Therapeutic Role of Heterocyclic Compounds in Neurodegenerative Diseases: Insights from Alzheimer’s and Parkinson’s Diseases
Abstract
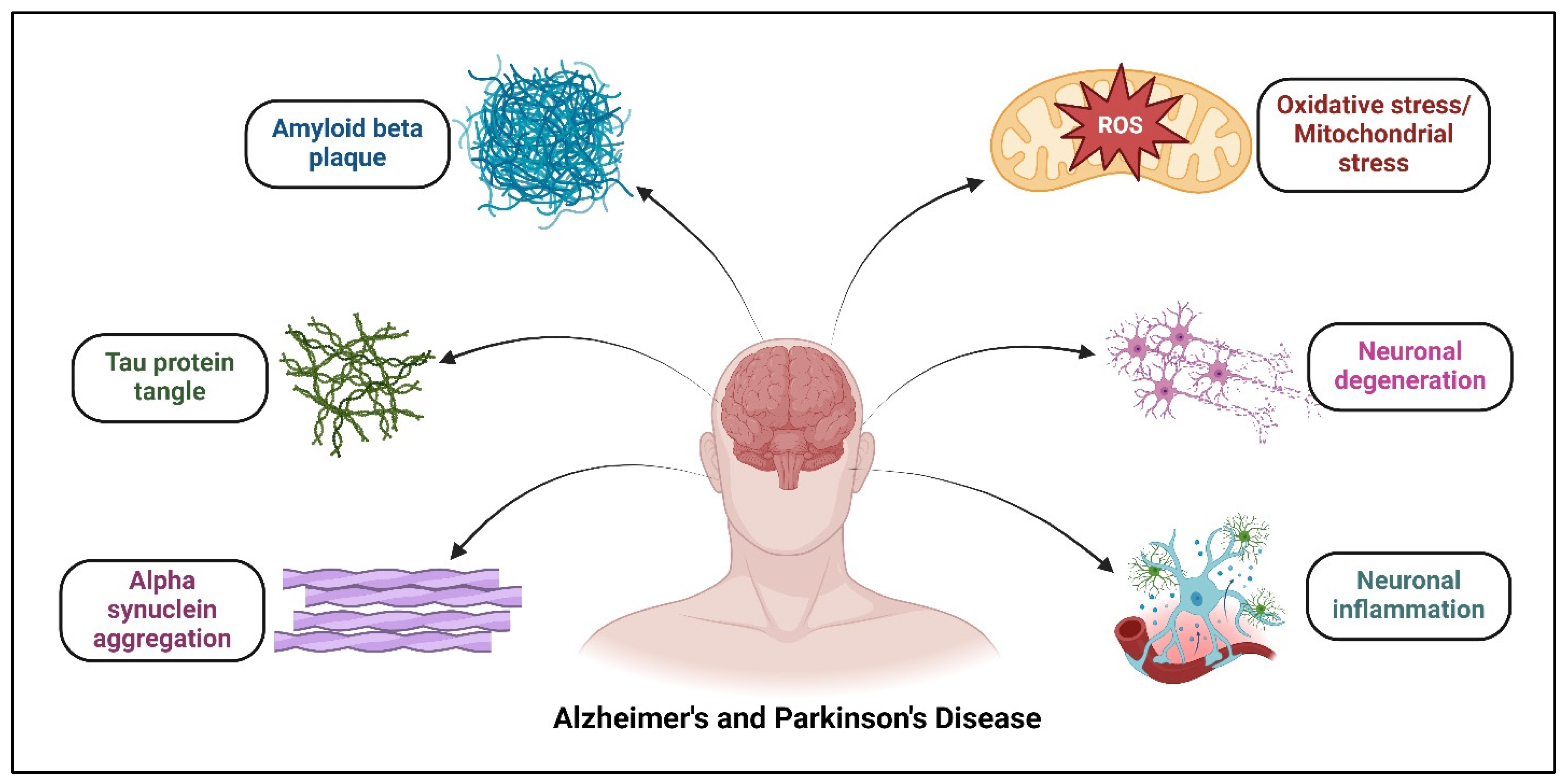
1. Introduction
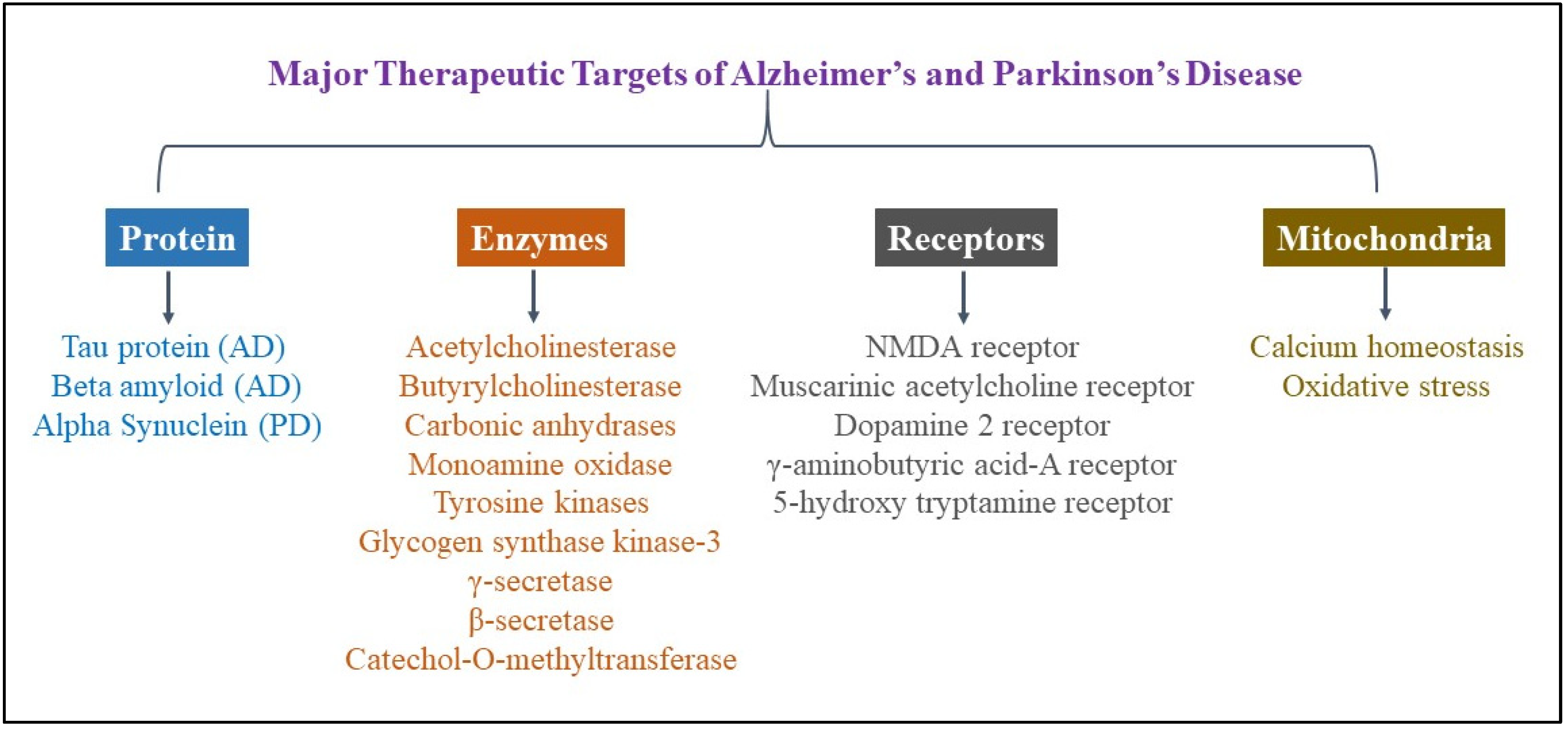
2. Therapeutic Targets in Alzheimer’s and Parkinson’s Disease
2.1. Proteins
2.1.1. Aggregated Protein
2.1.2. Dopamine Transporter (DAT)
2.2. Enzymes
2.2.1. Cholinesterase Enzymes
2.2.2. Carbonic Anhydrases (CAs)
2.2.3. Monoamine Oxidase Enzyme (MAO)
2.2.4. Catechol-O-Methyltransferase (COMT) Inhibitors
2.3. Mitochondrial Calcium Homeostasis and Oxidative Stress
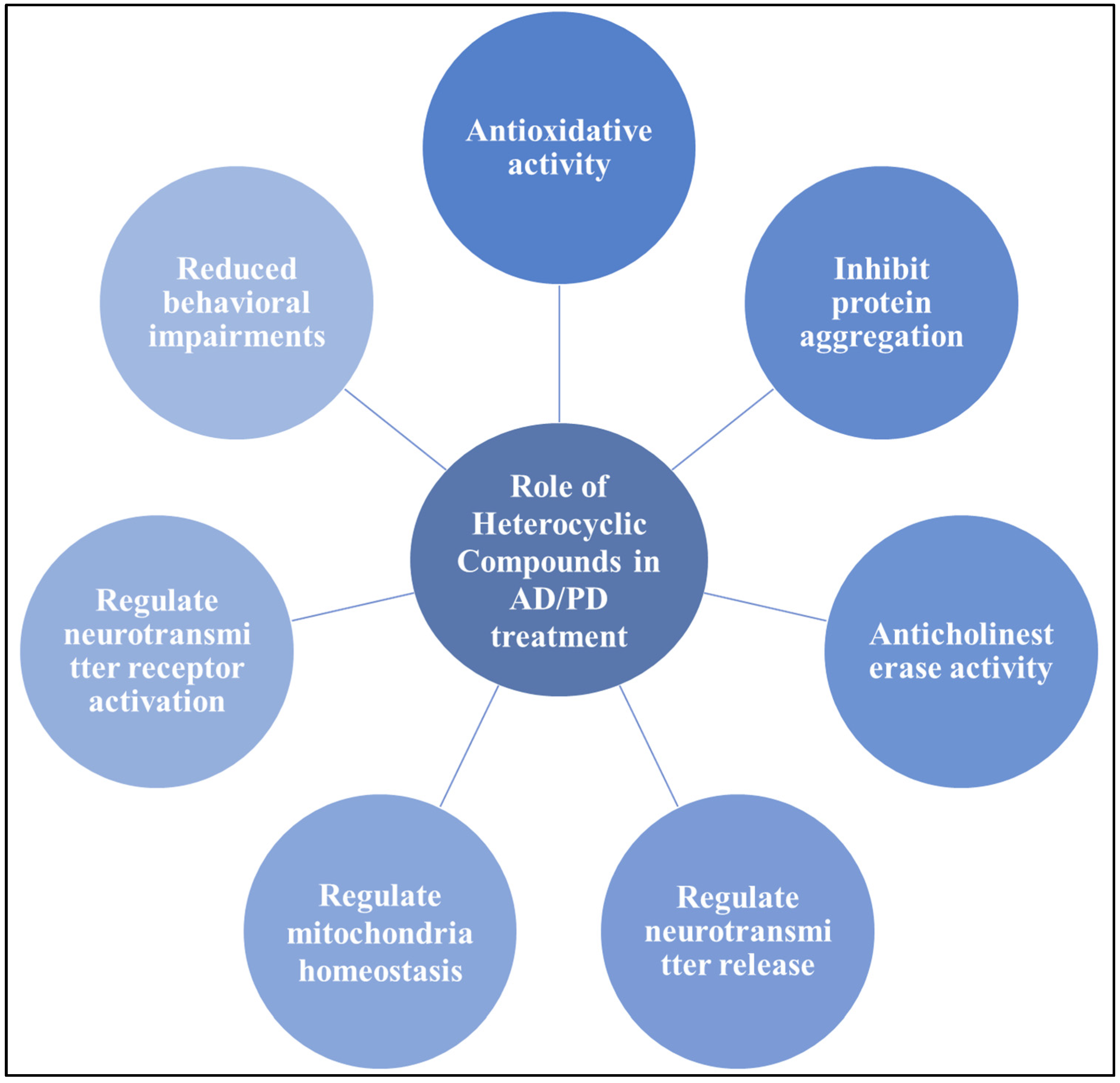
3. Therapeutic Potential of Heterocyclic Compounds in AD and PD Management
3.1. Nitrogen-Based Heterocyclic Moieties
| Compounds | Condition | Study Model | Dose and Time | Experimental Assays | Outcomes | Reference |
|---|---|---|---|---|---|---|
| N-Substituted 2-aryloxymethylpyrrolidines | AD | HepG2 and SH-SY5Y cells | 0.1–100 µM for 24 h | AChE and BChE inhibition activity; antioxidant activity; molecular modeling; cell viability assay | The compound shows dual BChE/FAAH inhibition activity and a high potential to cross BBB | [135] |
| Diosgenin–indole derivatives | AD | SH-SY5Y cells ICR mice | 0.01–100 mM for 2 h | In vitro: H2O2- and anti-6-OHDA-induced oxidant assay; anti-Ab assay In vivo: Morris water maze (MWM) test | Effectively improved memory and learning impairments in mice damaged by Aβ | [133] |
| Indole derivatives | PD | Human HMC3 microglial cells MPTP mice | In vitro: 1–10 µM for 8 h+ 20 h In vivo: 40 mg/kg for 6 weeks (5 times/week) | In vitro: antioxidant assay; In vivo: behavioral tests; neuroinflammation and OS analyses | It reduced MPP+-induced cytotoxicity, reduced NO, IL-1β, IL-6, and TNF-α production, suppressed NLRP3 inflammasome activation, and ameliorated motor deficits, nonmotor depression, and OS in mice | [141] |
| Indole-3-carbinol | PD | LPS rats | 25 and 50 mg/kg for 21 days | Cytokine assay, NF-κB inhibition assay; balance test, open field test (OFT), and MWM | A delay in neurodegeneration of neurons and improvement in motor functions and cognitive function | [142] |
| Benzothiazole and indole derivatives | PD | M17D-TR/αS-3 K::YFP neuroblastoma cells | 5 μM to 40 μM for 24 h | α-Syn inclusion-forming neuroblastoma cell experiment; tau fibril inhibition assay | Inhibit α-syn oligomer activity, but not tau oligomers | [143] |
| Indanone derivatives | AD and PD | Perphenazine (PPZ)-induced (PD) LPS-induced mice (AD) | PD mice model: 20 mg/kg, p.o.; AD mice model: 250 µg/kg, i.p | Memory assay | Improve cognitive function | [144] |
| 1,4-Dihydropyridine derivatives | NDD | SH-SY5Y; rat hippocampal slices; glia from cerebral cortex of Sprague Dawley rats | Cell line: 1 μM for 24 h; hippocampal slices: 10 µM for 6 h | Neuroprotection studies; anti-inflammatory capacity; GSK-3 inhibitory capacity; voltage-dependent calcium channel blockade assay | Have high antioxidant activity, potent anti-inflammatory capacity, GSK-3 inhibitory capacity, and VDCC antagonist activity; can cross the BBB | [145] |
| 3,5-Diarylpyrazole analogs | AD | MC65 cell lines; adult female Swiss albino mice | Cell line: 1–50 µM; mice: 30 mg/kg | Cholinesterase inhibitory activity and SAR studies; AChE enzyme kinetic assay; behavioral studies (in vivo) | Decreased metal-induced Aβ1-42 aggregation; better spontaneous alternation score and novel arm entries without influencing the locomotor activity | [146] |
