Cardiac Autonomic Modulation and Cognitive Performance in Community-Dwelling Older Adults: A Preliminary Study
Abstract
1. Introduction
2. Materials and Methods
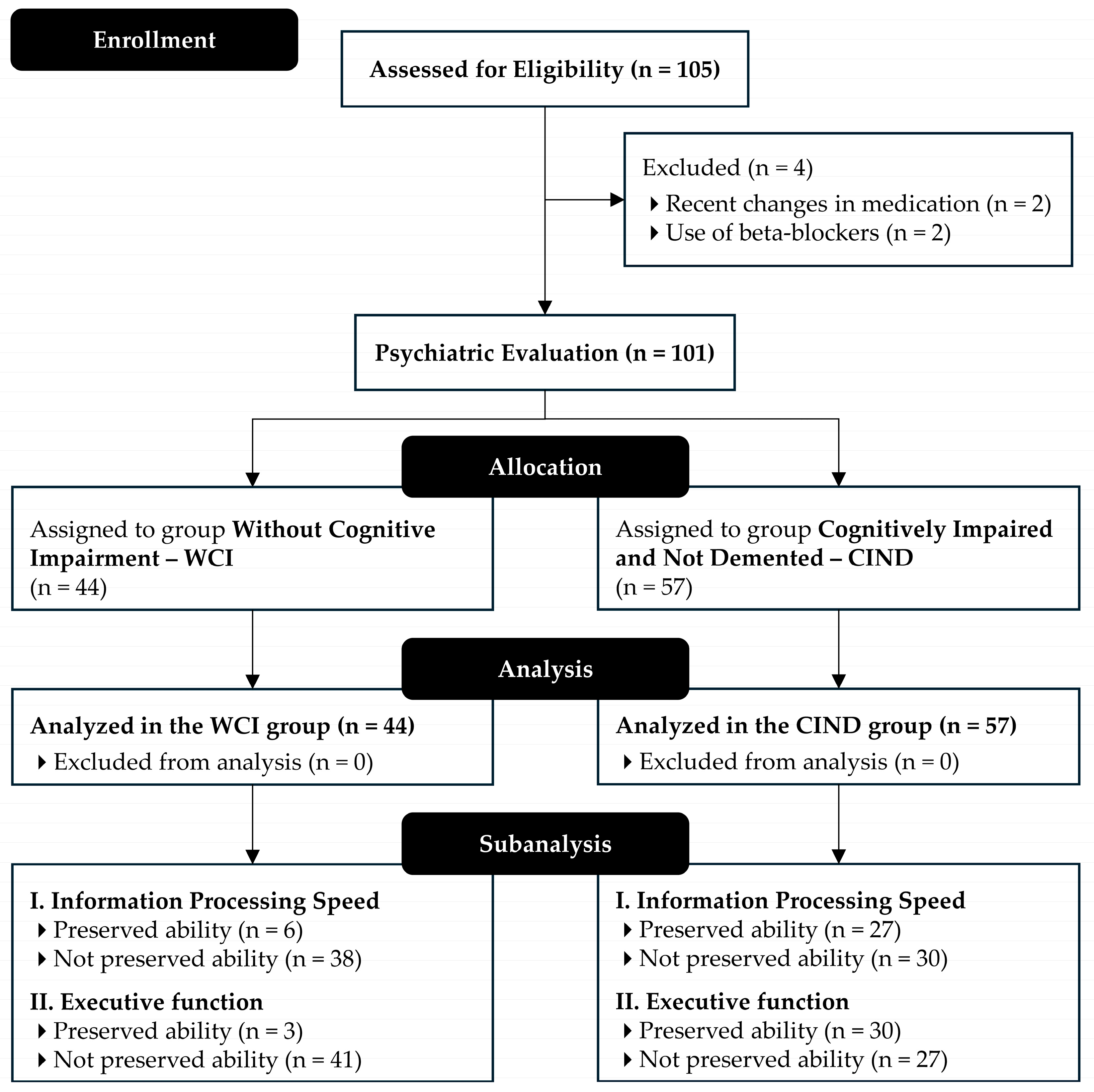
2.1. Study Design and Participants
2.2. Procedures
2.3. Haemodynamic Parameters and Cardiac Autonomic Function Assessments
2.4. Anthropometric Measurements
2.5. Autonomic Nervous System Dysfunction
2.6. Assessment of Activities of Daily Living and Instrumental Activities of Daily Living
2.7. Assessments of Information Processing Speed and Executive Function
2.8. Statistical Analysis
3. Results
3.1. Sample Characteristics
3.2. Haemodynamic Parameters
3.3. Heart Rate Variability
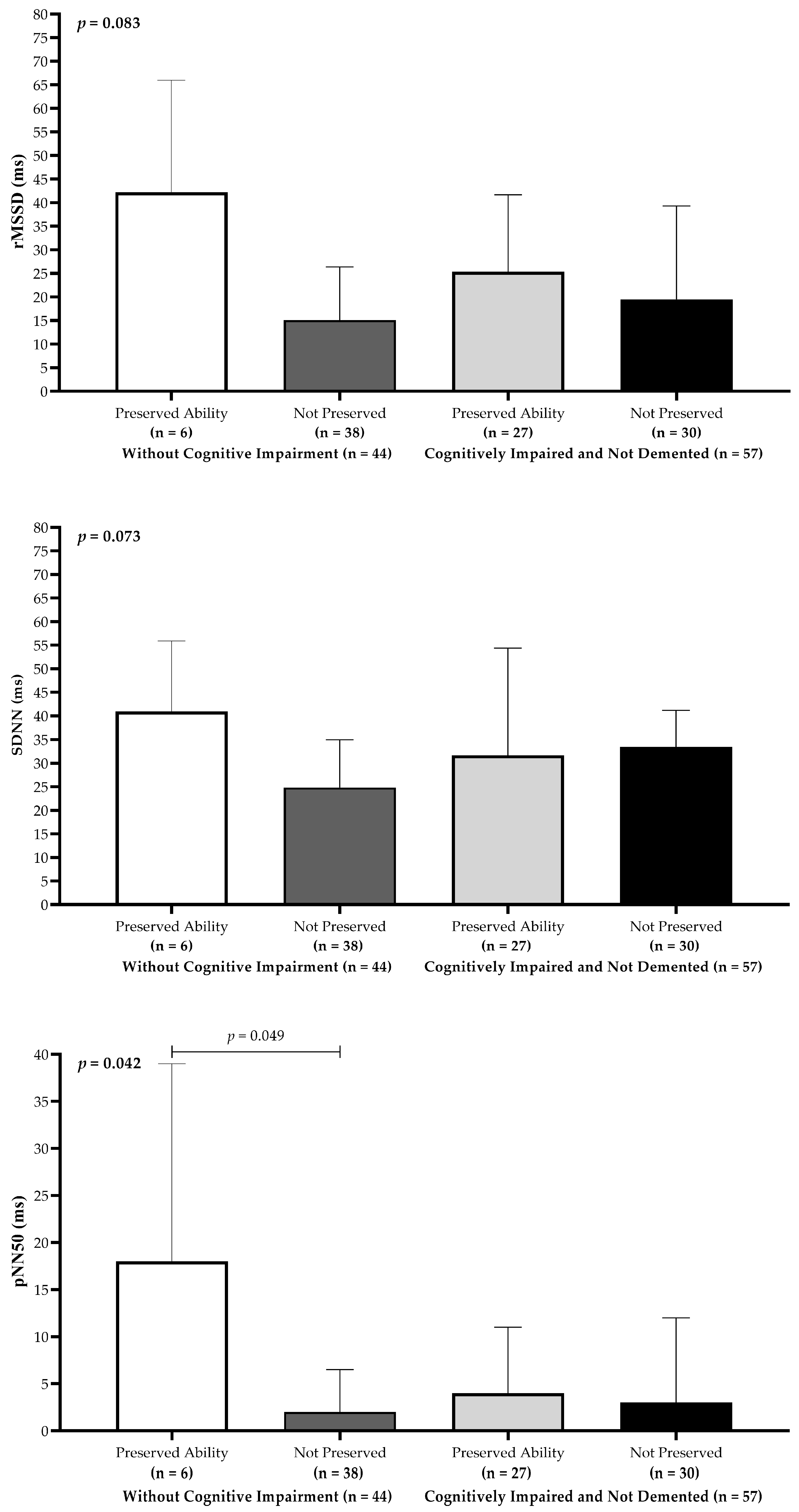
3.4. Heart Rate Variability and Cognitive Performance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 1Q | first quartile |
| 3Q | third quartile |
| ADLs | activities of daily living |
| ANCOVA | analysis of covariance |
| ANS | autonomic nervous system |
| BEI | baroreflex entropy index |
| BMI | body mass index |
| BP | blood pressure |
| CI | confidence interval |
| CIND | cognitively impaired and not demented |
| DBP | diastolic blood pressure |
| EBSERH | Brazilian Hospital Services Company |
| ECG | electrocardiogram |
| HF | high frequency |
| HRV | heart rate variability |
| HRVB | heart rate variability biofeedback |
| HU | Dr. Washington Antônio de Barros Teaching Hospital |
| IADLs | instrumental activities of daily living |
| ICF | informed consent form |
| LF | low frequency |
| MCI | mild cognitive impairment |
| MMSE | mini-mental state examination |
| η2p | partial eta squared |
| nVNS | non-invasive vagus nerve stimulation |
| OH | orthostatic hypotension |
| pNN50 | percentage of successive R–R intervals that differ by more than 50 milliseconds |
| rMSSD | root mean square of successive R–R interval differences |
| SAH | systemic arterial hypertension |
| SBP | systolic blood pressure |
| SDNN | standard deviation of normal-to-normal intervals |
| T2DM | type 2 diabetes mellitus |
| TBM | total body mass |
