Medications for Managing Central Neuropathic Pain as a Result of Underlying Conditions—A Systematic Review
Abstract
1. Introduction
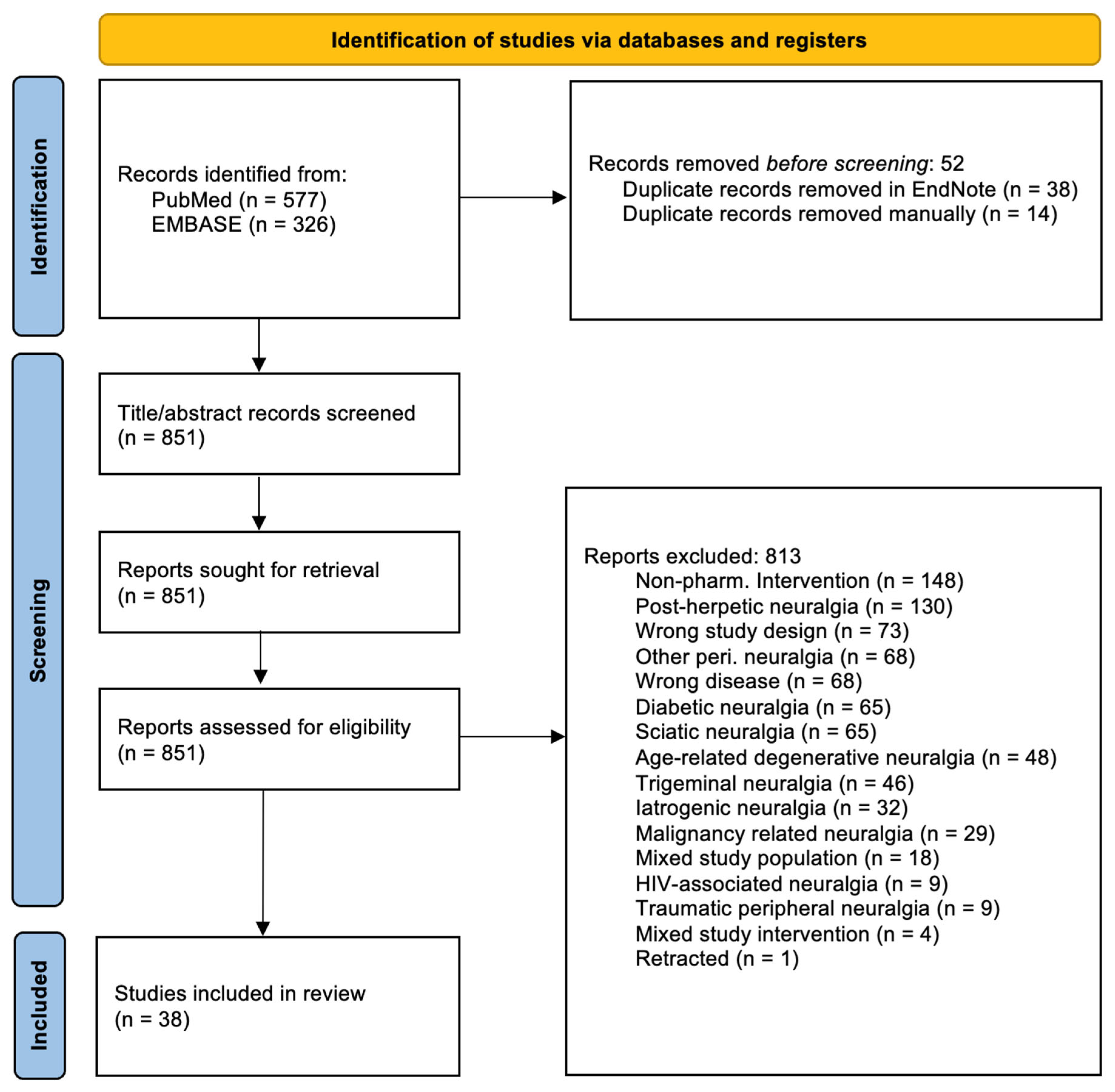
2. Materials and Methods
2.1. Study Selection
2.2. Data Extraction and Quality Assessment
2.3. Pain-Measuring Instruments
- Visual Analogue Scale (VAS)
- McGill Pain Questionnaire (MPQ)
- Short-form McGill Pain Questionnaire (SFMPQ)
- Short-form McGill Pain Questionnaire 2 (SFMPQ2)
- Borg Category Ratio Scale (CR-10)
- Duration-Adjusted Average Pain Change (DAAC)
- Numerical (Pain) Rating Scale (NRS/NPRS)
- Neuropathic Pain Scale (NPS)
- Neuropathic Pain Symptom Inventory (NPSI)
- Pain Disability Index (PDI)
- Multidimensional Pain Inventory (MPI)
- Brief Pain Inventory (BPI)
- Short-Form Health Questionnaire (SF-36)
- Complex Regional Pain Syndrome (CRPS) Score/Shoulder–Hand Syndrome Score
3. Results
3.1. Investigated First-Line Treatment
- Tricyclic antidepressants (TCAs)
- Serotonin norepinephrine reuptake inhibitors (SNRIs)
- Gabapentanoids
- Topicals
3.2. Investigated Second-Line Treatment
- Tramadol
3.3. Investigated Third-Line Treatment
- Selective serotonin reuptake inhibitors (SSRIs)
- Anticonvulsants
- N-Methyl-D-aspartate (NMDA) antagonists
3.4. Investigated None-Tier Medication
- Cannabinoids
- Anti-inflammatory drugs
- Sodium channel blockers
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Vranken, J.H.; Dijkgraaf, M.G.; Kruis, M.R.; van Dasselaar, N.T.; van der Vegt, M.H. Iontophoretic administration of S(+)-ketamine in patients with intractable central pain: A placebo-controlled trial. Pain 2005, 118, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Langford, R.M.; Mares, J.; Novotna, A.; Vachova, M.; Novakova, I.; Notcutt, W.; Ratcliffe, S. A double-blind, randomized, placebo-controlled, parallel-group study of THC/CBD oromucosal spray in combination with the existing treatment regimen, in the relief of central neuropathic pain in patients with multiple sclerosis. J. Neurol. 2013, 260, 984–997. [Google Scholar] [CrossRef]
- Agarwal, N.; Joshi, M. Effectiveness of amitriptyline and lamotrigine in traumatic spinal cord injury-induced neuropathic pain: A randomized longitudinal comparative study. Spinal Cord 2017, 55, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Svendsen, K.B.; Jensen, T.S.; Bach, F.W. Does the cannabinoid dronabinol reduce central pain in multiple sclerosis? Randomised double blind placebo controlled crossover trial. Br. Med. J. 2004, 329, 253–257. [Google Scholar] [CrossRef]
