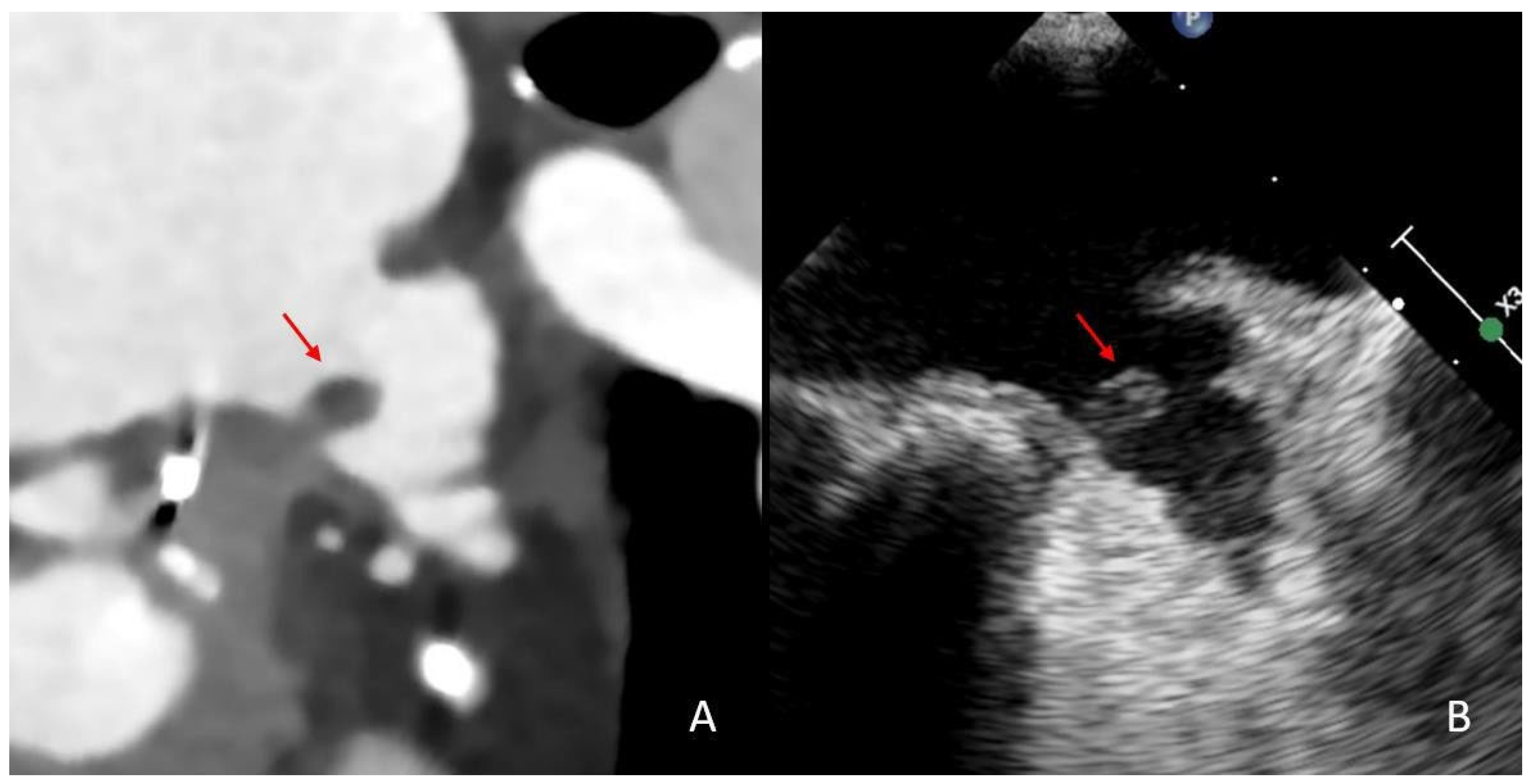
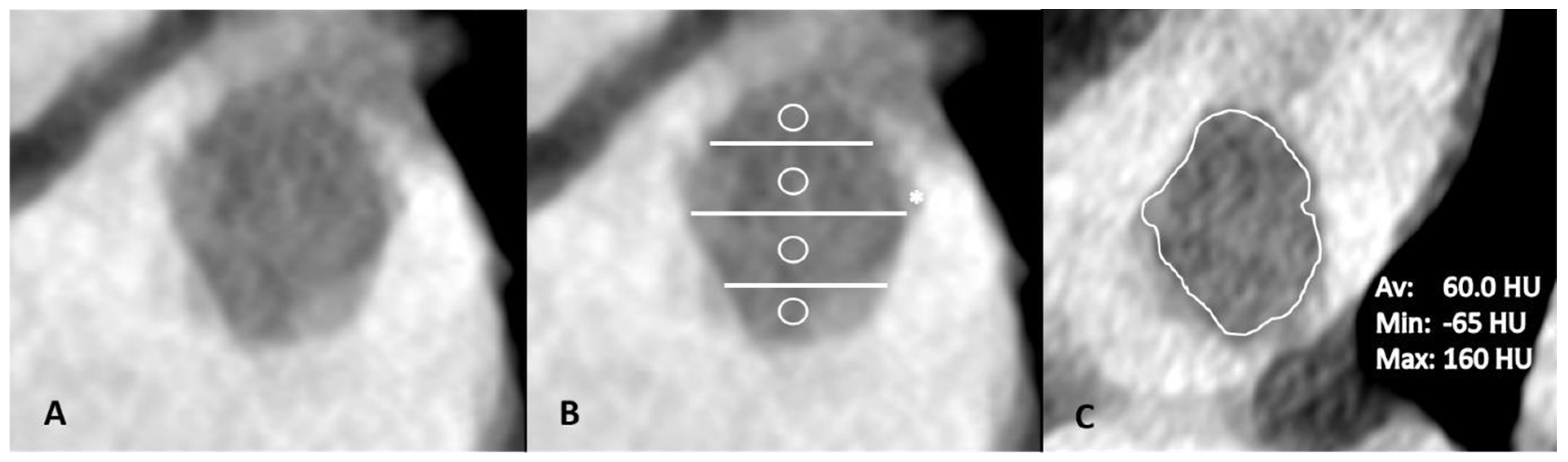
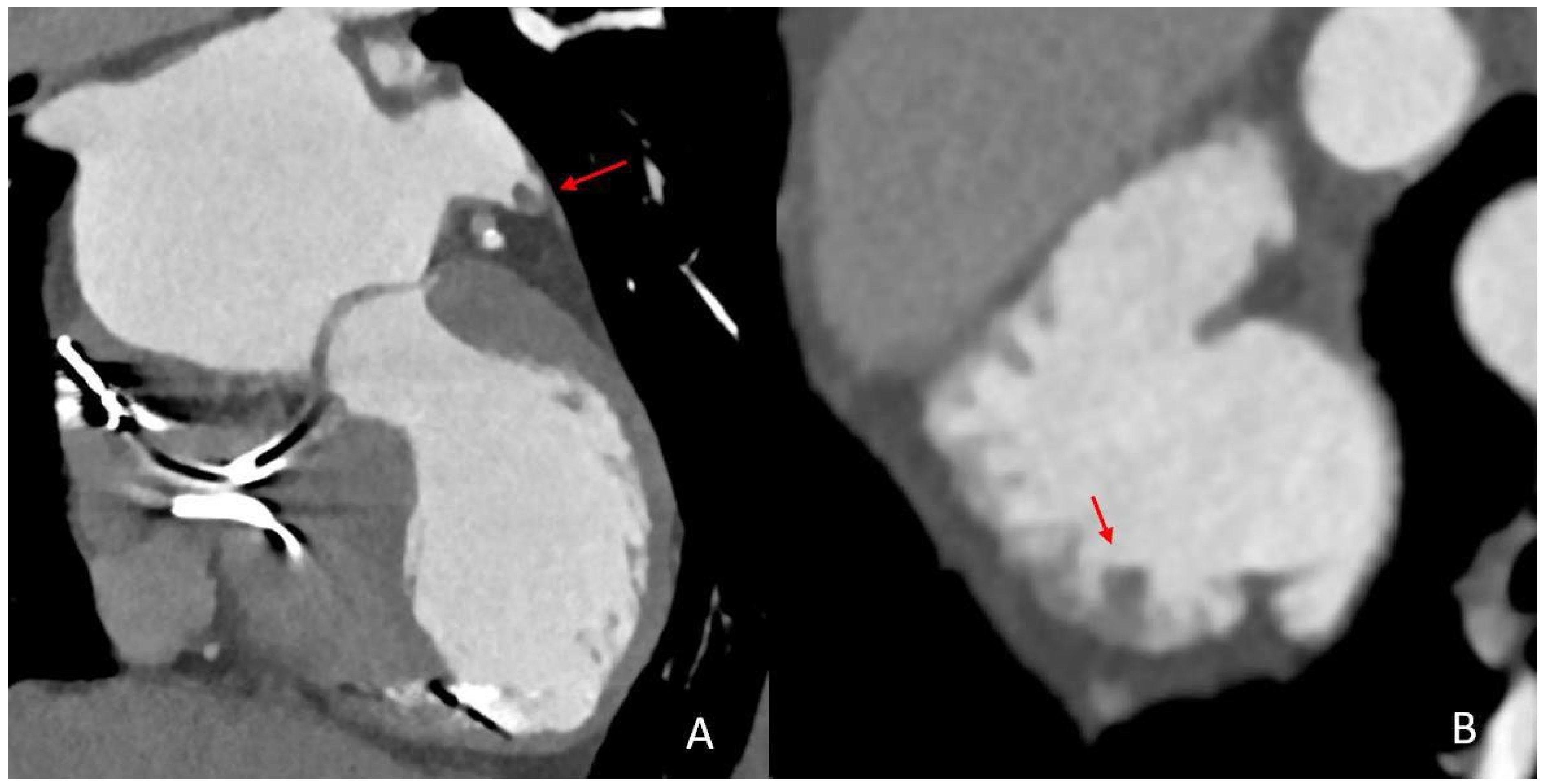
2.4. Imaging Analysis
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