| TMT-A | trail making test part A |
| TMT-B | trail making test part B |
| UNIVASF | Federal University of Vale do São Francisco |
| WCI | without cognitive impairment |
References
- Livingston, G.; Huntley, J.; Sommerlad, A.; Ames, D.; Ballard, C.; Banerjee, S.; Brayne, C.; Burns, A.; Cohen-Mansfield, J.; Cooper, C.; et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020, 396, 413–446. [Google Scholar] [CrossRef] [PubMed]
- Nelson, M.E.; Jester, D.J.; Petkus, A.J.; Andel, R. Cognitive reserve, Alzheimer’s neuropathology, and risk of dementia: A systematic review and meta-analysis. Neuropsychol. Rev. 2021, 31, 233–250. [Google Scholar] [CrossRef]
- Jack, C.R.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J.; et al. NIA-AA Research framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018, 14, 535–562. [Google Scholar] [CrossRef] [PubMed]
- Zulli, R.; Nicosia, F.; Borroni, B.; Agosti, C.; Prometti, P.; Donati, P.; De Vecchi, M.; Romanelli, G.; Grassi, V.; Padovani, A. QT Dispersion and heart rate variability abnormalities in Alzheimer’s disease and in mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 2135–2139. [Google Scholar] [CrossRef] [PubMed]
- Frewen, J.; Finucane, C.; Savva, G.M.; Boyle, G.; Coen, R.F.; Kenny, R.A. Cognitive function is associated with impaired heart rate variability in ageing adults: The Irish longitudinal study on ageing wave one results. Clin. Auton. Res. 2013, 23, 313–323. [Google Scholar] [CrossRef]
- Ramachandran, M.; Priyadarsini, N.; Kar, M.; Behera, K.K. Impact of cardiac autonomic dysfunction on cognitive event-related potential in type 2 diabetes mellitus patients: A cross-sectional study. Indian J. Endocrinol. Metab. 2023, 27, 506–512. [Google Scholar] [CrossRef]
- Shaffer, F.; McCraty, R.; Zerr, C.L. A Healthy Heart Is Not a Metronome: An Integrative Review of the Heart’s Anatomy and Heart Rate Variability. Front. Psychol. 2014, 5, 1040. [Google Scholar] [CrossRef]
