Treatment-Associated Neuroplastic Changes in People with Stroke-Associated Ataxia—An fMRI Study
Abstract
1. Introduction
- To verify existing fMRI motor paradigms for their usability in this specific population: Considering the relatively uninvestigated population, the first aim of this trial was to investigate the usefulness of fMRI paradigms, such as foot tapping and the motor imagery (MI) of a balancing task, for this specific population and outcome.
- To follow up on neuroplastic changes occurring due to four weeks of intensive supervised practice and eight weeks of home-exercise training, given the importance of a better understanding of neuronal recovery in stroke patients in general.
- To investigate the effects of different approaches in physiotherapy practice, especially of coordination exercises, as they are thought to compensate for difficulties in motor learning due to cerebellar damage according to their exercise structure.
2. Methods
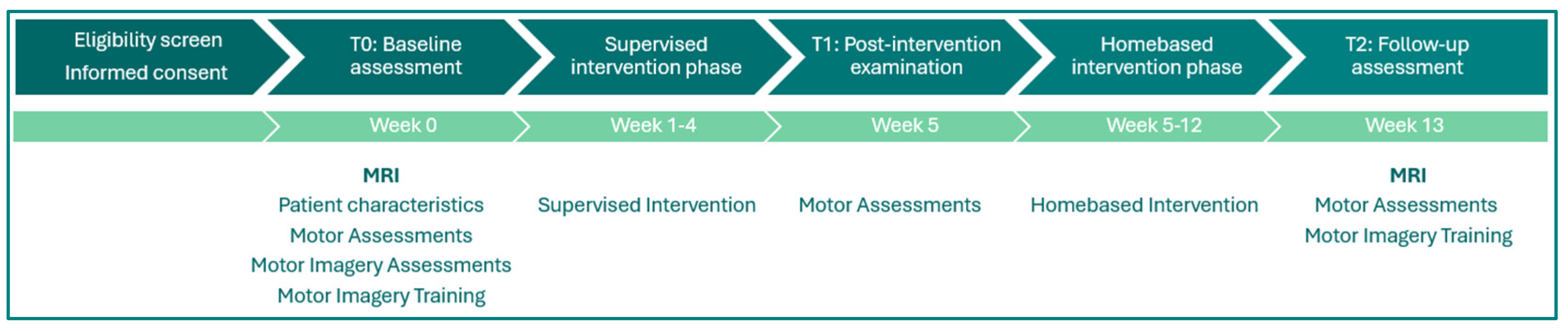
2.1. Participants and Study Design
2.2. Data Acquisition
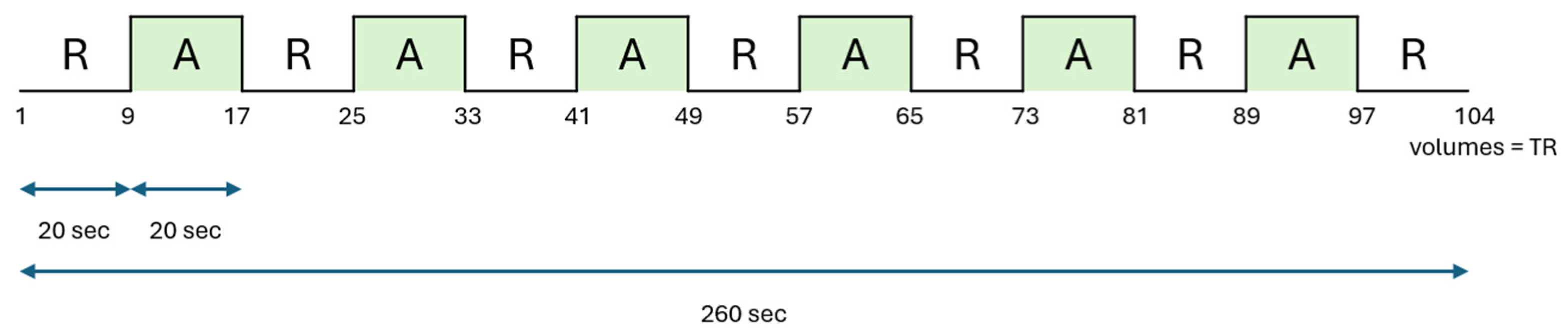
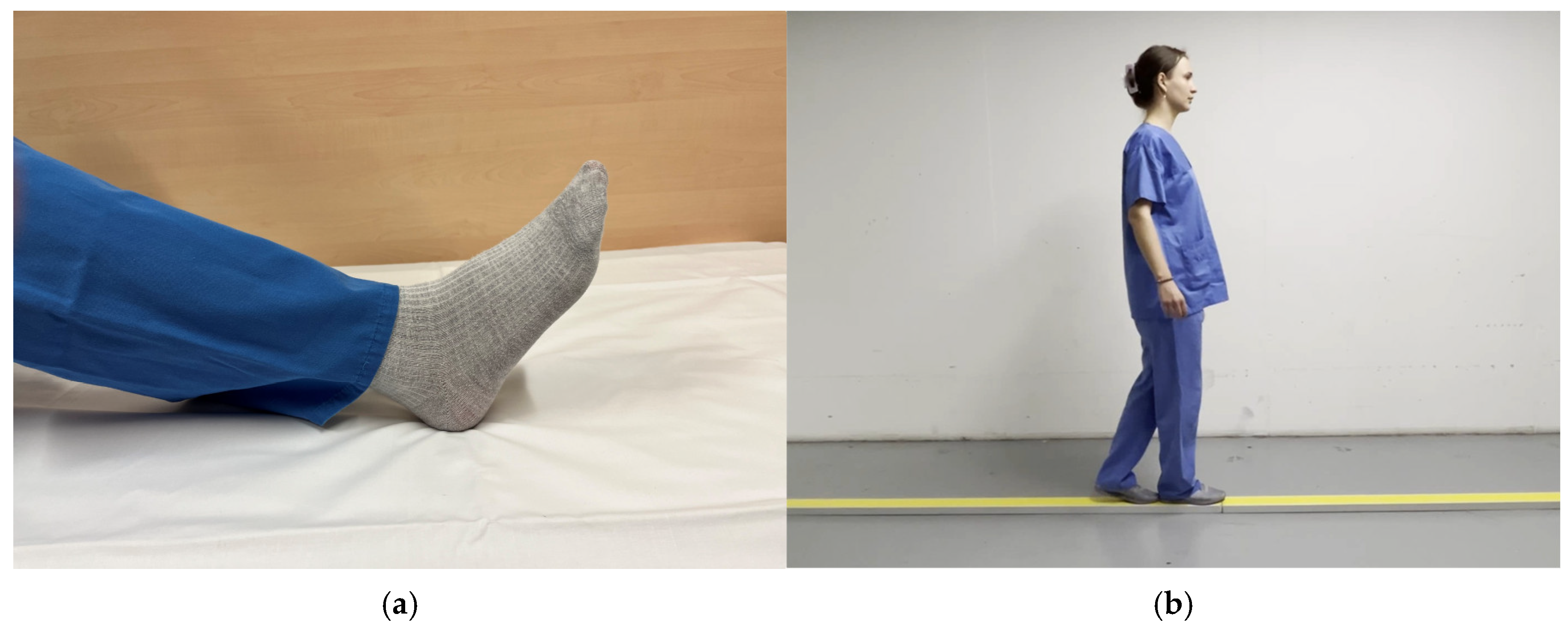
2.3. fMRI Design
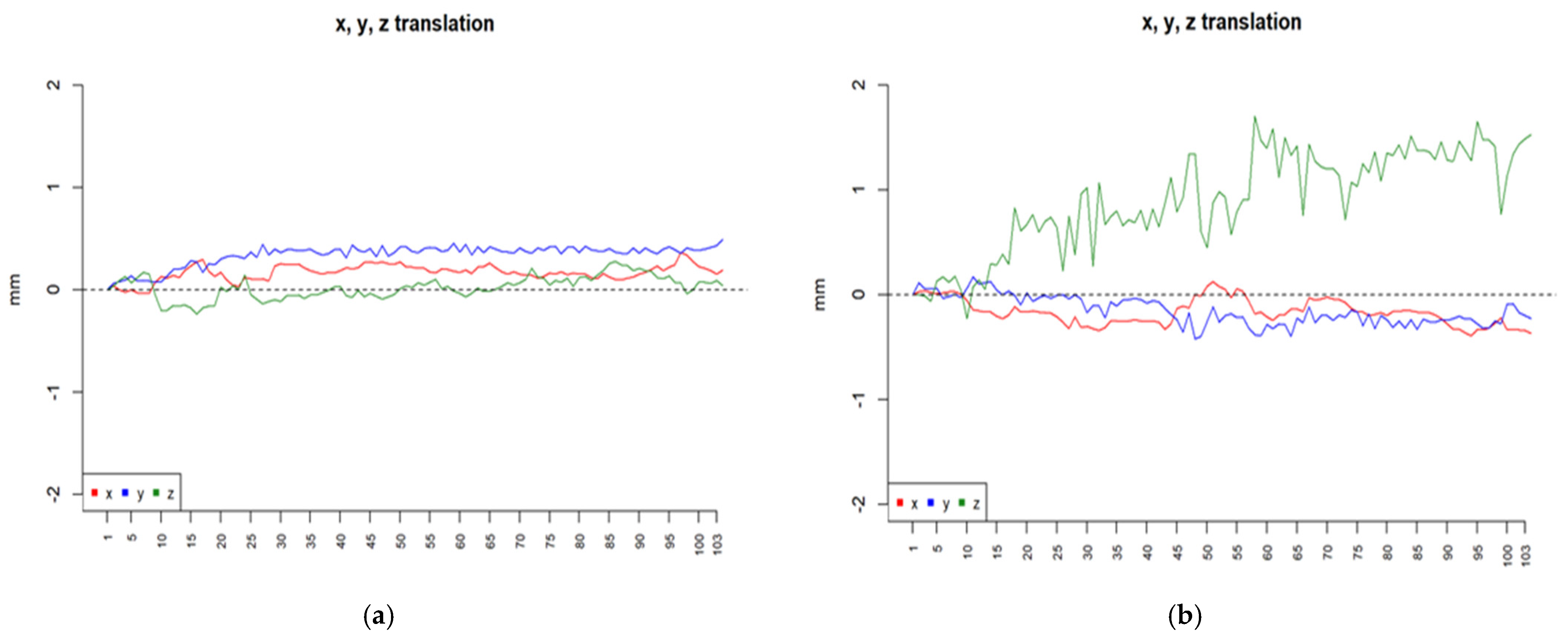
2.4. Data Analysis
3. Results
3.1. Utility of Chosen fMRI Paradigms in Acute Stroke Patients with Ataxic Symptoms
3.2. Changes in Activation Patterns During Recovery and Rehabilitation
3.3. Differences in Activation Due to Treatment Methods