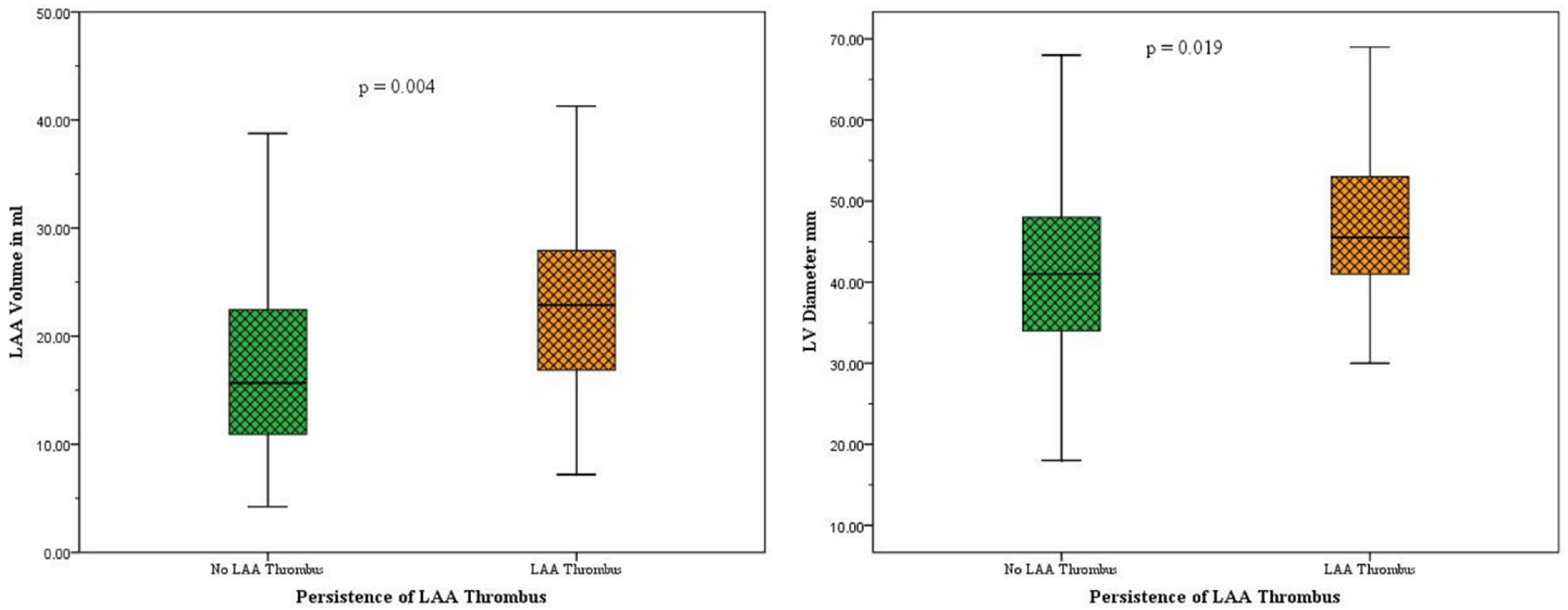
3.2. Subgroup Analysis: Patients with LAA Persistent Thrombi vs. Patients Without Persistent LAA Thrombi
3.3. Logistic Regression Analysis
3.4. Subgroup Analysis: LAA Thrombi Despite OAC Treatment vs. LAA Thrombi Without OAC Treatment
4. Discussion
- (1)
- (2)
- LAA volume, LAA stasis and LV diameter are independent predictors of thrombus persistence in the LAA irrespective of an adequate DOAC therapy (Table 2).