| Curcumin pyrazole and its derivative (N-(3-Nitrophenylpyrazole) | PD | SHSY5Y neuroblastoma cell line | 210 µM for 24 h | Determination of cytotoxicity; aggregation assays | Inhibit α-syn aggregation | [139] |
| CNB-001, a pyrazole derivative | PD | Adult male C57BL/6 mice | 24 mg/kg body wt. for 7 days | Behavioral assay; analysis of striatal dopamine and its metabolites | Mitigated motor impairments, reduced behavioral impairments, and significantly reduced striatal dopamine and its metabolite levels in mice while also protecting dopaminergic neurons from MPTP toxicity | [147] |
| Pyrazolo[3,4-b]quinoline and benzo[b]pyrazolo[4,3-g][1,8]naphthyridine derivatives | AD | 10 nM to 1 µM | SH-SY5Y cells | AChE/BuChE inhibitory activity; neuroprotective effect | Protect against rotenone/oligomycin A-induced neuronal death; inhibit AChE/BuChE activity in vitro | [148] |
| 3,4-Dimethyl coumarin scaffold derivatives | AD | SH-SY5Y cells | 1 and 10 μM for 24 h | AChE and MAO-B inhibitory assay | Inhibit hAChE and hMAO-B; reduce OS | [149] |
| Dibenzo[1,4,5]thiadiazepine | NDDs | Human neuroblastoma cell line SH-SY5Y | 3 mM for 24 h | Cholinesterase inhibitory activities, cell viability experiments, neuroprotection studies, cytosolic calcium concentration | Shows significant calcium channel modulation activity and is found to be effective in sequestering mitochondrial ROS | [150] |
| N-Heterocyclic amine | AD | In vitro biochemical assays and FRDA and neuronal (HT-22) cell line | 1 μM for 20 min | Antioxidant capacity and amyloid disaggregation | The compound protects amyloid from copper ions and disaggregated amyloid aggregates, with antioxidant activity observed in both cell lines | [151] |
| 3-Amidocoumarin derivatives (coumarins 1–17) | NDDs | Rat cortical neuron culture | 100 mm for 24 h | MAO in vitro inhibition; neuronal survival; PAMPA | Cross the BBB and thus exert activity in the CNS; notable neuroprotection from OS | [152] |
| Ethyl nipecotate (ethyl-piperidine-3-carboxylate) nipecotic acid | AD | Wistar rats | 0.15 mmol/kg for 3.5 h | In vitro lipid peroxidation inhibition; AChE inhibition activity | Significant antioxidant potential; GABA reuptake inhibitor; inhibits AChE and reduces rat paw edema | [153] |
| Pyridine-based hybrids linked to the 1,2,3-triazole unit | Neurological diseases | Outbred mice | 50 to 500 mg/kg | Anticonvulsant effect; psychotropic properties; OFT, elevated plus maze, forced swimming, and test for learning and memory | High anticonvulsant and psychotropic properties | [154] |
| 1,2,4-Thiadiazolylnitrones and furoxanylnitrones | NDDs | SH-SY5Y cells | 0.05–10 μM for 24 h | PAMPA, antioxidant activity | Compounds exhibit strong free radical scavenger properties and potential therapeutic applications in preventing cell death from OS and damage | [155] |
| Rosiglitazone | AD | K670N/M671L-transgenic mice overexpressing human hAPP | 5 mg/kg for 4 weeks | Object recognition and MWM tests; Aβ plaque deposition assay (ELISA) | Ameliorates memory deficits; rosiglitazone shown to decrease brain Aβ levels and Aβ plaque deposition; reduces p-tau aggregates | [156] |
| Riluzole | AD | Aβ25-35-induced rat | 10 mg/kg/day p.o. | MWM tests; AChE activity and OS marker assay | Improves spatial memory, retention, and recall in MWM and passive avoidance tasks, but is not neutralized by muscarinic or nicotinic receptor antagonists | [157] |
| Compounds | Condition | Experimental Assays | Outcomes | Reference |
|---|---|---|---|---|
| 6-Amino-substituted imidazo[1,2-b] pyridazines | NDDs | DPPH radical scavenging activity; AChE inhibition assay; molecular docking | Antioxidative/antiparkinsonian agents for important metabolic functions | [159] |