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feigin, V.L.; Forouzanfar, M.H.; Krishnamurthi, R.; Mensah, G.A.; Connor, M.; Bennett, D.A.; Moran, A.E.; Sacco, R.L.; Anderson, L.; Truelsen, T.; et al. Global and regional burden of stroke during 1990-2010: Findings from the Global Burden of Disease Study 2010. Lancet 2014, 383, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Caplan, L.R. Clinical Stroke Syndromes. Front. Neurol. Neurosci. 2016, 40, 72–92. [Google Scholar] [CrossRef] [PubMed]
- Belas Dos Santos, M.; Barros de Oliveira, C.; Dos Santos, A.; Garabello Pires, C.; Dylewski, V.; Arida, R.M. A Comparative Study of Conventional Physiotherapy versus Robot-Assisted Gait Training Associated to Physiotherapy in Individuals with Ataxia after Stroke. Behav. Neurol. 2018, 2018, 2892065. [Google Scholar] [CrossRef]
- Kruger, E.; Teasell, R.; Salter, K.; Foley, N.; Hellings, C. The rehabilitation of patients recovering from brainstem strokes: Case studies and clinical considerations. Top. Stroke Rehabil. 2007, 14, 56–64. [Google Scholar] [CrossRef]
- Marquer, A.; Barbieri, G.; Pérennou, D. The assessment and treatment of postural disorders in cerebellar ataxia: A systematic review. Ann. Phys. Rehabil. Med. 2014, 57, 67–78. [Google Scholar] [CrossRef]
- Intercollegiate Stroke Working Party. National Clinical Guideline for Stroke for the UK and Ireland 2023. Available online: www.strokeguideline.org (accessed on 16 February 2024).