- Alrosan, A.Z.; Heilat, G.B.; Alrosan, K.; Aleikish, A.A.; Rabbaa, A.N.; Shakhatreh, A.M.; Alshalout, E.M.; Al Momany, E.M.A. Autonomic brain functioning and age-related health concerns. Curr. Res. Physiol. 2024, 7, 100123. [Google Scholar] [CrossRef]
- Kim, D.; Lee, J.; Choi, J.Y.; Lee, H.J.; Min, J.Y.; Min, K.B. Association between health-related quality of life and heart rate variability in elderly individuals with cognitive impairment in Korea: Cross-sectional study. BMC Geriatr. 2023, 23, 847. [Google Scholar] [CrossRef]
- Collins, O.; Dillon, S.; Finucane, C.; Lawlor, B.; Kenny, R.A. Parasympathetic autonomic dysfunction is common in mild cognitive impairment. Neurobiol. Aging 2012, 33, 2324–2333. [Google Scholar] [CrossRef]
- Galluzzi, S.; Nicosia, F.; Geroldi, C.; Alicandri, A.; Bonetti, M.; Romanelli, G.; Zulli, R.; Frisoni, G.B. Cardiac autonomic dysfunction is associated with white matter lesions in patients with mild cognitive impairment. J. Gerontol. A Biol. Sci. Med. Sci. 2009, 64, 1312–1315. [Google Scholar] [CrossRef]
- Jandackova, V.K.; Scholes, S.; Britton, A.; Steptoe, A. Are changes in heart rate variability in middle-aged and older people normative or caused by pathological conditions? Findings from a large population-based longitudinal cohort study. J. Am. Heart Assoc. 2016, 5, e002365. [Google Scholar] [CrossRef]
- McIntosh, R.C.; Khambaty, T.; Llabre, M.M.; Perreira, K.M.; Gonzalez, H.M.; Kansal, M.M.; Tarraf, W.; Schneiderman, N. Paradoxical effect of cumulative stress exposure on information processing speed in Hispanics/Latinos with elevated heart rate variability. Int. J. Psychophysiol. 2021, 164, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Salthouse, T. Consequences of age-related cognitive declines. Annu. Rev. Psychol. 2012, 63, 201–226. [Google Scholar] [CrossRef]
- Diamond, A. Executive functions. Annu. Rev. Psychol. 2013, 64, 135–168. [Google Scholar] [CrossRef] [PubMed]
- Pettigrew, C.; Soldan, A. Defining cognitive reserve and implications for cognitive aging. Curr. Neurol. Neurosci. Rep. 2019, 19, 1. [Google Scholar] [CrossRef] [PubMed]