| 4-(Benzylideneamino)- and 4-(benzylamino)-benzenesulfonamide derivatives | AD | AChE activity determination, in vitro inhibition studies, ADMET analysis | Potential inhibitor properties for AChE | [160] |
| Thiazolyl-pyrazoline derivatives (3a-k) | AD | AChE activity assay, CA activity assay, AChE and CA kinetic analysis | Inhibit AChE and hCA activity | [161] |
| Isoindole-1,3-dione-substituted sulfonamides | AChE enzyme activity; molecular docking study | Inhibit AChE and hCA activity | [162] | |
| Isoindolines/isoindoline-1,3-diones | AD | Anticholinesterase activity assay; molecular docking | AChE inhibitors | [163] |
| Imidazo [2,1-B][1,3,4] thiadiazole | AD | BACE1 enzymatic assay; inhibitory activities against AChE and BChE | Superior BACE1 inhibitory activity; potential inhibitory activity against cholinesterase (AChE and BChE) | [164] |
| 1-Hydroxy-2(1H)-pyridinone-based chelators | PD | Modeling methods | Potential to inhibit COMT | [165] |
| N-substituted pyrazole-derived α-aminophosphonates | AD | Inhibition assay on cholinesterase; evaluation of the cytotoxic activity; antioxidant activity assay | Better AChE inhibitory activity; did not show any cytotoxicity and have promising antioxidant activities against DPPH and H2O2 scavenging | [166] |
| Nitrocatechol derivatives of chalcone | PD | MAO-A, MAO-B, and COMT inhibition assay | Potent inhibitors of MAO and COMT | [167] |
| 1,3- Oxazole analogs | AD | AChE and BChE inhibition activity | Ability to inhibit AChE and BChE | [168] |
| Nitrogen-based novel heterocyclic compounds | AD | AChE and α-glycosidase enzymes were evaluated | Potentially inhibit AChE and α-glycosidase | [124] |
| Heterocyclic amines (F3S4-m, F2S4-m, and F2S4-p) | AD | BACE1 and AChE inhibition activity and Aβ oligomerization assay | Inhibit Aβ1–42 aggregation and AChE and BACE1 enzyme activities | [169] |
| 6-Benzothiazolyl urea, thiourea, and guanidine derivatives | AD | ABAD’s enzymatic activity | Potent inhibitors of ABAD/17β-HSD10 and potential drugs for AD treatment | [170] |
3.2. Sulfur-Based Heterocycle Moieties
3.3. Oxygen-Based Heterocycle Moieties
4. Current Challenges and Future Prospects
Author Contributions
Funding
Conflicts of Interest
References
- Sweeney, P.; Park, H.; Baumann, M.; Dunlop, J.; Frydman, J.; Kopito, R.; McCampbell, A.; Leblanc, G.; Venkateswaran, A.; Nurmi, A.; et al. Protein misfolding in neurodegenerative diseases: Implications and strategies. Transl. Neurodegener. 2017, 6, 6. [Google Scholar] [CrossRef]
- Khanam, H.; Ali, A.; Asif, M.; Shamsuzzaman. Neurodegenerative diseases linked to misfolded proteins and their therapeutic approaches: A review. Eur. J. Med. Chem. 2016, 124, 1121–1141. [Google Scholar] [CrossRef] [PubMed]
- Puranik, N.; Yadav, D.; Song, M. Advancements in the Application of Nanomedicine in Alzheimer’s Disease: A Therapeutic Perspective. Int. J. Mol. Sci. 2023, 24, 14044. [Google Scholar] [CrossRef] [PubMed]
- 2023 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2023, 19, 1598–1695. [CrossRef] [PubMed]
- Zhang, J.; Zhang, Y.; Wang, J.; Xia, Y.; Zhang, J.; Chen, L. Recent advances in Alzheimer’s disease: Mechanisms, clinical trials and new drug development strategies. Signal Transduct. Target. Ther. 2024, 9, 211. [Google Scholar] [CrossRef]
- Puranik, N.; Song, M. Insights into the Role of microRNAs as Clinical Tools for Diagnosis, Prognosis, and as Therapeutic Targets in Alzheimer’s Disease. Int. J. Mol. Sci. 2024, 25, 9936. [Google Scholar] [CrossRef]