- Mahesh, B.; Singh, V.K.; Pathak, A.; Kumar, A.; Mishra, V.N.; Joshi, D.; Chaurasia, R.N. Efficacy of Duloxetine in Patients with Central Post-stroke Pain: A Randomized Double Blind Placebo Controlled Trial. Pain Med. 2023, 24, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Vranken, J.H.; Hollmann, M.W.; Van Der Vegt, M.H.; Kruis, M.R.; Heesen, M.; Vos, K.; Pijl, A.J.; Dijkgraaf, M.G.W. Duloxetine in patients with central neuropathic pain caused by spinal cord injury or stroke: A randomized, double-blind, placebo-controlled trial. Pain 2011, 152, 267–273. [Google Scholar] [CrossRef]
- Olusanya, A.; Yearsley, A.; Brown, N.; Braun, S.; Hayes, C.; Rose, E.; Connolly, B.; Dicks, M.; Beal, C.; Helmonds, B.; et al. Capsaicin 8% patch for spinal cord injury focal neuropathic pain, a randomized controlled trial. Pain Med. 2023, 24, 71–78. [Google Scholar] [CrossRef]
- Vranken, J.H.; Dijkgraaf, M.G.W.; Kruis, M.R.; van der Vegt, M.H.; Hollmann, M.W.; Heesen, M. Pregabalin in patients with central neuropathic pain: A randomized, double-blind, placebo-controlled trial of a flexible-dose regimen. Pain 2008, 136, 150–157. [Google Scholar] [CrossRef]
- Bates, D.; Schultheis, B.C.; Hanes, M.C.; Jolly, S.M.; Chakravarthy, K.V.; Deer, T.R.; Levy, R.M.; Hunter, C.W. A Comprehensive Algorithm for Management of Neuropathic Pain. Pain Med. 2019, 20, S2–S12. [Google Scholar] [CrossRef]
- Finnerup, N.B.; Attal, N.; Haroutounian, S.; McNicol, E.; Baron, R.; Dworkin, R.H.; Gilron, I.; Haanpää, M.; Hansson, P.; Jensen, T.S.; et al. Pharmacotherapy for neuropathic pain in adults: A systematic review and meta-analysis. Lancet Neurol. 2015, 14, 162–173. [Google Scholar] [CrossRef]
- Moore, R.A.; Chi, C.C.; Wiffen, P.J.; Derry, S.; Rice, A.S. Oral nonsteroidal anti-inflammatory drugs for neuropathic pain. Cochrane Database Syst. Rev. 2015, 2015, Cd010902. [Google Scholar] [CrossRef] [PubMed]
- Kvarnstrom, A.; Karlsten, R.; Quiding, H.; Gordh, T. The analgesic effect of intravenous ketamine and lidocaine on pain after spinal cord injury. Acta Anaesthesiol. Scand. 2004, 48, 498–506. [Google Scholar] [CrossRef]
- Kalita, J.; Misra, U.K.; Kumar, A.; Bhoi, S.K. Long-term prednisolone in post-stroke complex regional pain syndrome. Pain Physician 2016, 19, 565–574. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. Bmj 2021, 372, n160. [Google Scholar] [CrossRef] [PubMed]
- Zenodo Dataset. 2025. Available online: https://doi.org/10.5281/zenodo.14892940 (accessed on 6 May 2025).
- The Endnote Team. EndNote, EndNote 21, Clarivate: Philadelphia, PA, USA, 2021.
- NHLBI. Study Quality Assessment Tools—Quality Assessment of Controlled Intervention Studies. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 6 May 2025).
