Development of a Novel Method of Spinal Electrophysiological Assessment via Intrathecal Administration at Analgesic Doses
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Intrathecal Administration
2.3. Behavioral Tests
2.4. In Vivo Extracellular Recordings
2.5. Statistics
3. Results
3.1. Development of an Intrathecal Administration Method
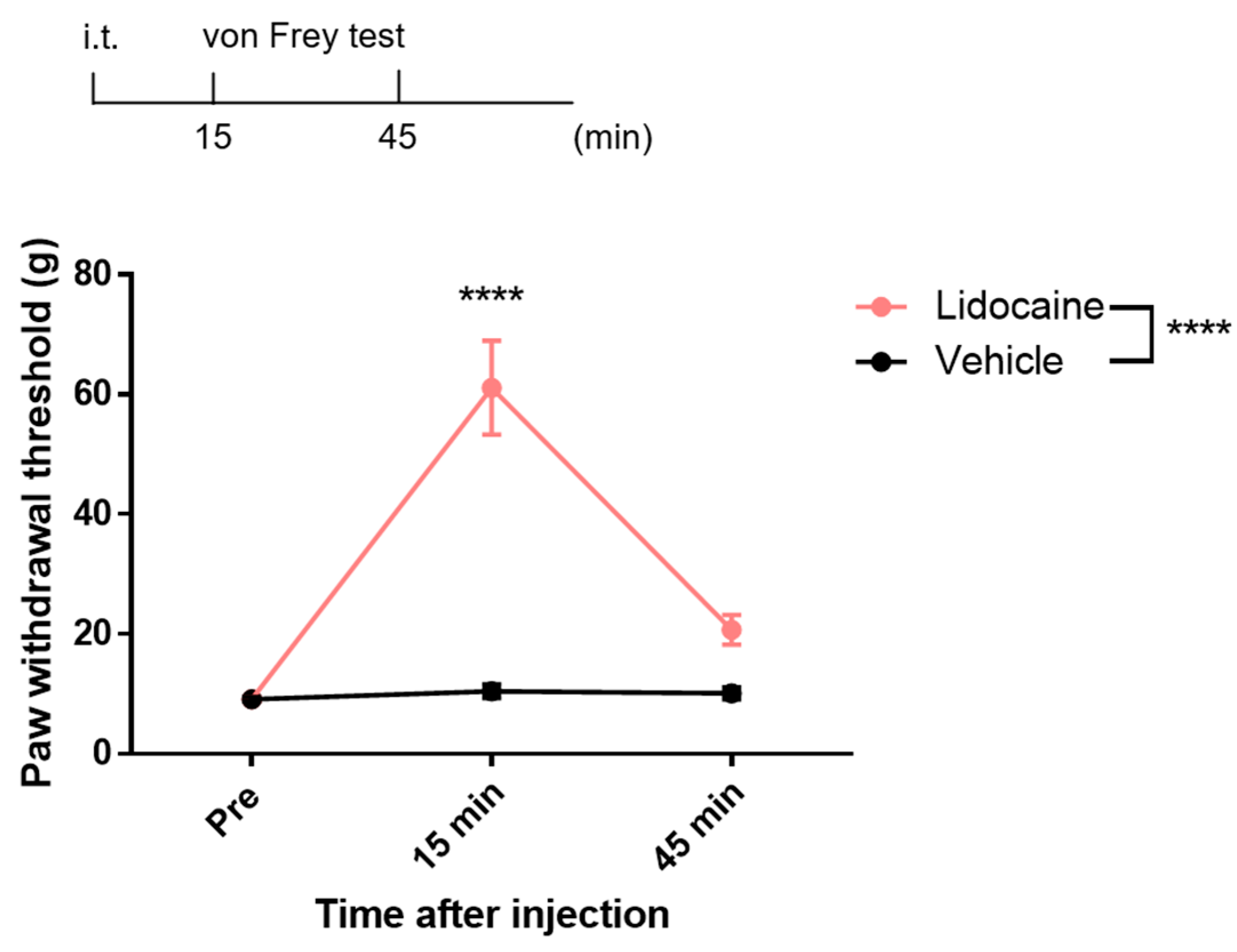
3.2. Analgesic Effects of Intrathecal Lidocaine
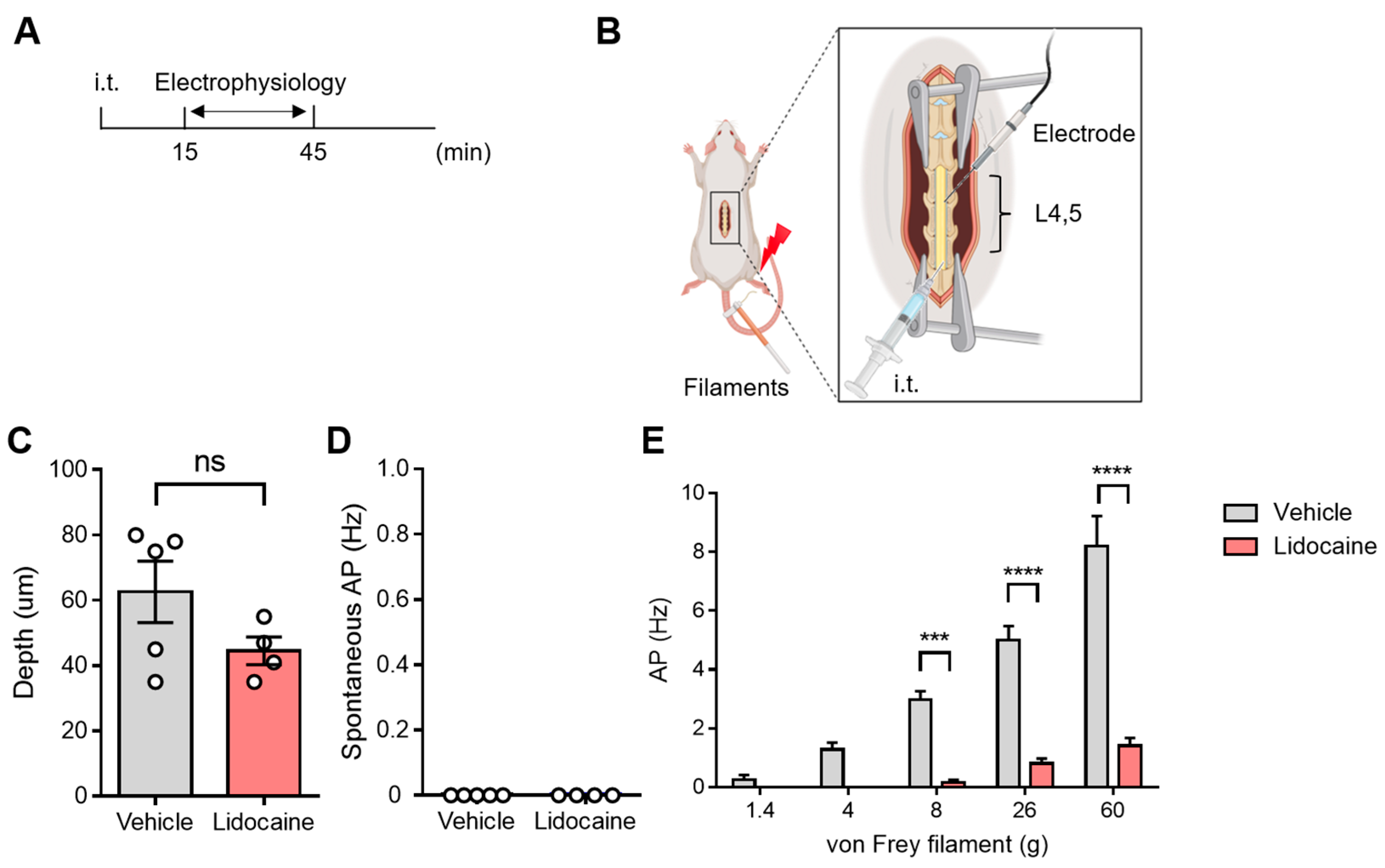
3.3. Evaluation of Spinal Neuronal Activity After Intrathecal Lidocaine
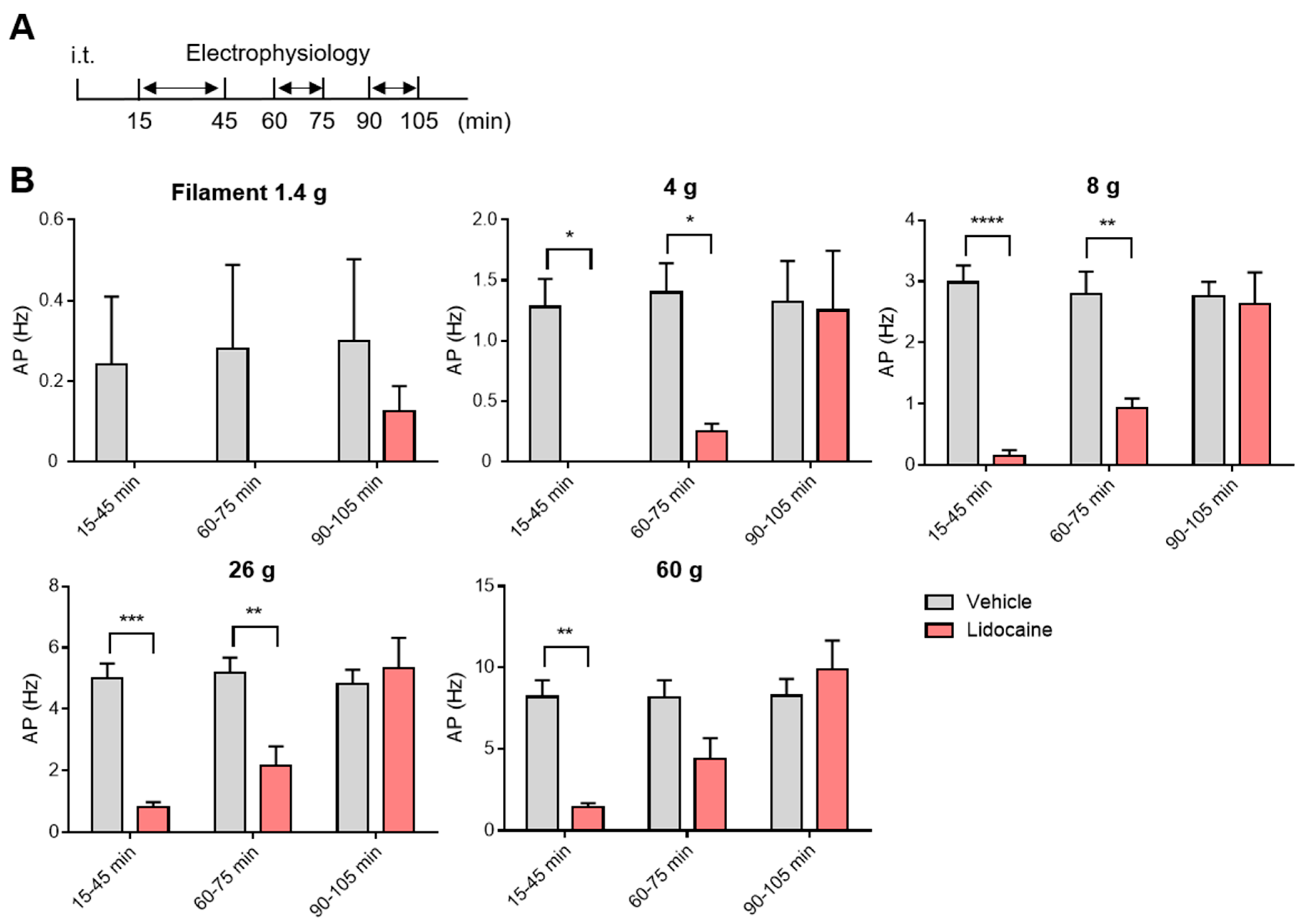
3.4. Washout and Long-Term Assessment of Lidocaine
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| i.t. | Intrathecal |
| i.p. | Intraperitoneal |
| CSF | Cerebrospinal fluid |
| vFF | von Frey filaments |
| AP | Action potential |
| CEC | Cauda equina compression |
| BBB | Blood–brain barrier |
| DRG | Dorsal root ganglia |
References
- Cohen, S.P.; Vase, L.; Hooten, W.M. Chronic pain: An update on burden, best practices, and new advances. Lancet 2021, 397, 2082–2097. [Google Scholar] [CrossRef] [PubMed]
- Scholz, J.; Finnerup, N.B.; Attal, N.; Aziz, Q.; Baron, R.; Bennett, M.I.; Benoliel, R.; Cohen, M.; Cruccu, G.; Davis, K.D.; et al. Classification Committee of the Neuropathic Pain Special Interest Group (NeuPSIG). The IASP classification of chronic pain for ICD-11: Chronic neuropathic pain. Pain 2019, 160, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Grace, P.M.; Hutchinson, M.R.; Maier, S.F.; Watkins, L.R. Pathological pain and the neuroimmune interface. Nat. Rev. Immunol. 2014, 14, 217–231. [Google Scholar] [CrossRef]