- (3)
4.1. Assessment of LAA Thrombi Under DOAC
4.2. Predictors of LAA Thrombus Persistence in AF Patients with Cardioembolic LVO Stroke
4.3. Summary and Clinical Implications
4.4. Limitations and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rennert, R.C.; Wali, A.R.; Steinberg, J.A.; Santiago-Dieppa, D.R.; Olson, S.E.; Pannell, J.S.; Khalessi, A.A. Epidemiology, Natural History, and Clinical Presentation of Large Vessel Ischemic Stroke. Neurosurgery 2019, 85, S4–S8. [Google Scholar] [CrossRef]
- Malhotra, K.; Gornbein, J.; Saver, J.L. Ischemic Strokes Due to Large-Vessel Occlusions Contribute Disproportionately to Stroke-Related Dependence and Death: A Review. Front. Neurol. 2017, 8, 651. [Google Scholar] [CrossRef]
- Smith, W.S.; Lev, M.H.; English, J.D.; Camargo, E.C.; Chou, M.; Johnston, S.C.; Gonzalez, G.; Schaefer, P.W.; Dillon, W.P.; Koroshetz, W.J.; et al. Significance of large vessel intracranial occlusion causing acute ischemic stroke and TIA. Stroke 2009, 40, 3834–3840. [Google Scholar] [CrossRef]
- Adams, H.P.; Bendixen, B.H.; Kappelle, L.J.; Biller, J.; Love, B.B.; Gordon, D.L.; Marsh, E.E. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993, 24, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M.; Noda, R.; Yamaguchi, S.; Tamai, Y.; Miyahara, M.; Yanagisawa, S.; Okamoto, K.; Hara, T.; Takeuchi, S.; Miki, K.; et al. Specific Factors to Predict Large-Vessel Occlusion in Acute Stroke Patients. J. Stroke Cerebrovasc. Dis. Off. J. Natl. Stroke Assoc. 2018, 27, 886–891. [Google Scholar] [CrossRef] [PubMed]
- Kamel, H.; Healey, J.S. Cardioembolic Stroke. Circ. Res. 2017, 120, 514–526. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.S.-H.; Lo, H.-Y.; Huang, K.-C.; Lin, T.-T.; Lee, J.-K. Efficacy and Safety of Direct Oral Anticoagulants for Stroke Prevention in Older Patients With Atrial Fibrillation: A Network Meta-Analysis of Randomized Controlled Trials. J. Am. Heart Assoc. 2023, 12, e030380. [Google Scholar] [CrossRef]
- Galea, R.; Seiffge, D.; Räber, L. Atrial Fibrillation and Ischemic Stroke despite Oral Anticoagulation. J. Clin. Med. 2023, 12, 5784. [Google Scholar] [CrossRef]
- Gladstone, D.J.; Spring, M.; Dorian, P.; Panzov, V.; Thorpe, K.E.; Hall, J.; Vaid, H.; O’Donnell, M.; Laupacis, A.; Côté, R.; et al. Atrial fibrillation in patients with cryptogenic stroke. N. Engl. J. Med. 2014, 370, 2467–2477. [Google Scholar] [CrossRef]
- Rose, D.Z.; Chang, J.Y.; Yi, X.; Kip, K.; Lu, Y.; Hilker, N.C.; Beltagy, A. Direct Oral Anticoagulant Failures in Atrial Fibrillation With Stroke: Retrospective Admission Analysis and Novel Classification System. Neurohospitalist 2023, 13, 256–265. [Google Scholar] [CrossRef]
- Connolly, S.J.; Ezekowitz, M.D.; Yusuf, S.; Eikelboom, J.; Oldgren, J.; Parekh, A.; Pogue, J.; Reilly, P.A.; Themeles, E.; Varrone, J.; et al. Dabigatran versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2009, 361, 1139–1151. [Google Scholar] [CrossRef]
- Granger, C.B.; Alexander, J.H.; McMurray, J.J.; Lopes, R.D.; Hylek, E.M.; Hanna, M.; Al-Khalidi, H.R.; Ansell, J.; Atar, D.; Avezum, A.; et al. Apixaban versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2011, 365, 981–992. [Google Scholar] [CrossRef]