- Delgado, D.A.; Lambert, B.S.; Boutris, N.; McCulloch, P.C.; Robbins, A.B.; Moreno, M.R.; Harris, J.D. Validation of Digital Visual Analog Scale Pain Scoring with a Traditional Paper-based Visual Analog Scale in Adults. J. Am. Acad. Orthop. Surg. Glob. Res. Rev. 2018, 2, e088. [Google Scholar] [CrossRef] [PubMed]
- Melzack, R. The McGill Pain Questionnaire: Major properties and scoring methods. Pain 1975, 1, 277–299. [Google Scholar] [CrossRef]
- Melzack, R. The short-form McGill Pain Questionnaire. Pain 1987, 30, 191–197. [Google Scholar] [CrossRef]
- Dworkin, R.H.; Turk, D.C.; Trudeau, J.J.; Benson, C.; Biondi, D.M.; Katz, N.P.; Kim, M. Validation of the Short-form McGill Pain Questionnaire-2 (SF-MPQ-2) in acute low back pain. J. Pain 2015, 16, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Borg, G. Borg’s Perceived Exertion and Pain Scales; American Psychological Association: Washington, DC, USA, 1998. [Google Scholar]
- Cardenas, D.D.; Nieshoff, E.C.; Suda, K.; Goto, S.I.; Sanin, L.; Kaneko, T.; Sporn, J.; Parsons, B.; Soulsby, M.; Yang, R.; et al. A randomized trial of pregabalin in patients with neuropathic pain due to spinal cord injury. Neurology 2013, 80, 533–539. [Google Scholar] [CrossRef]
- Hawker, G.A.; Mian, S.; Kendzerska, T.; French, M. Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF-36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP). Arthritis Care Res. 2011, 63 (Suppl. S11), S240–S252. [Google Scholar] [CrossRef]
- Galer, B.S.; Jensen, M.P. Development and preliminary validation of a pain measure specific to neuropathic pain: The Neuropathic Pain Scale. Neurology 1997, 48, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Bouhassira, D.; Attal, N.; Fermanian, J.; Alchaar, H.; Gautron, M.; Masquelier, E.; Rostaing, S.; Lanteri-Minet, M.; Collin, E.; Grisart, J.; et al. Development and validation of the Neuropathic Pain Symptom Inventory. Pain 2004, 108, 248–257. [Google Scholar] [CrossRef]
- Pollard, C.A. Preliminary validity study of the pain disability index. Percept. Mot. Ski. 1984, 59, 974. [Google Scholar] [CrossRef]
- Kerns, R.D.; Turk, D.C.; Rudy, T.E. The West Haven-Yale Multidimensional Pain Inventory (WHYMPI). Pain 1985, 23, 345–356. [Google Scholar] [CrossRef]
- Poquet, N.; Lin, C. The Brief Pain Inventory (BPI). J. Physiother. 2016, 62, 52. [Google Scholar] [CrossRef]
- Ware, J.E., Jr.; Sherbourne, C.D. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med. Care 1992, 30, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Braus, D.F.; Krauss, J.K.; Strobel, J. The shoulder-hand syndrome after stroke: A prospective clinical trial. Ann. Neurol. 1994, 36, 728–733. [Google Scholar] [CrossRef]
- Amr, Y.M. Multi-day low dose ketamine infusion as adjuvant to oral gabapentin in spinal cord injury related chronic pain: A prospective, randomized, double blind trial. Pain Physician 2010, 13, 245. [Google Scholar] [CrossRef] [PubMed]
- Chun, A.; Levy, I.; Yang, A.; Delgado, A.; Tsai, C.Y.; Leung, E.; Taylor, K.; Kolakowsky-Hayner, S.; Huang, V.; Escalon, M.; et al. Treatment of At-level Spinal Cord Injury Pain with Botulinum Toxin A. Arch. Phys. Med. Rehabil. 2019, 100, e137. [Google Scholar] [CrossRef]
- Escriba, P.V.; Gil-Agudo, A.M.; Vidal Samso, J.; Sanchez-Raya, J.; Salvador-de la Barrera, S.; Soto-Leon, V.; Leon-Alvarez, N.; Mendez Ferrer, B.; Membrilla-Mesa, M.D.; Redondo Galan, C.; et al. Randomised, double-blind, placebo-controlled, parallel-group, multicentric, phase IIA clinical trial for evaluating the safety, tolerability, and therapeutic efficacy of daily oral administration of NFX88 to treat neuropathic pain in individuals with spinal cord injury. Spinal Cord 2024, 62, 454–467. [Google Scholar] [CrossRef]
- Finnerup, N.B.; Biering-Sorensen, F.; Johannesen, I.L.; Terkelsen, A.J.; Juhl, G.I.; Kristensen, A.D.; Sindrup, S.H.; Bach, F.W.; Jensen, T.S. Intravenous lidocaine relieves spinal cord injury pain: A randomized controlled trial. Anesthesiology 2005, 102, 1023–1030. [Google Scholar] [CrossRef] [PubMed]