- Hunt, S.P.; Mantyh, P.W. The molecular dynamics of pain control. Nat. Rev. Neurosci. 2001, 2, 83–91. [Google Scholar] [CrossRef]
- Goodwin, G.; McMahon, S.B. The physiological function of different voltage-gated sodium channels in pain. Nat. Rev. Neurosci. 2021, 22, 263–274. [Google Scholar] [CrossRef]
- De Araujo, D.S.M.; Nassini, R.; Geppetti, P.; De Logu, F. TRPA1 as a therapeutic target for nociceptive pain. Expert Opin. Ther. Targets 2020, 24, 997–1008. [Google Scholar] [CrossRef]
- Uchytilova, E.; Spicarova, D.; Palecek, J. TRPV1 Antagonist Attenuates Postoperative Hypersensitivity by Central and Peripheral Mechanisms. Mol. Pain 2014, 10, 1744–8069. [Google Scholar] [CrossRef]
- Moon, J.Y.; Song, S.; Yoon, S.Y.; Roh, D.H.; Kang, S.Y.; Park, J.H.; Beitz, A.J.; Lee, J.H. The differential effect of intrathecal Nav1.8 blockers on the induction and maintenance of capsaicin- and peripheral ischemia-induced mechanical allodynia and thermal hyperalgesia. Anesth. Analg. 2012, 114, 215–223. [Google Scholar] [CrossRef]
- Cui, M.; Honore, P.; Zhong, C.; Gauvin, D.; Mikusa, J.; Hernandez, G.; Chandran, P.; Gomtsyan, A.; Brown, B.; Bayburt, E.K.; et al. TRPV1 receptors in the CNS play a key role in broad-spectrum analgesia of TRPV1 antagonists. J. Neurosci. 2006, 26, 9385–9393. [Google Scholar] [CrossRef]