- Nicolini, P.; Ciulla, M.M.; Asmundis, C.D.E.; Magrini, F.; Brugada, P. The prognostic value of heart rate variability in the elderly, changing the perspective: From sympathovagal balance to chaos theory. Pacing Clin. Electrophysiol. 2012, 35, 621–637. [Google Scholar] [CrossRef]
- Schaich, C.L.; Malaver, D.; Chen, H.; Shaltout, H.A.; Zeki Al Hazzouri, A.; Herrington, D.M.; Hughes, T.M. Association of heart rate variability with cognitive performance: The multi-ethnic study of atherosclerosis. J. Am. Heart Assoc. 2020, 9, e013827. [Google Scholar] [CrossRef]
- Malta, M.; Cardoso, L.O.; Bastos, F.I.; Magnanini, M.M.F.; da Silva, C.M.F.P. STROBE initiative: Guidelines on reporting observational studies. Rev. Saude Publica 2010, 44, 559–565. [Google Scholar] [CrossRef]
- Vandenbroucke, J.P.; von Elm, E.; Altman, D.G.; Gøtzsche, P.C.; Mulrow, C.D.; Pocock, S.J.; Poole, C.; Schlesselman, J.J.; Egger, M.; Blettner, M.; et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and elaboration. Int. J. Surg. 2014, 12, 1500–1524. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef]
- Chen, X. Exact computation of minimum sample size for estimating proportion of finite population. arXiv 2007, arXiv:0707.2115. [Google Scholar]
- Upton, J. Beck depression inventory (BDI). In Encyclopedia of Behavioral Medicine; Gellman, M.D., Turner, J.R., Eds.; Springer: New York, NY, USA, 2013; pp. 178–179. [Google Scholar] [CrossRef]
- Brucki, S.M.D.; Nitrin, R.; Caramelli, P.; Bertolucci, P.H.F.; Okamoto, I.H. Suggestions for utilization of the mini-mental state examination in Brazil. Arq. Neuropsiquiatr. 2003, 61, 777–781. [Google Scholar] [CrossRef]
- Stewart, A.; Marfell-Jones, M.; Olds, T.; De Ridder, H. International Standards for Anthropometric Assessment, 3rd ed.; International Society for the Advancement of Kinanthropometry: Lower Hutt, New Zealand, 2011. [Google Scholar]
- Barroso, W.K.S.; Rodrigues, C.I.S.; Bortolotto, L.A.; Mota-Gomes, M.A.; Brandão, A.A.; de Magalhães Feitosa, A.D.; Machado, C.A.; Poli-de-Figueiredo, C.E.; Amodeo, C.; Mion Júnior, D.; et al. Brazilian Guidelines of Hypertension-2020. Arq. Bras. Cardiol. 2021, 116, 516–658. [Google Scholar] [CrossRef] [PubMed]