- Bultmann, U.; Pierscianek, D.; Gizewski, E.R.; Schoch, B.; Fritsche, N.; Timmann, D.; Maschke, M.; Frings, M. Functional recovery and rehabilitation of postural impairment and gait ataxia in patients with acute cerebellar stroke. Gait Posture 2014, 39, 563–569. [Google Scholar] [CrossRef]
- Jung, C.; Kim, D.Y.; Kwon, S.; Chun, M.H.; Kim, J.; Kim, S.H. Morning Walk(®)-Assisted Gait Training Improves Walking Ability and Balance in Patients with Ataxia: A Randomized Controlled Trial. Brain Neurorehabil. 2020, 13, e23. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Shin, J.H.; Yang, S.P.; Shin, M.A.; Lee, S.H. Robot-assisted gait training for balance and lower extremity function in patients with infratentorial stroke: A single-blinded randomized controlled trial. J. Neuroeng. Rehabil. 2019, 16, 99. [Google Scholar] [CrossRef]
- Stoykov, M.E.; Stojakovich, M.; Stevens, J.A. Beneficial effects of postural intervention on prehensile action for an individual with ataxia resulting from brainstem stroke. NeuroRehabilitation 2005, 20, 85–89. [Google Scholar] [CrossRef]
- Meier, P. Plasticity and Motor Learning. In Functional Electrical Stimulation in Neurorehabilitation: Synergy Effects of Technology and Therapy; Schick, T., Ed.; Springer International Publishing: Cham, Switzerland, 2022; pp. 9–18. [Google Scholar] [CrossRef]
- Swayne, O.B.; Rothwell, J.C.; Ward, N.S.; Greenwood, R.J. Stages of motor output reorganization after hemispheric stroke suggested by longitudinal studies of cortical physiology. Cereb. Cortex 2008, 18, 1909–1922. [Google Scholar] [CrossRef]
- Hatakenaka, M.; Miyai, I.; Mihara, M.; Yagura, H.; Hattori, N. Impaired motor learning by a pursuit rotor test reduces functional outcomes during rehabilitation of poststroke ataxia. Neurorehabil. Neural Repair 2012, 26, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Hara, Y. Brain plasticity and rehabilitation in stroke patients. J. Nippon. Med. Sch. 2015, 82, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Calautti, C.; Jones, P.S.; Naccarato, M.; Sharma, N.; Day, D.J.; Bullmore, E.T.; Warburton, E.A.; Baron, J.C. The relationship between motor deficit and primary motor cortex hemispheric activation balance after stroke: Longitudinal fMRI study. J. Neurol. Neurosurg. Psychiatry 2010, 81, 788–792. [Google Scholar] [CrossRef]
- Bliss, T.V.; Lomo, T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J. Physiol. 1973, 232, 331–356. [Google Scholar] [CrossRef] [PubMed]
- Saywell, N.; Taylor, D. The role of the cerebellum in procedural learning—Are there implications for physiotherapists’ clinical practice? Physiother. Theory Pract. 2008, 24, 321–328. [Google Scholar] [CrossRef]
- Molinari, M.; Leggio, M.G.; Solida, A.; Ciorra, R.; Misciagna, S.; Silveri, M.C.; Petrosini, L. Cerebellum and procedural learning: Evidence from focal cerebellar lesions. Brain 1997, 120 Pt 10, 1753–1762. [Google Scholar] [CrossRef]
- Kelly, G.; Shanley, J. Rehabilitation of ataxic gait following cerebellar lesions: Applying theory to practice. Physiother. Theory Pract. 2016, 32, 430–437. [Google Scholar] [CrossRef]