- Gómez-Benito, M.; Granado, N.; García-Sanz, P.; Michel, A.; Dumoulin, M.; Moratalla, R. Modeling Parkinson’s Disease With the Alpha-Synuclein Protein. Front. Pharmacol. 2020, 11, 356. [Google Scholar] [CrossRef]
- Morris, H.R.; Spillantini, M.G.; Sue, C.M.; Williams-Gray, C.H. The pathogenesis of Parkinson’s disease. Lancet 2024, 403, 293–304. [Google Scholar] [CrossRef]
- Sayyaed, A.; Saraswat, N.; Vyawahare, N.; Kulkarni, A. A detailed review of pathophysiology, epidemiology, cellular and molecular pathways involved in the development and prognosis of Parkinson’s disease with insights into screening models. Bull. Natl. Res. Cent. 2023, 47, 70. [Google Scholar] [CrossRef]
- Rademacher, K.; Nakamura, K. Role of dopamine neuron activity in Parkinson’s disease pathophysiology. Exp. Neurol. 2024, 373, 114645. [Google Scholar] [CrossRef]
- Chakraborty, A.; Diwan, A. Alzheimer and It’s Possible Therapy: A Review. J. Exp. Neurol. 2020, 1, 115–122. [Google Scholar] [CrossRef]
- Gouda, N.A.; Elkamhawy, A.; Cho, J. Emerging Therapeutic Strategies for Parkinson’s Disease and Future Prospects: A 2021 Update. Biomedicines 2022, 10, 371. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.; Zhou, Y.; Lee, G.; Zhong, K.; Fonseca, J.; Cheng, F. Alzheimer’s disease drug development pipeline: 2023. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2023, 9, e12385. [Google Scholar] [CrossRef]
- McFarthing, K.; Buff, S.; Rafaloff, G.; Dominey, T.; Wyse, R.K.; Stott, S.R.W. Parkinson’s Disease Drug Therapies in the Clinical Trial Pipeline: 2020. J. Park. Dis. 2020, 10, 757–774. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Ahmad, R.; Khare, S.K. Alzheimer’s disease and its treatment by different approaches: A review. Eur. J. Med. Chem. 2021, 216, 113320. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.K.; Shin, Y.; Ju, S.; Han, S.; Kim, S.S.; Kang, I. Comprehensive Overview of Alzheimer’s Disease: Etiological Insights and Degradation Strategies. Int. J. Mol. Sci. 2024, 25, 6901. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, M.; Yin, X.; Chen, K.; Hu, Z.; Zhou, Q.; Cao, X.; Chen, Z.; Liu, D. The role of pathological tau in synaptic dysfunction in Alzheimer’s diseases. Transl. Neurodegener. 2021, 10, 45. [Google Scholar] [CrossRef]
- Bagga, S.; Kumar, M. Current Status of Alzheimer’s Disease and Pathological Mechanisms Investigating the Therapeutic Molecular Targets. Curr. Mol. Med. 2023, 23, 492–508. [Google Scholar] [CrossRef]
- Ju, Y.; Tam, K. Pathological mechanisms and therapeutic strategies for Alzheimer’s disease. Neural Regen. Res. 2022, 17, 543–549. [Google Scholar] [CrossRef]
- Cheong, S.L.; Tiew, J.K.; Fong, Y.H.; Leong, H.W.; Chan, Y.M.; Chan, Z.L.; Kong, E.W.J. Current Pharmacotherapy and Multi-Target Approaches for Alzheimer’s Disease. Pharmaceuticals 2022, 15, 1560. [Google Scholar] [CrossRef]
- Hynd, M.R.; Scott, H.L.; Dodd, P.R. Glutamate-mediated excitotoxicity and neurodegeneration in Alzheimer’s disease. Neurochem. Int. 2004, 45, 583–595. [Google Scholar] [CrossRef] [PubMed]
- Rajah Kumaran, K.; Yunusa, S.; Perimal, E.; Wahab, H.; Müller, C.P.; Hassan, Z. Insights into the Pathophysiology of Alzheimer’s Disease and Potential Therapeutic Targets: A Current Perspective. J. Alzheimer’s Dis. 2023, 91, 507–530. [Google Scholar] [CrossRef] [PubMed]
- Conway, M.E.; Conway, M.E. Alzheimer’s disease: Targeting the glutamatergic system. Biogerontology 2020, 21, 257–274. [Google Scholar] [CrossRef] [PubMed]