- Feitosa, A.D.D.M.; Barroso, W.K.S.; Mion Junior, D.; Nobre, F.; Mota-Gomes, M.A.; Jardim, P.C.B.V.; Amodeo, C.; Oliveira, A.C.; Alessi, A.; Sousa, A.L.L.; et al. Brazilian Guidelines for In-Office and Out-of-Office Blood Pressure Measurement-2023. Arq. Bras. Cardiol. 2024, 121, e20240113. [Google Scholar] [CrossRef] [PubMed]
- Gambassi, B.B.; Neves, V.R.; Brito, E.Z.A.; da Silva Fernandes, D.S.; Sá, C.A.; da Rocha Nogueira, R.M.; de Jesus Furtado Almeida, F.; de Araújo Cavalcanti, P.A.; Gomes Gonçalves E Silva, D.C.; Neto, D.S.; et al. A validation study of a smartphone application for heart rate variability assessment in asymptomatic adults. Am. J. Cardiovasc. Dis. 2020, 10, 219–229. [Google Scholar] [PubMed]
- Neves, V.R.; Takahashi, A.C.M.; Do Santos-Hiss, M.D.B.; Kiviniemi, A.M.; Tulppo, M.P.; De Moura, S.C.G.; Karsten, M.; Borghi-Silva, A.; Porta, A.; Montano, N.; et al. Linear and Nonlinear Analysis of Heart Rate Variability in Coronary Disease. Clin. Auton. Res. 2012, 22, 175–183. [Google Scholar] [CrossRef]
- Heart Rate Variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur. Heart J. 1996, 17, 354–381. [Google Scholar] [PubMed]
- Freeman, R.; Wieling, W.; Axelrod, F.B.; Benditt, D.G.; Benarroch, E.; Biaggioni, I.; Cheshire, W.P.; Chelimsky, T.; Cortelli, P.; Gibbons, C.H.; et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin. Auton. Res. 2011, 21, 69–72. [Google Scholar] [CrossRef]
- Katz, S.; Ford, A.B.; Moskowitz, R.W.; Jackson, B.A.; Jaffe, M.W. Studies of illness in the aged. The index of ADL: A standardized measure of biological and psychosocial function. JAMA 1963, 185, 914–919. [Google Scholar] [CrossRef]
- Lawton, M.P.; Brody, E.M. Assessment of older people: Self-maintaining and instrumental activities of daily living. Gerontologist 1969, 9, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Brasil; Ministério da Saúde; Secretaria de Atenção à Saúde; Departamento de Atenção Básica. Ageing and Health of the Elderly Person; Ministério da Saúde: Brasília, Brazil, 2006.