- Hassler, E.; Kneihsl, M.; Deutschmann, H.; Hinteregger, N.; Magyar, M.; Wießpeiner, U.; Haidegger, M.; Fandler-Höfler, S.; Eppinger, S.; Niederkorn, K.; et al. Relationship between stroke etiology and collateral status in anterior circulation large vessel occlusion. J. Neurol. 2020, 267, 3362–3370. [Google Scholar] [CrossRef]
- Patel, M.R.; Mahaffey, K.W.; Garg, J.; Pan, G.; Singer, D.E.; Hacke, W.; Breithardt, G.; Halperin, J.L.; Hankey, G.J.; Piccini, J.P.; et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N. Engl. J. Med. 2011, 365, 883–891. [Google Scholar] [CrossRef]
- Ruff, C.T.; Giugliano, R.P.; Braunwald, E.; Hoffman, E.B.; Deenadayalu, N.; Ezekowitz, M.D.; Camm, A.J.; Weitz, J.I.; Lewis, B.S.; Parkhomenko, A.; et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: A meta-analysis of randomised trials. Lancet 2014, 383, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Kohlhase, K.; Schäfer, J.H.; Tako, L.M.; Willems, L.M.; Hattingen, E.; Bohmann, F.O.; Grefkes, C.; Rosenow, F.; Strzelczyk, A. Large-vessel-occlusion in patients with previous ischemic stroke: An analysis of adherence to secondary preventive medication for different etiologies. Neurol. Res. Pract. 2023, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Koga, M.; Lee, K.J.; Kim, B.J.; Park, E.L.; Lee, J.; Mizoguchi, T.; Yoshimura, S.; Cha, J.K.; Lee, B.C.; et al. Atrial Fibrillation- Associated Ischemic Stroke Patients with Prior Anticoagulation Have Higher Risk for Recurrent Stroke. Stroke 2020, 51, 1150–1157. [Google Scholar] [CrossRef] [PubMed]
- Romoli, M.; Paciaroni, M.; Marrone, N.; Palaiodimou, L.; Tudisco, V.; Faini, C.; Katsanos, A.H.; Rubiera, M.; Eusebi, P.; Merlino, G. Causes and Risk Factors of Cerebral Ischemic Events in Patients With Atrial Fibrillation Treated With Non–Vitamin K Antagonist Oral Anticoagulants for Stroke Prevention. Neurology 2025, 105, 4. [Google Scholar] [CrossRef]
- Larsen, N.; Austein, F.; Klintz, T.; Campbell, G.; Sedaghat, S.; Aludin, S.; Schunk, D.; Both, M.; Jansen, O.; Langguth, P. Spectral cardiac CT in acute stroke patients. Sci. Rep. 2023, 13, 6781. [Google Scholar] [CrossRef]
- Austein, F.; Eden, M.; Engel, J.; Lebenatus, A.; Larsen, N.; Both, M.; Piesch, T.C.; Salehi Ravesh, M.; Meyne, J.; Jansen, O.; et al. Practicability and Diagnostic Yield of One-Stop Stroke CT with Delayed-Phase Cardiac CT in Detecting Major Cardioembolic Sources of Acute Ischemic Stroke: A Proof of Concept Study. Clin. Neuroradiol. 2021, 31, 911–920. [Google Scholar] [CrossRef]
- Thong, E.H.E.; Kong, W.K.F.; Poh, K.-K.; Wong, R.; Chai, P.; Sia, C.-H. Multimodal Cardiac Imaging in the Assessment of Patients Who Have Suffered a Cardioembolic Stroke: A Review. J. Cardiovasc. Dev. Dis. 2023, 11, 13. [Google Scholar] [CrossRef] [PubMed]
- Hur, J.; Kim, Y.J.; Lee, H.-J.; Ha, J.-W.; Heo, J.H.; Choi, E.-Y.; Shim, C.-Y.; Kim, T.H.; Nam, J.E.; Choe, K.O.; et al. Left atrial appendage thrombi in stroke patients: Detection with two-phase cardiac CT angiography versus transesophageal echocardiography. Radiology 2009, 251, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Hur, J.; Kim, Y.J.; Lee, H.-J.; Nam, J.E.; Hong, Y.J.; Kim, H.Y.; Lee, J.W.; Choi, B.W. Cardioembolic stroke: Dual-energy cardiac CT for differentiation of left atrial appendage thrombus and circulatory stasis. Radiology 2012, 263, 688–695. [Google Scholar] [CrossRef] [PubMed]