- McGaraughty, S.; Chu, K.L.; Scanio, M.J.C.; Kort, M.E.; Faltynek, C.R.; Jarvis, M.F. A selective Nav1.8 sodium channel blocker, A-803467 [5-4-chlorophenyl-N-(3,5-dimethoxyphenyl)furan-2-carboxamide], attenuates spinal neuronal activity in neuropathic rats. J. Pharmacol. Exp. Ther. 2008, 324, 1204–1211. [Google Scholar] [CrossRef] [PubMed]
- Uta, D.; Kiyohara, K.; Nagaoka, Y.; Kino, Y.; Fujita, T. Developing a Novel Method for the Analysis of Spinal Cord–Penile Neurotransmission Mechanisms. Int. J. Mol. Sci. 2023, 24, 1434. [Google Scholar] [CrossRef]
- Chaplan, S.R.; Bach, F.W.; Pogrel, J.W.; Chung, J.M.; Yaksh, T.L. Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods 1994, 53, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Uta, D.; Ishibashi, N.; Kawase, Y.; Tao, S.; Sawahata, M.; Kume, T. Relationship between Laser Intensity at the Peripheral Nerve and Inhibitory Effect of Percutaneous Photobiomodulation on Neuronal Firing in a Rat Spinal Dorsal Horn. J. Clin. Med. 2023, 12, 5126. [Google Scholar] [CrossRef]
- Tian, J.; Gu, Y.; Su, D.; Wu, Y.; Wang, X. Effects of intrathecal lidocaine on hyperalgesia and allodynia following chronic constriction injury in rats. Eur. J. Pain. 2009, 13, 130–137. [Google Scholar] [CrossRef]
- Rahman, M.M.; Lee, J.Y.; Kim, Y.H.; Park, C.K. Epidural and Intrathecal Drug Delivery in Rats and Mice for Experimental Research: Fundamental Concepts, Techniques, Precaution, and Application. Biomedicines 2023, 11, 1413. [Google Scholar] [CrossRef]