- Finnerup, N.B.; Grydehoj, J.; Bing, J.; Johannesen, I.L.; Biering-Sorensen, F.; Sindrup, S.H.; Jensen, T.S. Levetiracetam in spinal cord injury pain: A randomized controlled trial. Spinal Cord 2009, 47, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.A.; Song, D.H.; Oh, H.M.; Chung, M.E. Botulinum toxin type A for neuropathic pain in patients with spinal cord injury. Ann. Neurol. 2016, 79, 569–578. [Google Scholar] [CrossRef]
- Kaydok, E.; Levendoglu, F.; Ozerbil, M.O.; Karahan, A.Y. Comparison of the efficacy of gabapentin and pregabalin for neuropathic pain in patients with spinal cord injury: A crossover study. Acta Medica Mediterr. 2014, 30, 1343–1348. [Google Scholar]
- Kumru, H.; Benito-Penalva, J.; Kofler, M.; Vidal, J. Analgesic effect of intrathecal baclofen bolus on neuropathic pain in spinal cord injury patients. Brain Res. Bull. 2018, 140, 205–211. [Google Scholar] [CrossRef]
- Levendoglu, F.; Ogun, C.O.; Ozerbil, O.; Ogun, T.C.; Ugurlu, H. Gabapentin Is a First Line Drug for the Treatment of Neuropathic Pain in Spinal Cord Injury. Spine 2004, 29, 743–751. [Google Scholar] [CrossRef]
- Norrbrink, C.; Lundeberg, T. Tramadol in neuropathic pain after spinal cord injury a randomized, double-blind, placebo-controlled trial. Clin. J. Pain 2009, 25, 177–184. [Google Scholar] [CrossRef]
- Rintala, D.H.; Holmes, S.A.; Courtade, D.; Fiess, R.N.; Tastard, L.V.; Loubser, P.G. Comparison of the Effectiveness of Amitriptyline and Gabapentin on Chronic Neuropathic Pain in Persons With Spinal Cord Injury. Arch. Phys. Med. Rehabil. 2007, 88, 1547–1560. [Google Scholar] [CrossRef]
- Rintala, D.H.; Fiess, R.N.; Tan, G.; Holmes, S.A.; Bruel, B.M. Effect of dronabinol on central neuropathic pain after spinal cord injury: A pilot study. Am. J. Phys. Med. Rehabil./Assoc. Acad. Physiatr. 2010, 89, 840–848. [Google Scholar] [CrossRef]
- Siddall, P.J.; Cousins, M.J.; Otte, A.; Griesing, T.; Chambers, R.; Murphy, T.K. Pregabalin in central neuropathic pain associated with spinal cord injury: A placebo-controlled trial. Neurology 2006, 67, 1792–1800. [Google Scholar] [CrossRef]
- Tai, Q.; Kirshblum, S.; Chen, B.; Millis, S.; Johnston, M.; DeLisa, J.A. Gabapentin in the treatment of neuropathic pain after spinal cord injury: A prospective, randomized, double-blind, crossover trial. J. Spinal Cord Med. 2002, 25, 100–105. [Google Scholar] [CrossRef]
- Yang, M.L.; Li, J.J.; So, K.F.; Chen, J.Y.; Cheng, W.S.; Wu, J.; Wang, Z.M.; Gao, F.; Young, W. Efficacy and safety of lithium carbonate treatment of chronic spinal cord injuries: A double-blind, randomized, placebo-controlled clinical trial. Spinal Cord 2012, 50, 141–146. [Google Scholar] [CrossRef]
- Yilmaz, B.; Yasar, E.; Koroglu Omac, O.; Goktepe, A.S.; Tan, A.K. Gabapentin vs. pregabalin for the treatment of neuropathic pain in patients with spinal cord injury: A crossover study. Turk. Fiz. Tip. ve Rehabil. Derg. 2015, 61, 1–5. [Google Scholar] [CrossRef]
- Falah, M.; Madsen, C.; Holbech, J.V.; Sindrup, S.H. A randomized, placebo-controlled trial of levetiracetam in central pain in multiple sclerosis. Eur. J. Pain 2012, 16, 860–869. [Google Scholar] [CrossRef] [PubMed]
- Foley, P.; Parker, R.A.; de Angelis, F.; Connick, P.; Chandran, S.; Young, C.; Weir, C.J.; Chataway, J. Efficacy of Fluoxetine, Riluzole and Amiloride in treating neuropathic pain associated with secondary progressive multiple sclerosis. Pre-specified analysis of the MS-SMART double-blind randomised placebo-controlled trial. Mult. Scler. Relat. Disord. 2022, 63, 103925. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.; Mataluni, G.; Codeca, C.; Fiore, S.; Buttari, F.; Musella, A.; Castelli, M.; Bernardi, G.; Centonze, D. Effects of levetiracetam on chronic pain in multiple sclerosis: Results of a pilot, randomized, placebo-controlled study. Eur. J. Neurol. 2009, 16, 360–366. [Google Scholar] [CrossRef]
- Turcotte, D.; Doupe, M.; Torabi, M.; Gomori, A.; Ethans, K.; Esfahani, F.; Galloway, K.; Namaka, M. Nabilone as an adjunctive to gabapentin for multiple sclerosis-induced neuropathic pain: A randomized controlled trial. Pain Med. 2015, 16, 149–159. [Google Scholar] [CrossRef]