- Barnea, R.; Agmon, I.N.; Shafir, G.; Peretz, S.; Mendel, R.; Naftali, J.; Shiyovich, A.; Kornowski, R.; Auriel, E.; Hamdan, A.; et al. Cardiac CT for intra-cardiac thrombus detection in embolic stroke of undetermined source (ESUS). Eur. Stroke J. 2022, 7, 212–220. [Google Scholar] [CrossRef]
- Berge, E.; Whiteley, W.; Audebert, H.; De Marchis, G.M.; Fonseca, A.C.; Padiglioni, C.; de la Ossa, N.P.; Strbian, D.; Tsivgoulis, G.; Turc, G. European Stroke Organisation (ESO) guidelines on intravenous thrombolysis for acute ischaemic stroke. Eur. Stroke J. 2021, 6, I–LXII. [Google Scholar] [CrossRef]
- Joglar, J.A.; Chung, M.K.; Armbruster, A.L.; Benjamin, E.J.; Chyou, J.Y.; Cronin, E.M.; Deswal, A.; Eckhardt, L.L.; Goldberger, Z.D.; Gopinathannair, R.; et al. 2023 ACC/AHA/ACCP/HRS Guideline for the Diagnosis and Management of Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2024, 149, e1–e156. [Google Scholar] [CrossRef]
- Wang, Y.; Di Biase, L.; Horton, R.P.; Nguyen, T.; Morhanty, P.; Natale, A. Left atrial appendage studied by computed tomography to help planning for appendage closure device placement. J. Cardiovasc. Electrophysiol. 2010, 21, 973–982. [Google Scholar] [CrossRef]
- Lang, R.M.; Bierig, M.; Devereux, R.B.; Flachskampf, F.A.; Foster, E.; Pellikka, P.A.; Picard, M.H.; Roman, M.J.; Seward, J.; Shanewise, J.S.; et al. Recommendations for chamber quantification: A report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2005, 18, 1440–1463. [Google Scholar] [CrossRef]
- Kirchhof, K.; Welzel, T.; Mecke, C.; Zoubaa, S.; Sartor, K. Differentiation of white, mixed, and red thrombi: Value of CT in estimation of the prognosis of thrombolysis phantom study. Radiology 2003, 228, 126–130. [Google Scholar] [CrossRef]
- Chandler, A.B.; Hand, R.A. Phagocytized platelets: A source of lipids in human thrombi and atherosclerotic plaques. Science 1961, 134, 946–947. [Google Scholar] [CrossRef]
- Beigel, R.; Wunderlich, N.C.; Ho, S.Y.; Arsanjani, R.; Siegel, R.J. The left atrial appendage: Anatomy, function, and noninvasive evaluation. JACC Cardiovasc. Imaging 2014, 7, 1251–1265. [Google Scholar] [CrossRef]
- Di Biase, L.; Santangeli, P.; Anselmino, M.; Mohanty, P.; Salvetti, I.; Gili, S.; Horton, R.; Sanchez, J.E.; Bai, R.; Mohanty, S.; et al. Does the left atrial appendage morphology correlate with the risk of stroke in patients with atrial fibrillation? Results from a multicenter study. J. Am. Coll. Cardiol. 2012, 60, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Teunissen, C.; Habets, J.; Velthuis, B.K.; Cramer, M.J.; Loh, P. Double-contrast, single-phase computed tomography angiography for ruling out left atrial appendage thrombus prior to atrial fibrillation ablation. Int. J. Cardiovasc. Imaging 2017, 33, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-W.; Yan, L.-Q.; Wang, G.-S.; Sun, Y.-H.; Bao, W.-J.; Zhang, H.-W.; Yang, L.; Chen, C.; Li, M.-Y.; Yang, L.; et al. Left atrial appendage filling defects restricted to the early phase of cardiac computed tomography is significantly associated with ischemic stroke. Clin. Imaging 2023, 98, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Kannel, W.B.; Wolf, P.A.; Benjamin, E.J.; Levy, D. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: Population-based estimates. Am. J. Cardiol. 1998, 82, 2N–9N. [Google Scholar] [CrossRef]