- Hanajima, R.; Shadmehr, R.; Ohminami, S.; Tsutsumi, R.; Shirota, Y.; Shimizu, T.; Tanaka, N.; Terao, Y.; Tsuji, S.; Ugawa, Y.; et al. Modulation of error-sensitivity during a prism adaptation task in people with cerebellar degeneration. J. Neurophysiol. 2015, 114, 2460–2471. [Google Scholar] [CrossRef]
- Ilg, W.; Synofzik, M.; Brötz, D.; Burkard, S.; Giese, M.A.; Schöls, L. Intensive coordinative training improves motor performance in degenerative cerebellar disease. Neurology 2009, 73, 1823–1830. [Google Scholar] [CrossRef]
- Meier, P.; Mayer-Suess, L.; Kiechl, S.; Pachmann, U.; Greimann, R.; Kofler, M.; Brenneis, C.; Grams, A.; Steiger, R.; Seebacher, B.; et al. Recovery of balance and walking in people with ataxia after acute cerebral stroke: Study protocol for a prospective, monocentric, single-blinded, randomized controlled trial. Front. Stroke 2024, 3, 1388891. [Google Scholar] [CrossRef]
- Meier, P.; Mayer, L.; Schoenherr, G.; Knoflach, M.; Kiechl, S. Rehabilitation of gait ataxia in patients with acute stroke. Int. J. Stroke 2021, 16, 3–170. [Google Scholar]
- Malouin, F.; Richards, C.L.; Jackson, P.L.; Lafleur, M.F.; Durand, A.; Doyon, J. The Kinesthetic and Visual Imagery Questionnaire (KVIQ) for assessing motor imagery in persons with physical disabilities: A reliability and construct validity study. J. Neurol. Phys. Ther. 2007, 31, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Lam, H.S.; Lau, F.W.; Chan, G.K.; Sykes, K. The validity and reliability of a 6-Metre Timed Walk for the functional assessment of patients with stroke. Physiother. Theory Pract. 2010, 26, 251–255. [Google Scholar] [CrossRef]
- Malouin, F.; Richards, C.L.; Durand, A.; Doyon, J. Reliability of mental chronometry for assessing motor imagery ability after stroke. Arch. Phys. Med. Rehabil. 2008, 89, 311–319. [Google Scholar] [CrossRef]
- Malouin, F.; Richards, C.L. Mental practice for relearning locomotor skills. Phys. Ther. 2010, 90, 240–251. [Google Scholar] [CrossRef] [PubMed]
- Oostra, K.M.; Oomen, A.; Vanderstraeten, G.; Vingerhoets, G. Influence of motor imagery training on gait rehabilitation in sub-acute stroke: A randomized controlled trial. J. Rehabil. Med. 2015, 47, 204–209. [Google Scholar] [CrossRef]
- De Bartolo, D.; Belluscio, V.; Vannozzi, G.; Morone, G.; Antonucci, G.; Giordani, G.; Santucci, S.; Resta, F.; Marinozzi, F.; Bini, F.; et al. Sensorized Assessment of Dynamic Locomotor Imagery in People with Stroke and Healthy Subjects. Sensors 2020, 20, 4545. [Google Scholar] [CrossRef] [PubMed]
- Schuster, C.; Lussi, A.; Wirth, B.; Ettlin, T. Two assessments to evaluate imagery ability: Translation, test-retest reliability and concurrent validity of the German KVIQ and Imaprax. BMC Med. Res. Methodol. 2012, 12, 127. [Google Scholar] [CrossRef]
- Ma, Z.-Z.; Wu, J.-J.; Hua, X.-Y.; Zheng, M.-X.; Xing, X.-X.; Ma, J.; Li, S.-S.; Shan, C.-L.; Xu, J.-G. Brain Function and Upper Limb Deficit in Stroke With Motor Execution and Imagery: A Cross-Sectional Functional Magnetic Resonance Imaging Study. Front. Neurosci. 2022, 16, 806406. [Google Scholar] [CrossRef]
- Berg, K.; Wood-Dauphinee, S.; Williams, J.; Gayton, D. Measuring balance in the elderly: Preliminary development of an instrument. Physiother. Can. 1989, 41, 304–311. [Google Scholar] [CrossRef]