- van Amerongen, G.; Kanhai, K.; Baakman, A.C.; Heuberger, J.; Klaassen, E.; Beumer, T.L.; Strijers, R.L.M.; Killestein, J.; van Gerven, J.; Cohen, A.; et al. Effects on Spasticity and Neuropathic Pain of an Oral Formulation of DELTA9-tetrahydrocannabinol in Patients With Progressive Multiple Sclerosis. Clin. Ther. 2018, 40, 1467–1482. [Google Scholar] [CrossRef]
- Vollmer, T.L.; Robinson, M.J.; Risser, R.C.; Malcolm, S.K. A Randomized, Double-Blind, Placebo-Controlled Trial of Duloxetine for the Treatment of Pain in Patients with Multiple Sclerosis. Pain Pract. 2014, 14, 732–744. [Google Scholar] [CrossRef]
- Jungehulsing, G.J.; Israel, H.; Safar, N.; Taskin, B.; Nolte, C.H.; Brunecker, P.; Wernecke, K.D.; Villringer, A. Levetiracetam in patients with central neuropathic post-stroke pain—a randomized, double-blind, placebo-controlled trial. Eur. J. Neurol. 2013, 20, 331–337. [Google Scholar] [CrossRef]
- Ralph, S.J.; Weissenberger, A.; Bonev, V.; King, L.D.; Bonham, M.D.; Ferguson, S.; Smith, A.D.; Goodman-Jones, A.A.; Espinet, A.J. Phase I/II parallel double-blind randomized controlled clinical trial of perispinal etanercept for chronic stroke: Improved mobility and pain alleviation. Expert Opin. Investig. Drugs 2020, 29, 311–326. [Google Scholar] [CrossRef] [PubMed]
- Kalita, J.; Vajpayee, A.; Misra, U.K. Comparison of prednisolone with piroxicam in complex regional pain syndrome following stroke: A randomized controlled trial. QJM Int. J. Med. 2006, 99, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Iwaki, H.; Ando, R.; Tada, S.; Nishikawa, N.; Tsujii, T.; Yamanishi, Y.; Miyaue, N.; Yabe, H.; Nagai, M.; Nomoto, M. A double-blind, randomized controlled trial of duloxetine for pain in Parkinson’s disease. J. Neurol. Sci. 2020, 414, 116833. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.S.; Gustavsen, S.; Roshanisefat, H.; Kant, M.; Biering-Sorensen, F.; Andersen, C.; Olsson, A.; Chow, H.H.; Asgari, N.; Hansen, J.R.; et al. Cannabis-Based Medicine for Neuropathic Pain and Spasticity-A Multicenter, Randomized, Double-Blinded, Placebo-Controlled Trial. Pharmaceuticals 2023, 16, 1079. [Google Scholar] [CrossRef]

| Name, Year | Underlying Condition(s) | Analgesic(s) | Pop. | Pain-Measuring Instrument(s) | Significance | Effect Size | Risk of Bias |
|---|---|---|---|---|---|---|---|
| Agarwal et al., 2017 [3] | SCI | Amitriptyline Lamotrigine | 147 | SFMPQ2, 0–10 | Pain reduction from baseline, p = 0.000 * Difference between drugs, p = 0.648–0.819 | Amitriptyline: Days 7–14–21 Mean: 0.2149, s.d. 0.2075 Mean: 0.3593, s.d. 0.1939 Mean: 0.4312, s.d. 0.1812 Lamotrigine Days 7–14–21 Mean: 0.2048, s.d. 0.2245 Mean: 0.3622, s.d. 0.2403 Mean: 0.4432, s.d. 0.1812 | Fair |
| Amr, 2010 [32] | SCI | Ketamine add-on to gabapentin | 40 | VAS, 0–10 | Reduced pain in both groups, p < 0.05 * Reduction in pain vs pre-treatment, p = 0.0001 * Reduction in pain, 7 days of treatment + 2 weeks after stopping treatment, p = 0.0002 * 3 + 4 weeks after stopping treatment, p = 0.25–0.54 | VAS and % difference Days 1–7: 27.3 ± 8.9, 72.3 ± 5.4 (90.36%) 14.0 ± 5.6, 42.5 ± 14.5 (100.89%) 1st week, 2nd week after last infusion 21.50 ± 6.39, 43.00 ± 4.05 (66.67%) 22.4 ± 7.54, 44.00 ± 4.61 (65.06%) | Good |
| Cardenas et al., 2013 [23] | SCI | Pregabalin | 220 | DAAC, 0–10 NRS, 0–10 | DAAC, p = 0.003 * NRS pain, change from baseline, p = 0.007 * | DAAC, least squares, differences between groups: Placebo −1.07 Pregabalin −1.66 % difference (43.22%) Pain, NRS, least squares, difference in change from baseline pain: Placebo: −1.22 Pregabalin: −1.92 % difference (44.59%) | Fair |
| Chun et al., 2019 [33] | SCI | Botox-A | 8 | NPRS, 0–10 | Underpowered | N/A | Fair |
| Escribá et al. 2024 [34] | SCI | NFX88 add-on to pregabalin | 61 | VAS, 0–10 | Total patients, p < 0.2 | VAS, mean (nom. % chance) Visit 1, Visit 4 Placebo: 7.17, 5.50 (−23.29%) 1.05 g/day: 6.62, 5.92 (−10.57%) 2.1 g/day: 6.63, 4.32 (−34.84%) 4.2/day: 7.02, 6.01 (−14.39%) | Good |