- Kim, W.; Kim, E.J. Heart Failure as a Risk Factor for Stroke. J. Stroke 2018, 20, 33–45. [Google Scholar] [CrossRef]
- Elkholey, K.; Asad, Z.U.A.; Shehata, E.; Mustafina, I.; Fudim, M.; Stavrakis, S. Association between atrial fibrillation and heart failure patient reported outcomes across the ejection fraction spectrum. Am. Heart J. 2024, 273, 61–71. [Google Scholar] [CrossRef]
- Echocardiographic predictors of stroke in patients with atrial fibrillation: A prospective study of 1066 patients from 3 clinical trials. Arch. Intern. Med. 1998, 158, 1316–1320. [CrossRef]
- Uziębło-Życzkowska, B.; Krzesiński, P.; Jurek, A.; Kapłon-Cieślicka, A.; Gorczyca, I.; Budnik, M.; Gielerak, G.; Kiliszek, M.; Gawałko, M.; Scisło, P.; et al. Left Ventricular Ejection Fraction Is Associated with the Risk of Thrombus in the Left Atrial Appendage in Patients with Atrial Fibrillation. Cardiovasc. Ther. 2020, 2020, 3501749. [Google Scholar] [CrossRef]
- Song, Z.; Xu, K.; Hu, X.; Jiang, W.; Wu, S.; Qin, M.; Liu, X. A Study of Cardiogenic Stroke Risk in Non-valvular Atrial Fibrillation Patients. Front. Cardiovasc. Med. 2020, 7, 604795. [Google Scholar] [CrossRef]
- Wazni, O.M.; Saliba, W.I.; Nair, D.G.; Marijon, E.; Schmidt, B.; Hounshell, T.; Ebelt, H.; Skurk, C.; Oza, S.; Patel, C.; et al. Left Atrial Appendage Closure after Ablation for Atrial Fibrillation. N. Engl. J. Med. 2024, 392, 1277–1287. [Google Scholar] [CrossRef]
- Osmancik, P.; Herman, D.; Neuzil, P.; Hala, P.; Taborsky, M.; Kala, P.; Poloczek, M.; Stasek, J.; Haman, L.; Branny, M.; et al. Left Atrial Appendage Closure Versus Direct Oral Anticoagulants in High-Risk Patients With Atrial Fibrillation. J. Am. Coll. Cardiol. 2020, 75, 3122–3135. [Google Scholar] [CrossRef]
- Hu, J.; Wang, J.J.; Wang, L.; Cao, Y.H.; Wu, J.C. Combined Predictive Value of Neutrophil-to-Lymphocyte Ratio and CHA2DS2-VASc Score for Cardiogenic Cerebral Embolism in NVAF Patients. Int J Gen Med. 2025, 18, 3489–3499. [Google Scholar] [CrossRef]
- Buonacera, A.; Stancanelli, B.; Colaci, M.; Malatino, L. Neutrophil to Lymphocyte Ratio: An Emerging Marker of the Relationships between Immune System and Diseases. Int. J. Mol. Sci. 2022, 23, 3636. [Google Scholar] [CrossRef]
- Gosav, E.M.; Tanase, D.M.; Ouatu, A.; Buliga-Finis, O.N.; Popescu, D.; Dascalu, C.G.; Dima, N.; Badescu, M.C.; Rezus, C. The Role of Neutrophil-to-Lymphocyte Ratio and Platelet-to-Lymphocyte Ratio in Predicting Atrial Fibrillation and Its Comorbidities. Life 2025, 15, 960. [Google Scholar] [CrossRef]
- Corriere, T.; Di Marca, S.; Cataudella, E.; Pulvirenti, A.; Alaimo, S.; Stancanelli, B.; Malatino, L. Neutrophil-to-Lymphocyte Ratio is a strong predictor of atherosclerotic carotid plaques in older adults. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 23–27. [Google Scholar] [CrossRef]

| Overall (n = 169) | No LAA Thrombus (n = 146) | LAA Thrombus (n = 23) | p-Value | |
|---|---|---|---|---|
| Sex | 119 f, 50 m | 102 f, 41 m | 17 f, 6 m | |