- Kwakkel, G.; Stinear, C.; Essers, B.; Munoz-Novoa, M.; Branscheidt, M.; Cabanas-Valdés, R.; Lakičević, S.; Lampropoulou, S.; Luft, A.R.; Marque, P.; et al. rehabilitation after stroke: European Stroke Organisation (ESO) consensus-based definition and guiding framework. Eur. Stroke J. 2023, 8, 880–894. [Google Scholar] [CrossRef]
- Taube, W.; Mouthon, M.; Leukel, C.; Hoogewoud, H.M.; Annoni, J.M.; Keller, M. Brain activity during observation and motor imagery of different balance tasks: An fMRI study. Cortex 2015, 64, 102–114. [Google Scholar] [CrossRef]
- Ruffieux, J.; Mouthon, A.; Keller, M.; Mouthon, M.; Annoni, J.M.; Taube, W. Balance Training Reduces Brain Activity during Motor Simulation of a Challenging Balance Task in Older Adults: An fMRI Study. Front. Behav. Neurosci. 2018, 12, 10. [Google Scholar] [CrossRef] [PubMed]
- Le, A.; Vesia, M.; Yan, X.; Crawford, J.D.; Niemeier, M. Parietal area BA7 integrates motor programs for reaching, grasping, and bimanual coordination. J. Neurophysiol. 2017, 117, 624–636. [Google Scholar] [CrossRef]
- Mekbib, D.B.; Debeli, D.K.; Zhang, L.; Fang, S.; Shao, Y.; Yang, W.; Han, J.; Jiang, H.; Zhu, J.; Zhao, Z.; et al. A novel fully immersive virtual reality environment for upper extremity rehabilitation in patients with stroke. Ann. N. Y. Acad. Sci. 2021, 1493, 75–89. [Google Scholar] [CrossRef]
- Blickenstorfer, A.; Kleiser, R.; Keller, T.; Keisker, B.; Meyer, M.; Riener, R.; Kollias, S. Cortical and subcortical correlates of functional electrical stimulation of wrist extensor and flexor muscles revealed by fMRI. Hum. Brain Mapp. 2009, 30, 963–975. [Google Scholar] [CrossRef] [PubMed]
- Mouthon, A.; Ruffieux, J.; Mouthon, M.; Hoogewoud, H.M.; Annoni, J.M.; Taube, W. Age-Related Differences in Cortical and Subcortical Activities during Observation and Motor Imagery of Dynamic Postural Tasks: An fMRI Study. Neural Plast. 2018, 2018, 1598178. [Google Scholar] [CrossRef] [PubMed]
- Ben-Shabat, E.; Matyas, T.A.; Pell, G.S.; Brodtmann, A.; Carey, L.M. The Right Supramarginal Gyrus Is Important for Proprioception in Healthy and Stroke-Affected Participants: A Functional MRI Study. Front. Neurol. 2015, 6, 248. [Google Scholar] [CrossRef]
- Inagaki, Y.; Seki, K.; Makino, H.; Matsuo, Y.; Miyamoto, T.; Ikoma, K. Exploring Hemodynamic Responses Using Mirror Visual Feedback With Electromyogram-Triggered Stimulation and Functional Near-Infrared Spectroscopy. Front. Hum. Neurosci. 2019, 13, 60. [Google Scholar] [CrossRef]
- Rouiller, E.M.; Olivier, E. Functional recovery after lesions of the primary motor cortex. Prog. Brain Res. 2004, 143, 467–475. [Google Scholar] [CrossRef]
- Könönen, M.; Kuikka, J.T.; Husso-Saastamoinen, M.; Vanninen, E.; Vanninen, R.; Soimakallio, S.; Mervaala, E.; Sivenius, J.; Pitkänen, K.; Tarkka, I.M. Increased perfusion in motor areas after constraint-induced movement therapy in chronic stroke: A single-photon emission computerized tomography study. J. Cereb. Blood Flow. Metab. 2005, 25, 1668–1674. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.K.; Cho, S.H.; Jeon, H.-S.; Lee, Y.-H.; Song, J.C.; Jang, S.H.; Lee, C.-H.; Kwon, Y.H. Cortical effect and functional recovery by the electromyography-triggered neuromuscular stimulation in chronic stroke patients. Neurosci. Lett. 2008, 442, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Leff, D.R.; Elwell, C.E.; Orihuela-Espina, F.; Atallah, L.; Delpy, D.T.; Darzi, A.W.; Yang, G.Z. Changes in prefrontal cortical behaviour depend upon familiarity on a bimanual co-ordination task: An fNIRS study. Neuroimage 2008, 39, 805–813. [Google Scholar] [CrossRef]