- Burns, S.; Selman, A.; Sehar, U.; Rawat, P.; Reddy, A.P.; Reddy, P.H. Therapeutics of Alzheimer’s Disease: Recent Developments. Antioxidants 2022, 11, 2402. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; Zhang, C. Current therapeutics for Alzheimer’s disease and clinical trials. Open Explor. 2024, 3, 255–271. [Google Scholar] [CrossRef]
- Senkevich, K.; Rudakou, U.; Gan-Or, Z. New therapeutic approaches to Parkinson’s disease targeting GBA, LRRK2 and Parkin. Neuropharmacology 2022, 202, 108822. [Google Scholar] [CrossRef]
- Caramiello, A.M.; Pirota, V. Novel Therapeutic Horizons: SNCA Targeting in Parkinson’s Disease. Biomolecules 2024, 14, 949. [Google Scholar] [CrossRef]
- Polinski, N.K.; Martinez, T.N.; Ramboz, S.; Sasner, M.; Herberth, M.; Switzer, R.; Ahmad, S.O.; Pelligrino, L.J.; Clark, S.W.; Marcus, J.N.; et al. The GBA1 D409V mutation exacerbates synuclein pathology to differing extents in two alpha-synuclein models. Dis. Model. Mech. 2022, 15, dmm049192. [Google Scholar] [CrossRef]
- Tang, T.; Jian, B.; Liu, Z. Transmembrane Protein 175, a Lysosomal Ion Channel Related to Parkinson’s Disease. Biomolecules 2023, 13, 802. [Google Scholar] [CrossRef]
- Pickrell, A.M.; Huang, C.H.; Kennedy, S.R.; Ordureau, A.; Sideris, D.P.; Hoekstra, J.G.; Harper, J.W.; Youle, R.J. Endogenous Parkin Preserves Dopaminergic Substantia Nigral Neurons following Mitochondrial DNA Mutagenic Stress. Neuron 2015, 87, 371–381. [Google Scholar] [CrossRef]
- Vizziello, M.; Borellini, L.; Franco, G.; Ardolino, G. Disruption of Mitochondrial Homeostasis: The Role of PINK1 in Parkinson’s Disease. Cells 2021, 10, 3022. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Li, S.; Zeng, L.-H.; Tan, J. Tau-targeting therapy in Alzheimer’s disease: Critical advances and future opportunities. Ageing Neurodegener. Dis. 2022, 2, 11. [Google Scholar] [CrossRef]
- Congdon, E.E.; Ji, C.; Tetlow, A.M.; Jiang, Y.; Sigurdsson, E.M. Tau-targeting therapies for Alzheimer disease: Current status and future directions. Nat. Rev. Neurol. 2023, 19, 715–736. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Wan, H.; Chen, S.; Liu, G.P. Targeting tau in Alzheimer’s disease: From mechanisms to clinical therapy. Neural Regen. Res. 2024, 19, 1489–1498. [Google Scholar] [CrossRef] [PubMed]
- VandeVrede, L.; Boxer, A.L.; Polydoro, M. Targeting tau: Clinical trials and novel therapeutic approaches. Neurosci. Lett. 2020, 731, 134919. [Google Scholar] [CrossRef]
- Rao, C.V.; Asch, A.S.; Carr, D.J.J.; Yamada, H.Y. “Amyloid-beta accumulation cycle” as a prevention and/or therapy target for Alzheimer’s disease. Aging Cell 2020, 19, e13109. [Google Scholar] [CrossRef]
- Karran, E.; De Strooper, B. The amyloid hypothesis in Alzheimer disease: New insights from new therapeutics. Nat. Rev. Drug Discov. 2022, 21, 306–318. [Google Scholar] [CrossRef]
- Jeremic, D.; Jiménez-Díaz, L.; Navarro-López, J.D. Past, present and future of therapeutic strategies against amyloid-β peptides in Alzheimer’s disease: A systematic review. Ageing Res. Rev. 2021, 72, 101496. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, H.; Li, R.; Sterling, K.; Song, W. Amyloid β-based therapy for Alzheimer’s disease: Challenges, successes and future. Signal Transduct. Target. Ther. 2023, 8, 248. [Google Scholar] [CrossRef]
- Jasutkar, H.G.; Oh, S.E.; Mouradian, M.M. Therapeutics in the Pipeline Targeting α-Synuclein for Parkinson’s Disease. Pharmacol. Rev. 2022, 74, 207–237. [Google Scholar] [CrossRef]
- Teil, M.; Arotcarena, M.L.; Faggiani, E.; Laferriere, F.; Bezard, E.; Dehay, B. Targeting α-Synuclein for PD Therapeutics: A Pursuit on All Fronts. Biomolecules 2020, 10, 391. [Google Scholar] [CrossRef]