| Finnerup et al., 2005 [35] | SCI | Lidocaine | 26 | VAS, 0–10 | Total patients, p = 0.01 * | VAS average, 0–25 min: All patients 57.08, 39.00 % difference (−37.64%) | Good |
| Finnerup et al., 2009 [36] | SCI | Levetiracetam | 36 | NRS, 0–10 NPSI, 0–10 | NRS, p = 0.46 NPSI, p = 0.55–0.95 | Median NRS pain, placebo % change (−16.67%) Median NRS pain, levetiracetam: % change (0.00%) Levetiracetam vs. baseline, combined NPSI % change, (−2.11%) | Good |
| Han et al., 2016 [37] | SCI | Botox-A | 40 | VAS, 0–100 mm SFMPQ, 0–3 | VAS, p = 0.0027–0.0053 * SFMPQ Sensory, p = 0.0033 * Affective, p = 0.0086–0.0131 * Total scores, p = 0.0008–0.0197 * | VAS, Botox 0–4–8 weeks, 85.1 ± 13.6 (−21.86%) 66.5 ± 20.7 (−4.06%) 63.8 ± 27.5 VAS, Placebo, 0–4–8 weeks, 7.1 ± 14.0 (−3.37%) 74.5 ± 16.0 (3.09%) 76.8 ± 20.4 | Good |
| Kaydok et al., 2014 [38] | SCI | Gabapentin Pregabalin | 28 | VAS, 0–10 NPS, 0–10 | VAS, both drugs compared from baseline to 8 weeks, p < 0.001 * Pregabalin, pain reduction compared to gabapentin at 4 weeks, p = 0.045 * Pregabalin, pain reduction compared to gabapentin at 8 weeks, p > 0.05 NPS pregabalin compared to gabapentin, p > 0.05 | VAS, 0–4–8 week: Gabapentin: 7.78 ± 1.27, 4.36 ± 1.30 (−43.96%), 3.57 ± 1.21 (−54.11%) Pregabalin: 8.05 ± 1.26, 3.68 ± 1.15 (−54.29%), 3.36 ± 1.11 (−58.26%) NPS “Pain intensity” 0–8 week: Gabapentin: 52.1% ± 18.1 Pregabalin: 54.5% ± 17.8 | Poor |
| Kumru et al., 2018 [39] | SCI | Baclofen | 13 | NRS, 0–10 NPSI, 0–10 BPI, 0–10 | NRS, pain: 1, 2, 4, and 8 h from baseline, p < 0.05 * NPSI, continues pain: 4, 8, and 24 h from baseline, p < 0.05 * NPSI, paroxysmal pain: 4 and 8 h from baseline, p < 0.05 * NPSI, allodynia: 4 and 8 h from baseline, p < 0.05 * BPI, max. neuropathic pain: 4, 8, and 24 h, p < 0.05 * BPI, min. neuropathic pain: 4 and 24 h, p < 0.05 * | NRS and nom. % change from baseline 4 h, 8 h, and 24 h: Baclofen: 3.0 ± 2.2 (−51%), 2.6 ± 2.2 (−59%), 4.5 ± 1.9 (−23%) NPSI, continuous pain and nom., 2.0 ± 1.7, 1.9 ± 1.5, 3.0 ± 1.5 NPSI, paroxysmal pain and nom., 0.4 ± 1.0, 0.3 ± 0.6, 1.8 ± 2.6 NPSI, allodynia and nom. % change from baseline, 1.0 ± 1.2, 0.6 ± 1.0, 1.7 ± 1.1 BPI, baclofen % of improvement with treatment, 68.8% ± 23.0, 62.5% ± 30.6, 50.0% ± 25.6 | Fair |
| Kvarnström et al., 2004 [12] | SCI | Ketamine Lidocaine | 10 | VAS, 0–100 mm | Ketamine vs. placebo, p < 0.01 * Lidocaine vs. placebo, p < 0.60 | Mean VAS for all patients before infusion: 4.8 Mean % max pain reduction, ketamine, (−38%) Mean % max. pain reduction, lidocaine, (−10%) Mean % max. pain reduction, placebo, (−3%) | Good |
| Levendoglu et al., 2004 [40] | SCI | Gabapentin | 20 | VAS, 0–100 NPS, 0–10 | p = 0.000–0.001 * | Mean VAS points reduction during 0–8 week, gabapentin group: (60.7 ± 12.7%) Mean VAS points reduction during 0–8 week, placebo group: (10.3 ± 2.8%) Pain intensity, gabapentin group, 0–4–8 week, % change 0–8 week: 8.5 ± 0.9, 4.8 ± 1.1, 3.2 ± 1.2 (61.9 ± 14.3%) Pain intensity, placebo group, 0–4–8 week, % change 0–8 week: 6.0 ± 3.9, 5.3 ± 3.5, 5.5 ± 3.6 (13.2 ± 4.8%) | Good |
| Norrbrink et al., 2009 [41] | SCI | Tramadol | 36 | MPI, 0–6 CR-10, 0–10 | MPI, p < 0.05 * CR-10, p < 0.05 * | Tramadol median, 0–4 week, Present pain: 3, 3 General pain: 4, 3 The Worst pain: 7, 5 MPI-Pain: 3, 2.5 Placebo median, 0–4 week, Present pain: 5, 5.5 General pain: 7, 6.5 The Worst pain: 9, 8 MPI-Pain: 4, 4.13 | Poor |
| Olusanya et al., 2023 [7] | SCI | Capsaicin | 34 | MPI, 0–6 VAS, 0–10 | MPI, 2 weeks, p < 0.05 * MPI, 4 weeks, p < 0.05 * VAS, 2 weeks, p = 0.029 * VAS, 4 weeks, p = 0.049 * | VAS % change, 2nd week: −35% 4th week: −29% | Fair |
| Rintala et al., 2007 [42] | SCI | Amitriptyline Gabapentin | 38 | VAS, 0–100 mm NRS, 0–10 | VAS Amitriptyline vs. placebo from baseline, p = 0.035 * Gabapentin vs. placebo, p = 0.97 Amitriptyline vs. gabapentin, p = 0.061 | Mean decrease in average VAS pain, % change in VAS average pain, 0–8 week: Group 1, minor depression: Amitriptyline −1.58 (−31.5%) Gabapentin −0.84 (−134.9%) Placebo −0.40 (−16.5%) Group 2, major depression: Amitriptyline −3.21 (−40.6%) Gabapentin −0.70 (−11.3%) Placebo −0.74 (−8.7%) | Fair |