- Mihara, M.; Miyai, I.; Hatakenaka, M.; Kubota, K.; Sakoda, S. Sustained prefrontal activation during ataxic gait: A compensatory mechanism for ataxic stroke? Neuroimage 2007, 37, 1338–1345. [Google Scholar] [CrossRef] [PubMed]
- Timmann, D.; Shimansky, Y.; Larson, P.S.; Wunderlich, D.A.; Stelmach, G.E.; Bloedel, J.R. Visuomotor learning in cerebellar patients. Behav. Brain Res. 1996, 81, 99–113. [Google Scholar] [CrossRef]
- Ilg, W.; Christensen, A.; Mueller, O.M.; Goericke, S.L.; Giese, M.A.; Timmann, D. Effects of cerebellar lesions on working memory interacting with motor tasks of different complexities. J. Neurophysiol. 2013, 110, 2337–2349. [Google Scholar] [CrossRef]



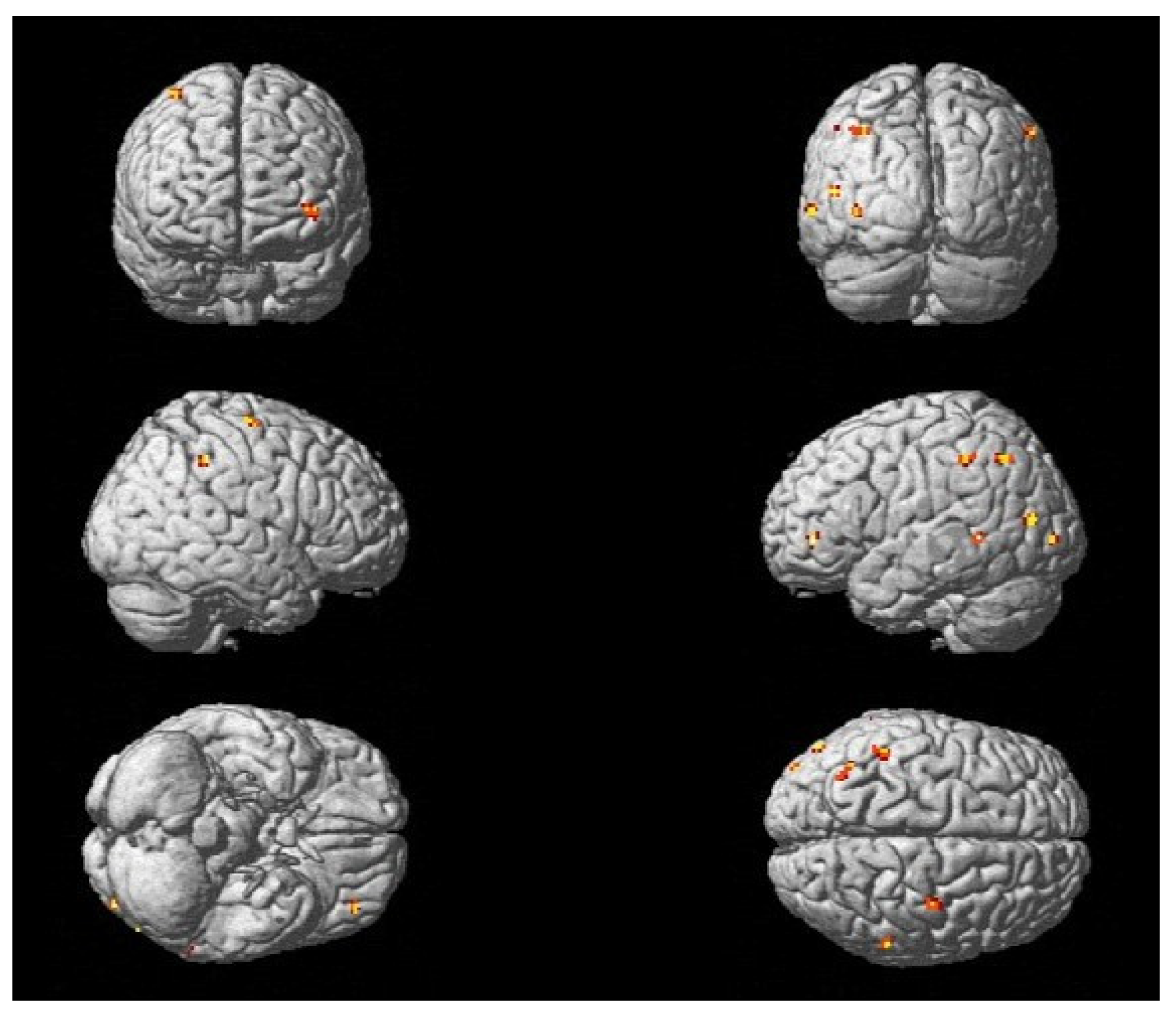
| Group | Age | Gender | Location | Lesion Side | BBS T0 * | BBS T2 ** | SARA T0 * | SARA T2 ** | |
|---|---|---|---|---|---|---|---|---|---|
| 01 | CG | 69 a | F | PICA | right | 47 | 56 | 6 | 0.5 |
| 02 | CG | 69 a | M | PICA | left | 21 | 55 | 13.5 | 2.5 |
| 03 | CG | 58 a | M | SCA and ACP | left | 26 | 54 | 12.5 | 6 |
| 04 | CG | 56 a | M | PICA (incl. vermis) and medulla oblongata | left | 6 | 51 | 20.5 | 4.5 |
| 05 | CG | 74 a | M | PICA | right | 43 | 53 | 9.5 | 6 |
| 06 | CG | 53 a | F | SCA (and pons; asymptomatic) | left | 21 | 48 | 12.5 | 9 |
| 07 | IG | 65 a | M | SCA and ponto-mesencephal | left | 43 | 56 | 7.5 | 1.5 |
| 08 | IG | 83 a | M | SCA and pons | left | 40 | 54 | 10 | 2 |
| 09 | IG | 31 a | F | PICA and SCA and dorsolateral medulla oblongata | left > right | 43 | 56 | 9.5 | 2 |
| Functional Region | Brodmann Area No. | Cluster | Peak | Coordinates * | |||
|---|---|---|---|---|---|---|---|
| p (FWE-Corr) | No. Voxels | T | X (mm) | Y (mm) | Z (mm) | ||
| PMC and SMA left | 6 | 0.000 | 2449 | 12.71 | −2 | −6 | 62 |
| PMC and SMA left | 6 | 0.000 | 672 | 10.34 | −30 | −8 | 50 |
| dlPFC left | 9 | 0.000 | 40 | 6.58 | −44 | 36 | 28 |
| V3 left | 19 | 0.000 | 15651 | 17.84 | −50 | −72 | 2 |
| STG right | 22 | 0.000 | 480 | 11.38 | 66 | −30 | 18 |
| FG left | 37 | 0.000 | 26 | 6.70 | −42 | −40 | −20 |
| SMG left | 40 | 0.000 | 347 | 10.32 | −50 | −34 | 24 |
| Broca left | 44 | 0.000 | 415 | 9.25 | −50 | 8 | 8 |
| Broca right | 44 | 0.000 | 152 | 7.19 | 54 | 14 | 14 |
| Broca left | 44 | 0.000 | 34 | 6.78 | −36 | 14 | 10 |
| Cerebellum right | - | 0.000 | 59 | 6.81 | 32 | −48 | −54 |
| Functional Region | Brodmann Area No. | Cluster | Peak | Coordinates * | |||
|---|---|---|---|---|---|---|---|
| p (FWE-Corr) | No. Voxels | T | X (mm) | Y (mm) | Z (mm) | ||
| S1 right | 1 | 0.000 | 38 | 6.56 | 48 | −20 | 34 |
| Insula right | 13 | 0.000 | 83 | 6.87 | 34 | 18 | 8 |
| V3 left | 19 | 0.000 | 31,363 | 17.35 | −50 | −74 | 8 |
| SMG right | 40 | 0.000 | 549 | 10.67 | 64 | −30 | 20 |
| SMG left | 40 | 0.000 | 63 | 7.23 | −48 | −38 | 22 |