- Ashendorf, L.; Jefferson, A.L.; O’Connor, M.K.; Chaisson, C.; Green, R.C.; Stern, R.A. Trail Making Test errors in normal aging, mild cognitive impairment, and dementia. Arch. Clin. Neuropsychol. 2008, 23, 129–137. [Google Scholar] [CrossRef]
- Tombaugh, T.N. Trail Making Test A and B: Normative Data Stratified by Age and Education. Arch. Clin. Neuropsychol. 2004, 19, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Erceg-Hurn, D.M.; Mirosevich, V.M. Modern robust statistical methods: An easy way to maximize the accuracy and power of your research. Am. Psychol. 2008, 63, 591–601. [Google Scholar] [CrossRef]
- Grissom, R.J. Probability of the superior outcome of one treatment over another. J. Appl. Psychol. 1994, 79, 314–316. [Google Scholar] [CrossRef]
- Grässler, B.; Dordevic, M.; Darius, S.; Herold, F.; Forte, G.; Langhans, C.; Halfpaap, N.; Müller, P.; Glanz, W.; Dantas, E.H.M.; et al. Is there a link between heart rate variability and cognitive decline? A cross-sectional study on patients with mild cognitive impairment and cognitively healthy controls. Arq. Neuropsiquiatr. 2023, 81, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Stenfors, C.U.D.; Hanson, L.M.; Theorell, T.; Osika, W.S. Executive cognitive functioning and cardiovascular autonomic regulation in a population-based sample of working adults. Front. Psychol. 2016, 7, 1536. [Google Scholar] [CrossRef]
- Lane, R.D.; McRae, K.; Reiman, E.M.; Chen, K.; Ahern, G.L.; Thayer, J.F. Neural correlates of heart rate variability during emotion. Neuroimage 2009, 44, 213–222. [Google Scholar] [CrossRef]
- Rocha, A.S.L.; Siqueira, V.D.B.; Maduro, P.A.; Batista, L.D.S.P.; Schwingel, P.A. Reference values for heart rate variability in older adults: A systematic review. Psychophysiology 2024, 61, e14661. [Google Scholar] [CrossRef]
- Alharbi, E.A.; Jones, J.M.; Alomainy, A. Non-invasive solutions to identify distinctions between healthy and mild cognitive impairments participants. IEEE J. Transl. Eng. Health Med. 2022, 10, 2700206. [Google Scholar] [CrossRef]
- Nicolini, P.; Ciulla, M.M.; Malfatto, G.; Abbate, C.; Mari, D.; Rossi, P.D.; Pettenuzzo, E.; Magrini, F.; Consonni, D.; Lombardi, F. Autonomic dysfunction in mild cognitive impairment: Evidence from power spectral analysis of heart rate variability in a cross-sectional case-control study. PLoS ONE 2014, 9, e96656. [Google Scholar] [CrossRef]
- Thayer, J.F.; Lane, R.D. Claude Bernard and the heart-brain connection: Further elaboration of a model of neurovisceral integration. Neurosci. Biobehav. Rev. 2009, 33, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Czopek-Rowinska, J.; de Bruin, E.D.; Manser, P. Diagnostic accuracy of heart rate variability as a screening tool for mild neurocognitive disorder. Front. Aging Neurosci. 2024, 16, 1498687. [Google Scholar] [CrossRef]
- El-Kotob, R.; Craven, B.C.; Mathur, S.; Ditor, D.S.; Oh, P.; Miyatani, M.; Verrier, M.C. Assessing heart rate variability as a surrogate measure of cardiac autonomic function in chronic traumatic spinal cord injury. Top. Spinal Cord. Inj. Rehabil. 2018, 24, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Zulfiqar, U.; Jurivich, D.A.; Gao, W.; Singer, D.H. Relation of high heart rate variability to healthy longevity. Am. J. Cardiol. 2010, 105, 1181–1185. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Liao, X.; Lin, Y.; Chen, J.; Wu, H. Lead II electrocardiograph-derived entropy index for autonomic function assessment in type 2 diabetes mellitus. Biocybern. Biomed. Eng. 2024, 44, 513–520. [Google Scholar] [CrossRef]