| Rintala et al., 2010 [43] | SCI | Dronabinol | 7 | BPI, 0–10 | p = 0.102 | Pain intensity at its worst, 8.1 ± 1.6 Mean change from baseline, dronabinol group, 0.20 ± 0.837 Mean change from baseline, placebo group, −1.80 ± 2.49 | Fair |
| Siddall et al., 2006 [44] | SCI | Pregabalin | 137 | NRS, 0–10 VAS, 0–100 SFMPQ, 0–3 | VAS, p < 0.001 * NRS, p < 0.001 * SFMPQ, p ≤ 0.001–0.002 * | Placebo 0–12 week, NRS: 6.73, 6.27 Sensory: 14.0, 13.3 VAS: 73.1, 68.5 Pregabalin 0–12 week NRS: 6.54, 4.62 Sensory: 13.4, 9.3 VAS: 69.1, 49.2 | Fair |
| Tai et al., 2002 [45] | SCI | Gabapentin | 14 | NPS, 0–10 | 4th week for “unpleasant feeling” p = 0.028 * Rest of NPS, p > 0.05 | Average unpleasantness (0 week), 7.26 Average unpleasantness (4th week), Gabapentin, 3.60; Placebo, 5.33 | Fair |
| Yang et al., 2012 [46] | SCI | Lithium | 40 | VAS, 0–100 | 0–6 months: VAS, p = 0.237–0.97 | VAS Pain, 0–6 weeks, Placebo, −1 ± 3.97 Lithium, 8.833 ± 14.861 VAS Pain, 6 weeks–6 months, Placebo, 0.778 ± 17.176 Lithium, 9.389 ± 15.232 | Fair |
| Yilmaz et al., 2015 [47] | SCI | Gabapentin Pregabalin | 21 | VAS, 0–10 | p > 0.05 | Pain VAS, 1st period, 0–8 weeks Pregabalin 7.05 ± 1.92, 3.83 ± 3.40 Gabapentin 7.02 ± 1.63, 4.77 ± 2.77 Pain VAS, 2nd period, 0–8 weeks Pregabalin 7.42 ± 1.66, 2.53 ± 1.98 Gabapentin 8.60 ± 1.34, 5.00 ± 1.41 | Poor |
| Falah et al., 2012 [48] | MS | Levetiracetam | 37 | 6-point verbal scale | Total patients, p = 0.157–169 | “Total pain” 0–6 week: Levetiracetam, 5.8, 5.3 % change (−8.62%) Placebo: 5.8, 5.7 % change (−1.72%) % difference (7.27%) “Pain relief” 0–6 week: Levetiracetam 2.4, 2.1 % change (12.5%) | Good |
| Foley et al., 2022 [49] | MS | Fluoxetine Riluzole Amiloride | 445 | NPS, 0–10 BPI, 0–10 | NPS, p = 0.14 p = 0.26 p = 0.27 BPI, p = 0.57 p = 0.92 p = 0.18 | NPS pain relief: Fluoxetine–placebo: 0.52 Riluzole–placebo: 0.40 Amiloride–placebo: 0.38 BPI Pain interference: Fluoxetine–placebo: 0.18 Riluzole–placebo: −0.03 Amiloride–placebo: 0.42 | Fair |
| Langford et al., 2013 [2] | MS | Add-on THC/CBD | 393 | NRS, 0–10 SF-36, 0–5 | NRS: p = 0.234 SF-36 “Bodily pain”, p = 0.494 | Bodily pain, THC/CBD, Placebo, % difference 11.36, 10.01, 12.63% | Good |
| Rossi et al., 2009 [50] | MS | Levetiracetam | 20 | VAS, 0–100 mm | Mean pain intensity, p < 0.005 * Mean pain difference, p < 0.005 * % of patients with clinically significant pain reduction p < 0.05 * | Mean pain VAS score Placebo, Levetiracetam T1: −12.5%, −18.2% T2: −12.5%, −72.7% T3: −14.3%, −81.8% | Poor |
| Svendsen et al., 2004 [4] | MS | Dronabinol | 24 | NRS, 0–10 | NRS: p = 0.02–0.039 * SF-36 Bodily pain, p = 0.037 * | Active–placebo, placebo–active Spontaneous pain, −1.0, 0, 0, −1.5 Pain relief, 3.0, 0.5, 0, 4.0 Bodily pain, 41.0, 26.5, 42.0, 61.0 | Good |
| Turcotte et al., 2015 [51] | MS | Nabilone add-on to gabapentin | 15 | VAS, 0–100 mm | VAS pain, p < 0.01 * VAS pain impact, p < 0.01 * | N/A | Fair |
| van Amerongen et al., 2018 [52] | MS | THC | 24 | NRS, 0–10 | p = 0.6581 | NRS Pain, 0–4 week Placebo, 2.57 THC, 2.10 % difference (−20.13%) | Good |
| Vollmer et al. 2014 [53] | MS | Duloxetine | 239 | BPI, 0–10 | p = 0.001 * | Average pain intensity, baseline: Duloxetine, 6.5; Placebo, 6.3 Mean change in weekly API: Duloxetine, −1.83; Placebo, −1.07 % difference (−41.53%) | Fair |
| Jungehulsing et al., 2013 [54] | CPSP | Levetiracetam | 42 | NRS, 0–10 | p > 0.05 | N/A | Fair |
| Mahesh et al., 2023 [5] | CPSP | Duloxetine | 82 | NRS, 0–10 SFMPQ2, 0–10 | NRS, p = 0.002 * SFMPQ2, p = 0.032 * | NRS, 0–4 week and % change Duloxetine group: 6.51 ± 1.03, 3.02 ± 1.70, (−53.61%) Placebo group: 6.37 ± 1.41, 4.40 ± 1.77, (−30.93%) SFMPQ2, 0–4 week, % change, Duloxetine group: 19.53 ± 6.34, 8.85 ± 4.02, (−54.69%) Placebo group: 20.29 ± 7.10,13.29 ± 5.74, (−34.50%) | Good |