| Broca left | 44 | 0.000 | 449 | 9.31 | −50 | 10 | 10 |
| dlPFC left | 46 | 0.000 | 1088 | 7.51 | −44 | 44 | 4 |
| Cerebellum right | - | 0.000 | 45 | 6.82 | 40 | −54 | −30 |
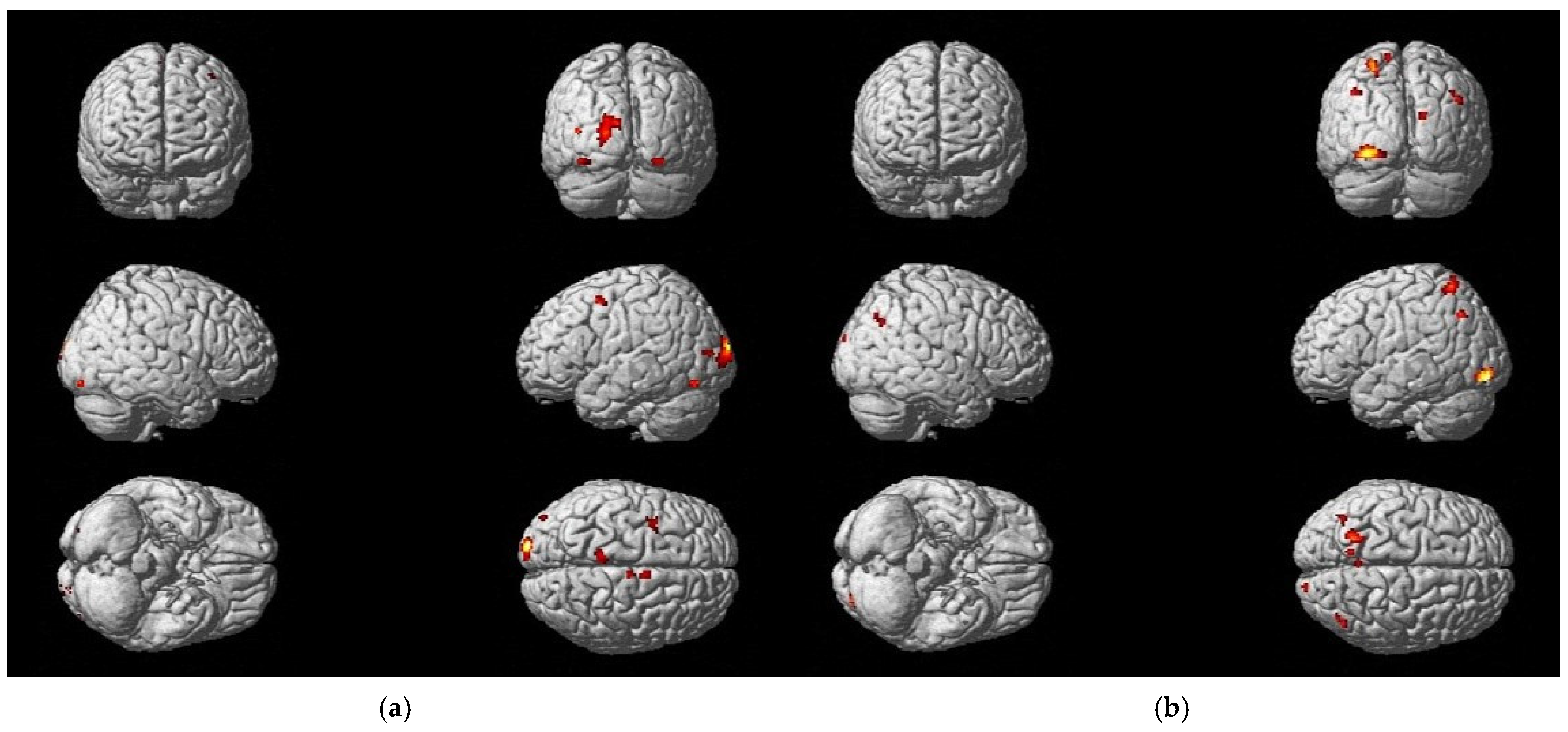
| Functional Region | Brodmann Area No. | Cluster | Peak | Coordinates * | |||
|---|---|---|---|---|---|---|---|
| p (FWE-Corr) | No. Voxels | T | X (mm) | Y (mm) | Z (mm) | ||
| Parietal cortex left | 7 | 0.001 | 26 | 5.68 | −12 | −62 | 70 |
| V2 left | 18 | 0.000 | 63 | 6.30 | −22 | −82 | −10 |
| Functional Region | Brodmann Area No. | Cluster | Peak | Coordinates * | |||
|---|---|---|---|---|---|---|---|
| p (FWE-Corr) | No. Voxels | T | X (mm) | Y (mm) | Z (mm) | ||
| M1 left | 4 | 0.000 | 134 | 5.04 | −10 | −28 | 80 |
| Parietal cortex left | 7 | 0.000 | 320 | 7.35 | −20 | −58 | 54 |
| Parietal cortex right | 7 | 0.000 | 304 | 6.90 | 22 | −68 | 54 |
| Parietal cortex right | 7 | 0.000 | 194 | 6.83 | 20 | −44 | 74 |
| Parietal cortex left | 7 | 0.000 | 134 | 6.66 | −2 | −36 | 74 |
| Parietal cortex right | 7 | 0.000 | 138 | 6.13 | 34 | −48 | 56 |
| Parietal cortex left | 7 | 0.000 | 40 | 5.87 | −12 | −60 | 70 |
| Parietal cortex right | 7 | 0.000 | 33 | 5.60 | 28 | −78 | 36 |
| Parietal cortex left | 7 | 0.000 | 44 | 5.29 | −20 | −76 | 40 |
| PFC right | 10 | 0.001 | 27 | 5.48 | 32 | 58 | −14 |
| V2 right | 18 | 0.000 | 95 | 7.14 | 40 | −82 | 4 |
| V2 left | 18 | 0.000 | 328 | 6.32 | −12 | −86 | −4 |
| V3 right | 19 | 0.000 | 101 | 6.52 | 42 | −76 | 24 |
| V3 right | 19 | 0.000 | 233 | 6.34 | 16 | −76 | 2 |
| V3 right | 19 | 0.000 | 84 | 6.22 | 30 | −82 | 16 |
| V3 left | 19 | 0.000 | 181 | 6.21 | −38 | −82 | 20 |
| V3 right | 19 | 0.000 | 56 | 6.02 | 52 | −70 | 12 |
| V3 left | 19 | 0.001 | 23 | 5.33 | −46 | −72 | −4 |
| V3 right | 19 | 0.000 | 33 | 5.24 | 34 | −74 | −10 |
| FG right | 37 | 0.002 | 16 | 5.26 | 42 | −40 | −20 |
| Cerebellum left | - | 0.000 | 161 | 6.15 | −8 | −72 | −24 |
| Cerebellum left | - | 0.002 | 16 | 5.37 | −32 | −40 | −34 |
| Functional Region | Brodmann Area No. | Cluster | Peak | Coordinates * | |||
|---|---|---|---|---|---|---|---|
| p (FWE-Corr) | No. Voxels | T | X (mm) | Y (mm) | Z (mm) | ||
| PMC and SMA right | 6 | 0.001 | 19 | 5.58 | 38 | −14 | 66 |
| Parietal cortex left | 7 | 0.000 | 26 | 5.38 | −34 | −64 | 46 |
| V2 left | 18 | 0.001 | 18 | 5.65 | −38 | −90 | 0 |
| V3 left | 19 | 0.000 | 24 | 6.03 | −50 | −78 | 12 |
| MTG left | 21 | 0.001 | 17 | 5.74 | −62 | −48 | 2 |
| SMG right | 40 | 0.001 | 21 | 5.49 | 58 | −40 | 44 |
| SMG left | 40 | 0.000 | 25 | 5.42 | −46 | −40 | 44 |
| dlPFC left | 46 | 0.000 | 25 | 5.35 | −40 | 44 | 0 |
| Functional Region | Brodmann Area No. | Cluster | Peak | Coordinates * | |||
|---|---|---|---|---|---|---|---|
| p (FWE-Corr) | No. Voxels | T | X (mm) | Y (mm) | Z (mm) | ||
| PMC and SMA right | 6 | 0.000 | 32 | 5.84 | 6 | −2 | 64 |
| PMC and SMA right | 6 | 0.001 | 27 | 5.71 | 6 | −16 | 62 |
| PMC and SMA left | 6 | 0.000 | 38 | 5.22 | −38 | 6 | 54 |
| V2 left | 18 | 0.000 | 219 | 6.45 | −16 | −100 | 18 |
| V2 right | 18 | 0.000 | 36 | 6.41 | 24 | −88 | −14 |
| V2 left | 18 | 0.001 | 24 | 6.02 | −8 | −84 | 18 |
| V3 left | 19 | 0.000 | 48 | 5.81 | −30 | −76 | −14 |