- Walczak-Nowicka, Ł.J.; Herbet, M. Acetylcholinesterase Inhibitors in the Treatment of Neurodegenerative Diseases and the Role of Acetylcholinesterase in their Pathogenesis. Int. J. Mol. Sci. 2021, 22, 9290. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Hu, Q.; Wang, X.; Li, C.; Chen, X.; Zhao, D.; Qiu, Y.; Xu, H.; Wang, J.; Ren, L.; et al. Dual-target inhibitors based on acetylcholinesterase: Novel agents for Alzheimer’s disease. Eur. J. Med. Chem. 2024, 279, 116810. [Google Scholar] [CrossRef] [PubMed]
- Benfante, R.; Di Lascio, S.; Cardani, S.; Fornasari, D. Acetylcholinesterase inhibitors targeting the cholinergic anti-inflammatory pathway: A new therapeutic perspective in aging-related disorders. Aging Clin. Exp. Res. 2019, 33, 823–834. [Google Scholar] [CrossRef] [PubMed]
- Akıncıoğlu, H.; Gülçin, İ. Potent Acetylcholinesterase Inhibitors: Potential Drugs for Alzheimer’s Disease. Mini-Rev. Med. Chem. 2020, 20, 703–715. [Google Scholar] [CrossRef]
- Marucci, G.; Buccioni, M.; Ben, D.D.; Lambertucci, C.; Volpini, R.; Amenta, F. Efficacy of acetylcholinesterase inhibitors in Alzheimer’s disease. Neuropharmacology 2021, 190, 108352. [Google Scholar] [CrossRef]
- Nordberg, A.; Ballard, C.; Bullock, R.; Darreh-Shori, T.; Somogyi, M. A Review of Butyrylcholinesterase as a Therapeutic Target in the Treatment of Alzheimer’s Disease. Prim. Care Companion CNS Disord. 2013, 15, PCC.12r01412. [Google Scholar] [CrossRef]
- Ha, Z.Y.; Mathew, S.; Yeong, K.Y. Butyrylcholinesterase: A Multifaceted Pharmacological Target and Tool. Curr. Protein Pept. Sci. 2019, 21, 99–109. [Google Scholar] [CrossRef]
- Fu, L.; Liu, B. Role of receptor tyrosine kinases in neurodegenerative disorders. In Receptor Tyrosine Kinases in Neurodegenerative and Psychiatric Disorders; Elsevier: Amsterdam, The Netherlands, 2023; pp. 279–299. [Google Scholar] [CrossRef]
- Turkistani, A.; Al-kuraishy, H.M.; Al-Gareeb, A.I.; Albuhadily, A.K.; Alexiou, A.; Papadakis, M.; Elfiky, M.M.; Saad, H.M.; Batiha, G.E. Therapeutic Potential Effect of Glycogen Synthase Kinase 3 Beta (GSK-3β) Inhibitors in Parkinson Disease: Exploring an Overlooked Avenue. Mol. Neurobiol. 2024, 61, 7092–7108. [Google Scholar] [CrossRef]
- Lauretti, E.; Dincer, O.; Praticò, D. Glycogen synthase kinase-3 signaling in Alzheimer’s disease. Biochim. Biophys. Acta Mol. Cell Res. 2020, 1867, 118664. [Google Scholar] [CrossRef]
- Yu, H.; Xiong, M.; Zhang, Z. The role of glycogen synthase kinase 3 beta in neurodegenerative diseases. Front. Mol. Neurosci. 2023, 16, 1209703. [Google Scholar] [CrossRef] [PubMed]
- Swarup, G.; Kanik, P.; Shekhar, V.; Gupta, S. β- and γ-secretases as therapeutic targets for Alzheimer’s disease. In Targeted Therapy for the Central Nervous System; Elsevier: Amsterdam, The Netherlands, 2025; pp. 239–263. [Google Scholar] [CrossRef]
- Wolfe, M.S. γ-Secretase: Once and future drug target for Alzheimer’s disease. Expert. Opin. Drug Discov. 2024, 19, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Özdemir, Z.; Alagöz, M.A.; Bahçecioğlu, Ö.F.; Gök, S. Monoamine Oxidase-B (MAO-B) Inhibitors in the Treatment of Alzheimer’s and Parkinson’s Disease. Curr. Med. Chem. 2021, 28, 6045–6065. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Ross, P.J.; Ellis, J.; Salter, M.W. Targeting NMDA receptors in neuropsychiatric disorders by drug screening on human neurons derived from pluripotent stem cells. Transl. Psychiatry 2022, 12, 243. [Google Scholar] [CrossRef]
- Bading, H. Therapeutic targeting of the pathological triad of extrasynaptic NMDA receptor signaling in neurodegenerations. J. Exp. Med. 2017, 214, 569. [Google Scholar] [CrossRef]