- Terreros-Roncal, J.; Moreno-Jiménez, E.P.; Flor-García, M.; Rodríguez-Moreno, C.B.; Trinchero, M.F.; Cafini, F.; Rábano, A.; Llorens-Martín, M. Impact of neurodegenerative diseases on human adult hippocampal neurogenesis. Science 2021, 374, 1106–1113. [Google Scholar] [CrossRef]
- Gunning-Dixon, F.M.; Raz, N. The cognitive correlates of white matter abnormalities in normal aging: A quantitative review. Neuropsychology 2000, 14, 224–232. [Google Scholar] [CrossRef]
- Shaffer, F.; Ginsberg, J.P. An overview of heart rate variability metrics and norms. Front. Public Health 2017, 5, 258. [Google Scholar] [CrossRef]
- Kim, H.G.; Cheon, E.J.; Bai, D.S.; Lee, Y.H.; Koo, B.H. Stress and heart rate variability: A meta-analysis and review of the literature. Psychiatry Investig. 2018, 15, 235–245. [Google Scholar] [CrossRef]
- Sessa, F.; Anna, V.; Messina, G.; Cibelli, G.; Monda, V.; Marsala, G.; Ruberto, M.; Biondi, A.; Cascio, O.; Bertozzi, G.; et al. Heart rate variability as predictive factor for sudden cardiac death. Aging 2018, 10, 166–177. [Google Scholar] [CrossRef] [PubMed]
- Burger, A.M.; D’Agostini, M.; Verkuil, B.; Van Diest, I. Moving beyond Belief: Moving beyond belief: A narrative review of potential biomarkers for transcutaneous vagus nerve stimulation. Psychophysiology 2020, 57, e13571. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, F.; Meehan, Z.M. A Practical guide to resonance frequency assessment for heart rate variability biofeedback. Front. Neurosci. 2020, 14, 570400. [Google Scholar] [CrossRef]
- De Couck, M.; Caers, R.; Spiegel, D.; Gidron, Y. The role of the vagus nerve in cancer prognosis: A systematic and a comprehensive review. J. Oncol. 2018, 2018, 1236787. [Google Scholar] [CrossRef] [PubMed]

| Variables | Total (n = 101) | WCI (n = 44) | CIND (n = 57) | p |
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | ||
| Age, years | 69.1 ± 6.6 | 66.2 ± 4.0 | 71.4 ± 7.4 | <0.001 |
| Female sex, n (%) | 68 (67.3) | 31 (70.5) | 37 (64.9) | 0.556 |
| Schooling, years | 8.3 ± 3.3 | 9.6 ± 3.1 | 7.4 ± 3.1 | 0.001 |
| Mini-mental state examination, n | 24.1 ± 4.0 | 26.6 ± 2.3 | 22.3 ± 4.1 | <0.001 |
| Total body mass, kg | 70.6 ± 15.2 | 72.9 ± 15.9 | 68.8 ± 14.5 | 0.184 |
| Height, cm | 157.2 ± 7.6 | 159.9 ± 6.6 | 155.8 ± 8.0 | 0.033 |
| Body mass index, kg/m2 | 28.5 ± 5.4 | 28.7 ± 5.6 | 28.3 ± 5.3 | 0.682 |
| Obesity, n (%) | 56 (55.4) | 26 (59.1) | 30 (52.6) | 0.517 |
| Alcohol use, n (%) | 9 (8.9) | 5 (11.4) | 4 (7.0) | 0.447 |
| Tobacco, n (%) | 6 (5.9) | 1 (2.3) | 5 (8.8) | 0.171 |
| Coronavirus infection, n (%) | 23 (22.8) | 13 (29.5) | 10 (17.5) | 0.154 |
| Systemic arterial hypertension, n (%) | 73 (72.3) | 31 (70.5) | 42 (73.7) | 0.719 |
| Activities of daily living (ADLs), n | 5.1 ± 1.1 | 6.0 ± 0.1 | 4.3 ± 1.0 | <0.001 |
| Instrumental ADLs, n | 23.5 ± 3.4 | 25.8 ± 0.8 | 21.7 ± 3.6 | <0.001 |