| V3 left | 19 | 0.001 | 23 | 5.75 | −40 | −86 | 10 |
| Functional Region | Brodmann Area No. | Cluster | Peak | Coordinates * | |||
|---|---|---|---|---|---|---|---|
| p (FWE-Corr) | No. Voxels | T | X (mm) | Y (mm) | Z (mm) | ||
| Parietal cortex left | 7 | 0.000 | 110 | 6.22 | −24 | −58 | 64 |
| Parietal cortex left | 7 | 0.001 | 26 | 5.62 | −12 | −60 | 70 |
| Parietal cortex left | 7 | 0.002 | 15 | 5.52 | −2 | −54 | 52 |
| V2 left | 18 | 0.000 | 316 | 7.83 | −30 | −86 | −8 |
| V2 right | 18 | 0.001 | 24 | 5.66 | 16 | −98 | 22 |
| PCC right | 31 | 0.000 | 110 | 5.78 | 12 | −58 | 28 |
| PCC left | 31 | 0.000 | 33 | 5.55 | −8 | −60 | 20 |
| PCC left | 31 | 0.002 | 15 | 5.39 | −12 | −50 | 40 |
| AG right | 39 | 0.000 | 34 | 5.54 | 46 | −66 | 36 |
| AG left | 39 | 0.000 | 31 | 5.47 | −38 | −66 | 42 |
| Functional Region | Brodmann Area No. | Cluster | Peak | Coordinates * | |||
|---|---|---|---|---|---|---|---|
| p (FWE-Corr) | No. Voxels | T | X (mm) | Y (mm) | Z (mm) | ||
| Parietal cortex left | 7 | 0.000 | 467 | 6.43 | −34 | −64 | 46 |
| Parietal cortex right | 7 | 0.000 | 64 | 5.58 | 20 | −62 | 62 |
| Parietal cortex left | 7 | 0.002 | 16 | 5.50 | −8 | −66 | 52 |
| antPFC left | 10 | 0.001 | 19 | 5.46 | −26 | 44 | 10 |
| V1 right | 17 | 0.001 | 26 | 5.51 | 14 | −76 | 6 |
| V2 left | 18 | 0.000 | 296 | 7.91 | −36 | −92 | −6 |
| V2 right | 18 | 0.000 | 47 | 6.08 | 18 | −96 | 20 |
| V3 left | 19 | 0.000 | 67 | 6.85 | −50 | −78 | 12 |
| V3 right | 19 | 0.000 | 156 | 6.36 | 48 | −72 | 8 |
| FG left | 37 | 0.000 | 50 | 6.58 | −64 | −50 | 0 |
| FG left | 37 | 0.001 | 21 | 5.29 | −56 | −58 | −8 |
| FG left | 37 | 0.000 | 28 | 5.09 | −44 | −64 | −20 |
| AG right | 39 | 0.000 | 31 | 5.44 | 30 | −76 | 24 |
| Cerebellum left | - | 0.000 | 46 | 6.00 | −28 | −78 | −20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meier, P.; Siedentopf, C.; Mayer-Suess, L.; Knoflach, M.; Kiechl, S.; Schönherr, G.S.; Grams, A.E.; Gizewski, E.R.; Lamina, C.; Galijasevic, M.; et al. Treatment-Associated Neuroplastic Changes in People with Stroke-Associated Ataxia—An fMRI Study. Neurol. Int. 2025, 17, 84. https://doi.org/10.3390/neurolint17060084
Meier P, Siedentopf C, Mayer-Suess L, Knoflach M, Kiechl S, Schönherr GS, Grams AE, Gizewski ER, Lamina C, Galijasevic M, et al. Treatment-Associated Neuroplastic Changes in People with Stroke-Associated Ataxia—An fMRI Study. Neurology International. 2025; 17(6):84. https://doi.org/10.3390/neurolint17060084
Chicago/Turabian StyleMeier, Patricia, Christian Siedentopf, Lukas Mayer-Suess, Michael Knoflach, Stefan Kiechl, Gudrun Sylvest Schönherr, Astrid E. Grams, Elke R. Gizewski, Claudia Lamina, Malik Galijasevic, and et al. 2025. "Treatment-Associated Neuroplastic Changes in People with Stroke-Associated Ataxia—An fMRI Study" Neurology International 17, no. 6: 84. https://doi.org/10.3390/neurolint17060084
APA StyleMeier, P., Siedentopf, C., Mayer-Suess, L., Knoflach, M., Kiechl, S., Schönherr, G. S., Grams, A. E., Gizewski, E. R., Lamina, C., Galijasevic, M., & Steiger, R. (2025). Treatment-Associated Neuroplastic Changes in People with Stroke-Associated Ataxia—An fMRI Study. Neurology International, 17(6), 84. https://doi.org/10.3390/neurolint17060084