- Chang, M.F.; Hsieh, J.H.; Chiang, H.; Kan, H.W.; Huang, C.M.; Chellis, L.; Lin, B.S.; Miaw, S.C.; Pan, C.L.; Chao, C.C.; et al. Effective gene expression in the rat dorsal root ganglia with a non-viral vector delivered via spinal nerve injection. Sci. Rep. 2016, 6, 35612. [Google Scholar] [CrossRef]
- Minami, K.; Tamano, R.; Kasai, E.; Oyama, H.; Hasegawa, M.; Shinohara, S.; Asaki, T. Effects of duloxetine on pain and walking distance in neuropathic pain models via modulation of the spinal monoamine system. Eur. J. Pain 2018, 22, 355–369. [Google Scholar] [CrossRef]
- Bendnar, D.A. Cauda equina syndrome from lumbar disc herniation. CMAJ 2016, 188, 284. [Google Scholar] [CrossRef]
- Pan, H.L.; Song, H.K.; Eisenach, J.C. Effects of Intrathecal Neostigmine, Bupivacaine, and Their Combination on Sympathetic Nerve Activity in Rats. J. Am. Soc. Anesthesiol. 1998, 88, 481–486. [Google Scholar] [CrossRef]
- Kim, K.H.; Byeon, G.J.; Kim, H.Y.; Baek, S.H.; Shin, S.W.; Koo, S.T. Mechanical Antiallodynic Effect of Intrathecal Nefopam in a Rat Neuropathic Pain Model. J. Korean Med. Sci. 2015, 30, 1189–1196. [Google Scholar] [CrossRef]
- Orbach-Zinger, S.; Jadon, A.; Lucas, D.N.; Sia, A.T.; Tsen, L.C.; Van de Velde, M.; Heesen, M. Intrathecal catheter use after accidental dural puncture in obstetric patients: Literature review and clinical management recommendations. Anaesthesia 2021, 76, 1111–1121. [Google Scholar] [CrossRef] [PubMed]