- Carles, A.; Freyssin, A.; Perin-Dureau, F.; Rubinstenn, G.; Maurice, T. Targeting N-Methyl-d-Aspartate Receptors in Neurodegenerative Diseases. Int. J. Mol. Sci. 2024, 25, 3733. [Google Scholar] [CrossRef]
- Jiang, S.; Li, Y.; Zhang, C.; Zhao, Y.; Bu, G.; Xu, H.; Zhang, Y.W. M1 muscarinic acetylcholine receptor in Alzheimer’s disease. Neurosci. Bull. 2014, 30, 295–307. [Google Scholar] [CrossRef]
- Scarpa, M.; Hesse, S.; Bradley, S.J. M1 muscarinic acetylcholine receptors: A therapeutic strategy for symptomatic and disease-modifying effects in Alzheimer’s disease? Adv. Pharmacol. 2020, 88, 277–310. [Google Scholar] [CrossRef]
- Tozzi, A.; Tantucci, M.; Marchi, S.; Mazzocchetti, P.; Morari, M.; Pinton, P.; Mancini, A.; Calabresi, P. Dopamine D2 receptor-mediated neuroprotection in a G2019S Lrrk2 genetic model of Parkinson’s disease. Cell Death Dis. 2018, 9, 204. [Google Scholar] [CrossRef]
- Rangel-Barajas, C.; Coronel, I.; Florán, B. Dopamine Receptors and Neurodegeneration. Aging Dis. 2015, 6, 349. [Google Scholar] [CrossRef]
- Alharbi, B.; Al-kuraishy, H.M.; Al-Gareeb, A.I.; Elekhnawy, E.; Alharbi, H.; Alexiou, A.; Papadakis, M.; El-Saber Batiha, G. Role of GABA pathway in motor and non-motor symptoms in Parkinson’s disease: A bidirectional circuit. Eur. J. Med. Res. 2024, 29, 205. [Google Scholar] [CrossRef] [PubMed]
- Carello-Collar, G.; Bellaver, B.; Ferreira, P.C.L.; Ferrari-Souza, J.P.; Ramos, V.G.; Therriault, J.; Tissot, C.; De Bastiani, M.A.; Soares, C.; Pascoal, T.A.; et al. The GABAergic system in Alzheimer’s disease: A systematic review with meta-analysis. Mol. Psychiatry 2023, 28, 5025–5036. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, M.J. 5-HT6 receptors and Alzheimer’s disease. Alzheimers Res. Ther. 2013, 5, 15. [Google Scholar] [CrossRef] [PubMed]
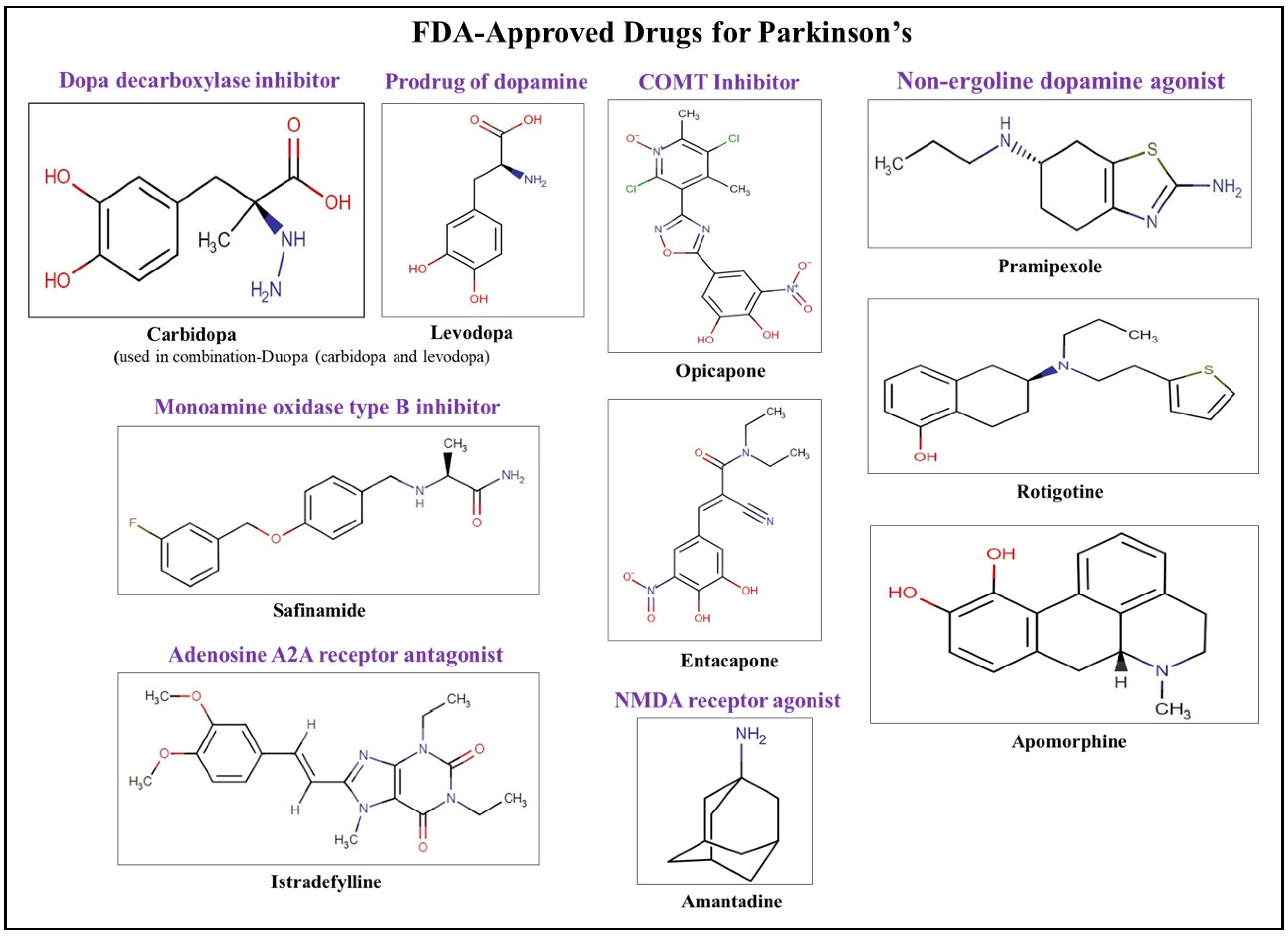
- Rai, S.N.; Singh, P.; Varshney, R.; Chaturvedi, V.K.; Vamanu, E.; Singh, M.P.; Singh, B.K. Promising drug targets and associated therapeutic interventions in Parkinson’s disease. Neural Regen. Res. 2021, 16, 1730. [Google Scholar] [CrossRef]
- Pingale, T.; Gupta, G.L. Current and emerging therapeutic targets for Parkinson’s disease. Metab. Brain Dis. 2021, 36, 13–27. [Google Scholar] [CrossRef]
- Kumari, S.; Maddeboina, K.; Bachu, R.D.; Boddu, S.H.S.; Trippier, P.C.; Tiwari, A.K. Pivotal role of nitrogen heterocycles in Alzheimer’s disease drug discovery. Drug Discov. Today 2022, 27, 103322. [Google Scholar] [CrossRef]
- Hiremathad, A.; Piemontese, L. Heterocyclic compounds as key structures for the interaction with old and new targets in Alzheimer’s disease therapy. Neural Regen. Res. 2017, 12, 1256–1261. [Google Scholar] [CrossRef]
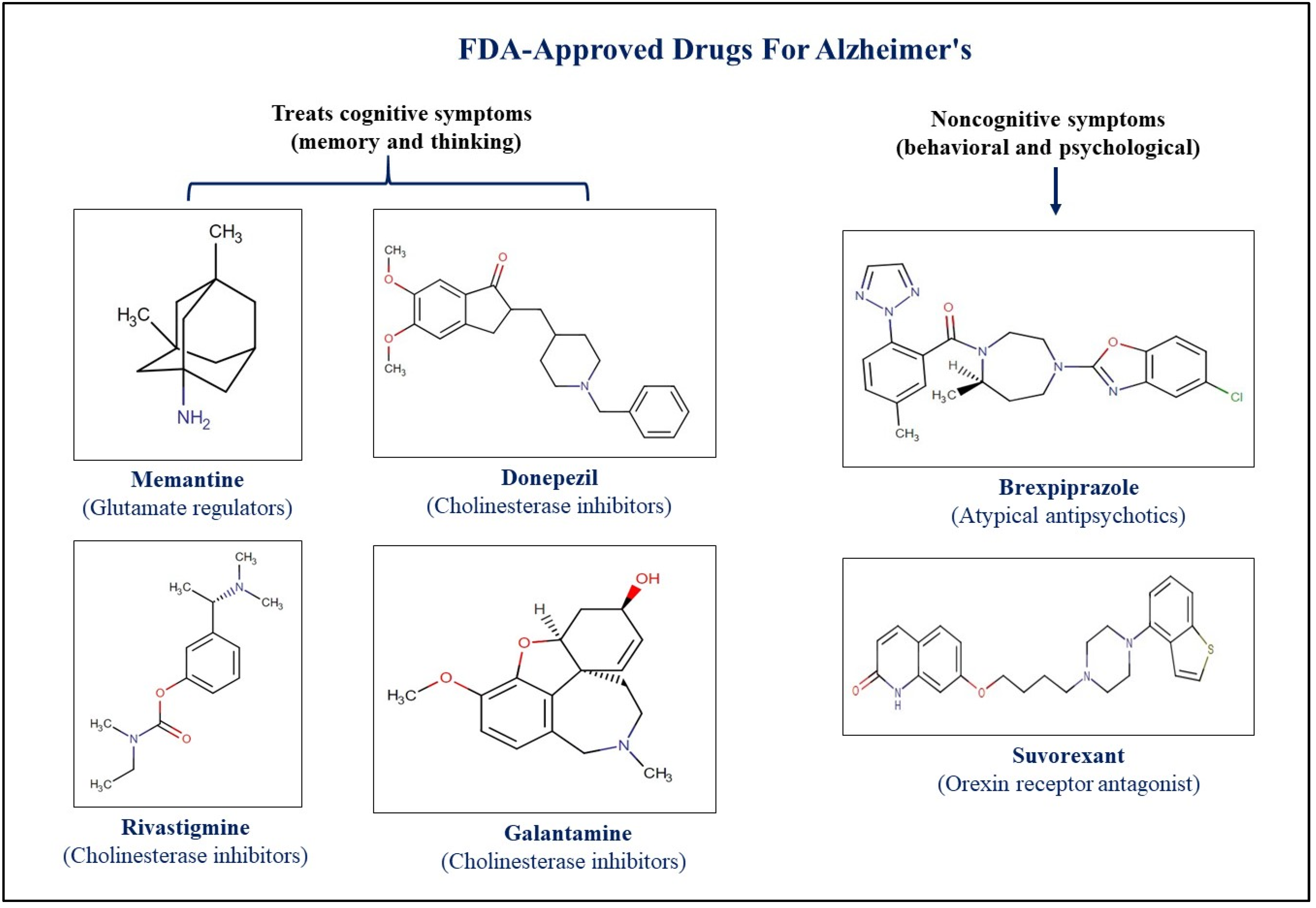
- Varadharajan, A.; Davis, A.D.; Ghosh, A.; Jagtap, T.; Xavier, A.; Menon, A.J.; Roy, D.; Gandhi, S.; Gregor, T. Guidelines for pharmacotherapy in Alzheimer’s disease—A primer on FDA-approved drugs. J. Neurosci. Rural. Pr. 2023, 14, 566. [Google Scholar] [CrossRef]
- Alhazmi, H.A.; Albratty, M. An update on the novel and approved drugs for Alzheimer disease. Saudi Pharm. J. SPJ 2022, 30, 1755. [Google Scholar] [CrossRef]
- Chopade, P.; Chopade, N.; Zhao, Z.; Mitragotri, S.; Liao, R.; Chandran Suja, V. Alzheimer’s and Parkinson’s disease therapies in the clinic. Bioeng. Transl. Med. 2023, 8, e10367. [Google Scholar] [CrossRef]