| Ralph et al., 2020 [55] | CPSP | Etanercept | 20 | VAS, 0–100 NPRS-FPS, 0–10 | VAS: “Worst pain”, p = 0.01 * “Average pain”, p = 0.029 * NPRS-FPS: p = 0.012 * | NPRS-FPS “Worst pain” Etanercept group Median decrease: 27.5 points Mean decrease: 33.5 ± 10.4 Placebo group Median decrease 17.5 Mean decrease 13.33 ± 6.7 | Good |
| Kalita et al., 2006 [56] | PS-CRPS | Prednisolone Piroxicam | 60 | CRPS, 0–5 | Prednisolone pain reduction against piroxicam p < 0.0001 * | CRPS pain, prednisolone group, 0–1 month: 3.97 ± 0.85, 1.13 ± 1.31 (−71.54%) CRPS pain, piroxicam group, 0–1 month: 4.00 ± 0.87, 3.67 ± 1.35 (−8.25%) | Fair |
| Kalita et al., 2016 [13] | PS-CRPS | Prednisolone | 77 | VAS, 0–10 CRPS, 0–5 | Prednisolone vs. baseline 0–1 month VAS, p < 0.001 * CRPS, p < 0.001 * Prednisolone vs. placebo, 0–1 month VAS, p > 0.05 CRPS, p < 0.01 * | VAS, 0–1 month, nom. % change Prednisolone continued group, 3.6 ± 1.1, 2.4 ± 1.0, (−33.33%) Prednisolone discontinued group, 3.5 ± 1.0, 4.9 ± 2.1, (−40.00%) CRPS “pain score” 0–1 month, nom. % change: Prednisolone-continued group, 4.3 ± 0.9, 0.8 ± 0.5, (−81.40%) Prednisolone-discontinued group, 4.6 ± 1.5, 2.1 ± 1.2, (−54.35%) | Poor |
| Iwaki et al., 2020 [57] | Parkinson’s disease | Duloxetine | 47 | VAS, 0–100 mm | p > 0.05 | Mean change, % change from baseline Duloxetine, −0.83, (−2.28%) Placebo, −1.91, (−4.99%) | Fair |
| Hansen et al., 2023 [58] | SCI MS | THC CBD THC+CBD | 134 | NPSI, 0–10 | p > 0.05 | Difference from baseline, NPSI, total difference: THC, −1.3 CBD, −1.5 THC+CBD, −0.8 | Fair |
| Vranken et al., 2011 [6] | SCI Stroke | Duloxetine | 48 | VAS, 0–10 SF-36, 0–5 PDI, 0–10 | VAS: p = 0.056 SF-36, bodily pain: p = 0.035 * PDI: p = 0.063 | VAS mean pain intensity, 0–8 weeks: Placebo, 7.2 ± 0.8, 6.1 ± 1.7 (−15%) Duloxetine, 7.1 ± 0.8, 5.0 ± 2.0 (−29.6%) SF-36, 0–8 weeks: Placebo, 31 ± 12, 35 ± 14 Duloxetine, 33 ± 13, 45 ± 17 PDI, 0–8 weeks: Placebo, 38 ± 14.3, 36 ± 13.3 Duloxetine, 33 ± 11.2, 28 ± 12.2 | Good |
| Vranken et al., 2005 [1] | Stroke MS Parkinson’s disease Thalamus lesion Brainstem pathology SCI | Ketamine | 33 | VAS, 0–10 SF-36, 0–5 PDI, 0–10 | VAS, p > 0.05 SF-36, bodily pain 75 mg/day after 7 days of treatment, p = 0.025 * PDI, 75 mg/day after 7 days of treatment, p = 0.025 * | VAS mean pain intensity, 0–1 week: Placebo, 7.1, 6.4, (−9.86%) Ketamine 50 mg, 7.3, 6.2, (−15.07%) Ketamine 75 mg, 7.3, 5.7, (−21.92%) SF-36, 0–1 week: Placebo, 16.5 ± 14.2, 22.4 ± 16.2 Ketamine 50 mg, 14.5 ± 13.4, 34.4 ± 11.7 Ketamine 75 mg, 13.1 ± 9.6, 66.6 ± 13.1 PDI, 0–1 week: Placebo, 43.6 ± 10.2, 42.7 ± 11.1 Ketamine 50 mg, 45.5 ± 11.9, 45.0 ± 13.2 Ketamine 75 mg, 45.6 ± 5.7, 19.5 ± 12.9 | Good |
| Vranken et al., 2008 [8] | Stroke Thalamus lesion Brainstem pathology SCI | Pregabalin | 40 | VAS, 0–10 SF36, 0–5 PDI, 0–10 | From baseline: VAS, p = 0.01–0.016 * SF-36, bodily pain, p = 0.009 * PDI, p = 0.111 | VAS mean pain intensity, 0–4 weeks: Placebo, 7.4 ± 1.0, 7.3 ± 2.0, (−1.35%) Pregabalin, 7.6 ± 0.8, 5.1 ± 2.9, (−32.90%) SF-36, 0–4 weeks: Placebo, 26.2 ± 15.4, 27.8 ± 19.4 Pregabalin, 30.7 ± 16.1, 46.3 ± 20.2 PDI, 0–4 weeks: Placebo, 41.7 ± 14.8, 43.3 ± 14.7 Duloxetine, 39.9 ± 13.2, 35.7 ± 14.9 | Good |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Houlind, B.K.; Jensen, H.B. Medications for Managing Central Neuropathic Pain as a Result of Underlying Conditions—A Systematic Review. Neurol. Int. 2025, 17, 77. https://doi.org/10.3390/neurolint17050077
Houlind BK, Jensen HB. Medications for Managing Central Neuropathic Pain as a Result of Underlying Conditions—A Systematic Review. Neurology International. 2025; 17(5):77. https://doi.org/10.3390/neurolint17050077
Chicago/Turabian StyleHoulind, Bjarke Kaae, and Henrik Boye Jensen. 2025. "Medications for Managing Central Neuropathic Pain as a Result of Underlying Conditions—A Systematic Review" Neurology International 17, no. 5: 77. https://doi.org/10.3390/neurolint17050077
APA StyleHoulind, B. K., & Jensen, H. B. (2025). Medications for Managing Central Neuropathic Pain as a Result of Underlying Conditions—A Systematic Review. Neurology International, 17(5), 77. https://doi.org/10.3390/neurolint17050077