| Age, years | 80.4 ± 10.6 | 80.6 ± 10.8 | 79.3 ± 9.5 | 0.50 |
| Imaging Findings | ||||
| LAA Chicken Wing, n | 81 (47.9%) | 72 (49.3%) | 9 (39.1%) | 0.39 |
| LAA Cauliflower, n | 37 (21.9%) | 30 (20.5%) | 7 (30.4%) | 0.27 |
| LAA Cactus, n | 15 (8.9%) | 14 (9.6%) | 1 (4.3%) | 0.42 |
| LAA Windsock, n | 38 (22.5%) | 32 (21.9%) | 6 (26.1%) | 0.63 |
| LAA Volume (ml), avg. | 18.5 ± 9.8 mL | 17.7 ± 9.5 mL | 23.7 ± 10.3 mL | 0.004 ** |
| LAA Stasis, n | 111 (65.7%) | 90 (61.2%) | 21 (91.3%) | 0.004 ** |
| Mean LV Diameter (mm), avg. | 42.3 ± 10.6 mm | 41.5 ± 10.1 mm | 48.5 ± 12.1 mm | 0.019 ** |
| HU Ratio Aorta/Stasis, avg. | 2.9 ± 1.7 | 2.7 ± 1.6 | 3.3 ± 1.2 | 0.12 |
| Clinical and Laboratory Findings | ||||
| History of Smoking, n | 33 (19.5%) | 29 (19.9%) | 4 (17.4%) | 0.80 |
| Diabetes, n | 30 (17.8%) | 25 (17.1%) | 5 (21.7%) | 0.57 |
| Dyslipidemia, n | 38 (22.5%) | 35 (24.0%) | 3 (13.0%) | 0.26 |
| Hypertension, n | 115 (68.0%) | 101 (69.2%) | 14 (60.9%) | 0.49 |
| History of Stroke, n | 34 (20.1%) | 27 (18.5%) | 7 (30.4%) | 0.17 |
| History of heart failure, n | 18 (10.7%) | 16 (11.0%) | 2 (8.7%) | 0.33 |
| Adjustment | Factors | Odds-Ratio | CI 95% | p-Value |
|---|---|---|---|---|
| Unadjusted | LV Diameter | 1.06 | 1.02–1.11 | 0.005 ** |
| LAA Volume | 1.05 | 1.01–1.10 | 0.010 * | |
| LAA Stasis | 6.73 | 1.52–29.78 | 0.012 * | |
| Adjusted for DOAC | LV Diameter | 1.06 | 1.02–1.11 | 0.006 ** |
| LAA Volume | 1.05 | 1.01–1.11 | 0.011 * | |
| LAA Stasis | 6.14 | 1.49–29.55 | 0.013 * | |
| DOAC Interaction Effect | LV Diameter | --- | --- | 0.143 |
| LAA Volume | --- | --- | 0.639 | |
| LAA Stasis | --- | --- | 0.224 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mostafa, K.; Krutmann, S.; Wünsche, C.; Larsen, N.; Seiler, A.; Seoudy, H.; Schunk, D.; Jansen, O.; Langguth, P. Exploring Left Atrial Appendage Thrombi in Large Vessel Occlusion Stroke by Cardiac CT: Thrombus Features, LAA Characteristics and the Impact of Direct Oral Anticoagulation. Neurol. Int. 2025, 17, 127. https://doi.org/10.3390/neurolint17080127
Mostafa K, Krutmann S, Wünsche C, Larsen N, Seiler A, Seoudy H, Schunk D, Jansen O, Langguth P. Exploring Left Atrial Appendage Thrombi in Large Vessel Occlusion Stroke by Cardiac CT: Thrombus Features, LAA Characteristics and the Impact of Direct Oral Anticoagulation. Neurology International. 2025; 17(8):127. https://doi.org/10.3390/neurolint17080127
Chicago/Turabian StyleMostafa, Karim, Sarah Krutmann, Cosima Wünsche, Naomi Larsen, Alexander Seiler, Hatim Seoudy, Domagoj Schunk, Olav Jansen, and Patrick Langguth. 2025. "Exploring Left Atrial Appendage Thrombi in Large Vessel Occlusion Stroke by Cardiac CT: Thrombus Features, LAA Characteristics and the Impact of Direct Oral Anticoagulation" Neurology International 17, no. 8: 127. https://doi.org/10.3390/neurolint17080127
APA StyleMostafa, K., Krutmann, S., Wünsche, C., Larsen, N., Seiler, A., Seoudy, H., Schunk, D., Jansen, O., & Langguth, P. (2025). Exploring Left Atrial Appendage Thrombi in Large Vessel Occlusion Stroke by Cardiac CT: Thrombus Features, LAA Characteristics and the Impact of Direct Oral Anticoagulation. Neurology International, 17(8), 127. https://doi.org/10.3390/neurolint17080127