- Uta, D.; Ishibashi, N.; Konno, T.; Okada, Y.; Kawase, Y.; Tao, S.; Kume, T. Near-Infrared Photobiomodulation of the Peripheral Nerve Inhibits the Neuronal Firing in a Rat Spinal Dorsal Horn Evoked by Mechanical Stimulation. Int. J. Mol. Sci. 2023, 24, 2352. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uta, D.; Yamane, T.; Yoneda, S.; Kasai, E.; Kume, T. Development of a Novel Method of Spinal Electrophysiological Assessment via Intrathecal Administration at Analgesic Doses. Neurol. Int. 2025, 17, 78. https://doi.org/10.3390/neurolint17050078
Uta D, Yamane T, Yoneda S, Kasai E, Kume T. Development of a Novel Method of Spinal Electrophysiological Assessment via Intrathecal Administration at Analgesic Doses. Neurology International. 2025; 17(5):78. https://doi.org/10.3390/neurolint17050078
Chicago/Turabian StyleUta, Daisuke, Takuya Yamane, Sosuke Yoneda, Erika Kasai, and Toshiaki Kume. 2025. "Development of a Novel Method of Spinal Electrophysiological Assessment via Intrathecal Administration at Analgesic Doses" Neurology International 17, no. 5: 78. https://doi.org/10.3390/neurolint17050078
APA StyleUta, D., Yamane, T., Yoneda, S., Kasai, E., & Kume, T. (2025). Development of a Novel Method of Spinal Electrophysiological Assessment via Intrathecal Administration at Analgesic Doses. Neurology International, 17(5), 78. https://doi.org/10.3390/neurolint17050078