| Information processing speed, seconds | 71.8 ± 47.6 | 49.7 ± 18.3 | 88.8 ± 55.7 | <0.001 |
| Executive function, seconds | 225.1 ± 117.1 | 147.3 ± 63.2 | 285.2 ± 114.0 | <0.001 |
| Variables | Total (n = 101) | WCI (n = 44) | CIND (n = 57) | p | d (95% CI) |
|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | |||
| Resting heart rate, bpm | 64.4 ± 11.1 | 65.5 ± 10.9 | 63.5 ± 11.3 | 0.378 | 0.18 (−0.22–0.57) |
| Systolic blood pressure, mmHg | 115.1 ± 12.8 | 114.8 ± 14.2 | 115.3 ± 11.9 | 0.828 | 0.04 (−0.44–0.35) |
| Diastolic blood pressure, mmHg | 73.8 ± 8.0 | 74.2 ± 8.3 | 73.5 ± 7.8 | 0.667 | 0.09 (−0.31–0.48) |
| Orthostatic hypotension, n (%) | 40 (39.6) | 18 (40.9) | 22 (38.6) | 0.814 | 0.03 (−0.43–0.34) |
| Variables | Total (n = 101) | WCI (n = 44) | CIND (n = 57) | p | d (95% CI) |
|---|---|---|---|---|---|
| (1Q–3Q) | (1Q–3Q) | (1Q–3Q) | |||
| Mean R–R interval, ms | 849.3 (742.4–945.1) | 856.4 (737.6–954.2) | 833.0 (749.3–939.3) | 0.795 | 0.05 (−0.32–0.46) |
| SDNN, ms | 20.4 (12.0–37.0) | 21.2 (14.6–41.5) | 15.6 (9.3–28.9) | 0.035 | 0.44 (0.04–0.83) |
| rMSSD, ms | 30.7 (21.4–41.5) | 32.6 (25.5–41.8) | 26.2 (18.0–40.6) | 0.049 | 0.39 (0.01–0.79) |
| pNN50, % | 3.0 (2.0–9.5) | 4.0 (2.5–10.0) | 2.5 (2.0–9.5) | 0.047 | 0.40 (0.01–0.80) |
| Low frequency (LF), ms2 | 106.0 (56.9–197.2) | 109.1 (62.8–208.0) | 100.2 (49.8–165.1) | 0.515 | 0.13 (−0.26–0.53) |
| High frequency (HF), ms2 | 80.4 (36.0–165.8) | 87.3 (44.0–180.6) | 68.9 (30.8–163.4) | 0.411 | 0.16 (−0.23–0.56) |
| LF/HF ratio, % | 1.3 (0.7–2.7) | 1.2 (0.6–2.4) | 1.4 (0.7–2.9) | 0.547 | 0.12 (−0.25–0.54) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maduro, P.A.; Maduro, L.A.R.; Lima, P.E.; Silva, A.C.C.; Silva, R.d.C.M.d.; Rocha, A.S.L.; Ribeiro, M.J.S.; Matoso, J.M.D.; Bavaresco Gambassi, B.; Schwingel, P.A. Cardiac Autonomic Modulation and Cognitive Performance in Community-Dwelling Older Adults: A Preliminary Study. Neurol. Int. 2025, 17, 74. https://doi.org/10.3390/neurolint17050074
Maduro PA, Maduro LAR, Lima PE, Silva ACC, Silva RdCMd, Rocha ASL, Ribeiro MJS, Matoso JMD, Bavaresco Gambassi B, Schwingel PA. Cardiac Autonomic Modulation and Cognitive Performance in Community-Dwelling Older Adults: A Preliminary Study. Neurology International. 2025; 17(5):74. https://doi.org/10.3390/neurolint17050074
Chicago/Turabian StyleMaduro, Paula Andreatta, Luiz Alcides Ramires Maduro, Polyana Evangelista Lima, Ana Clara Castro Silva, Rita de Cássia Montenegro da Silva, Alaine Souza Lima Rocha, Maria Jacqueline Silva Ribeiro, Juliana Magalhães Duarte Matoso, Bruno Bavaresco Gambassi, and Paulo Adriano Schwingel. 2025. "Cardiac Autonomic Modulation and Cognitive Performance in Community-Dwelling Older Adults: A Preliminary Study" Neurology International 17, no. 5: 74. https://doi.org/10.3390/neurolint17050074
APA StyleMaduro, P. A., Maduro, L. A. R., Lima, P. E., Silva, A. C. C., Silva, R. d. C. M. d., Rocha, A. S. L., Ribeiro, M. J. S., Matoso, J. M. D., Bavaresco Gambassi, B., & Schwingel, P. A. (2025). Cardiac Autonomic Modulation and Cognitive Performance in Community-Dwelling Older Adults: A Preliminary Study. Neurology International, 17(5), 74. https://doi.org/10.3390/neurolint17050074