- Barresi, E.; Baglini, E.; Poggetti, V.; Castagnoli, J.; Giorgini, D.; Salerno, S.; Taliani, S.; Da Settimo, F. Indole-Based Compounds in the Development of Anti-Neurodegenerative Agents. Molecules 2024, 29, 2127. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wei, W.; Zhao, M.; Ma, L.; Jiang, X.; Pei, H.; Cao, Y.; Li, H. Interaction between Aβ and Tau in the Pathogenesis of Alzheimer’s Disease. Int. J. Biol. Sci. 2021, 17, 2181. [Google Scholar] [CrossRef] [PubMed]
- Roda, A.; Serra-Mir, G.; Montoliu-Gaya, L.; Tiessler, L.; Villegas, S. Amyloid-beta peptide and tau protein crosstalk in Alzheimer’s disease. Neural Regen. Res. 2022, 17, 1666–1674. [Google Scholar] [CrossRef] [PubMed]
- Busche, M.A.; Hyman, B.T. Synergy between amyloid-β and tau in Alzheimer’s disease. Nat. Neurosci. 2020, 23, 1183–1193. [Google Scholar] [CrossRef]
- Abyadeh, M.; Gupta, V.; Paulo, J.A.; Mahmoudabad, A.G.; Shadfar, S.; Mirshahvaladi, S.; Gupta, V.; Nguyen, C.T.O.; Finkelstein, D.I.; You, Y.; et al. Amyloid-beta and tau protein beyond Alzheimer’s disease. Neural Regen. Res. 2024, 19, 1262–1276. [Google Scholar] [CrossRef]
- Pan, L.; Meng, L.; He, M.; Zhang, Z. Tau in the Pathophysiology of Parkinson’s Disease. J. Mol. Neurosci. 2021, 71, 2179–2191. [Google Scholar] [CrossRef]
- Srinivasan, E.; Chandrasekhar, G.; Chandrasekar, P.; Anbarasu, K.; Vickram, A.S.; Karunakaran, R.; Rajasekaran, R.; Srikumar, P.S. Alpha-Synuclein Aggregation in Parkinson’s Disease. Front. Med. 2021, 8, 736978. [Google Scholar] [CrossRef]
- Vidović, M.; Rikalovic, M.G. Alpha-Synuclein Aggregation Pathway in Parkinson’s Disease: Current Status and Novel Therapeutic Approaches. Cells 2022, 11, 1732. [Google Scholar] [CrossRef]
- Burré, J.; Edwards, R.H.; Halliday, G.; Lang, A.E.; Lashuel, H.A.; Melki, R.; Murayama, S.; Outeiro, T.F.; Papa, S.M.; Stefanis, L.; et al. Research Priorities on the Role of α-Synuclein in Parkinson’s Disease Pathogenesis. Mov. Disord. 2024, 39, 1663–1678. [Google Scholar] [CrossRef]
- Tofaris, G.K. Initiation and progression of α-synuclein pathology in Parkinson’s disease. Cell. Mol. Life Sci. 2022, 79, 210. [Google Scholar] [CrossRef]
- Pan, L.; Li, C.; Meng, L.; Tian, Y.; He, M.; Yuan, X.; Zhang, G.; Zhang, Z.; Xiong, J.; Chen, G.; et al. Tau accelerates α-synuclein aggregation and spreading in Parkinson’s disease. Brain 2022, 145, 3454–3471. [Google Scholar] [CrossRef] [PubMed]
- Ichimata, S.; Yoshida, K.; Li, J.; Rogaeva, E.; Lang, A.E.; Kovacs, G.G. The molecular spectrum of amyloid-beta (Aβ) in neurodegenerative diseases beyond Alzheimer’s disease. Brain Pathol. 2024, 34, e13210. [Google Scholar] [CrossRef] [PubMed]
- Bu, M.; Farrer, M.J.; Khoshbouei, H. Dynamic control of the dopamine transporter in neurotransmission and homeostasis. Npj Park. Dis. 2021, 7, 22. [Google Scholar] [CrossRef] [PubMed]
- Palermo, G.; Giannoni, S.; Bellini, G.; Siciliano, G.; Ceravolo, R. Dopamine Transporter Imaging, Current Status of a Potential Biomarker: A Comprehensive Review. Int. J. Mol. Sci. 2021, 22, 11234. [Google Scholar] [CrossRef]
- Shaikh, A.; Ahmad, F.; Teoh, S.L.; Kumar, J.; Yahaya, M.F. Targeting dopamine transporter to ameliorate cognitive deficits in Alzheimer’s disease. Front. Cell Neurosci. 2023, 17, 1292858. [Google Scholar] [CrossRef]
- Kang, S.; Jeon, S.; Lee, Y.-G.; Ye, B.S. Striatal dopamine transporter uptake, parkinsonism and cognition in Alzheimer’s disease. Eur. J. Neurol. 2023, 30, 3105–3113. [Google Scholar] [CrossRef]
- Obaid, R.J.; Naeem, N.; Mughal, E.U.; Al-Rooqi, M.M.; Sadiq, A.; Jassas, R.S.; Moussa, Z.; Ahmed, S.A. Inhibitory potential of nitrogen, oxygen and sulfur containing heterocyclic scaffolds against acetylcholinesterase and butyrylcholinesterase. RSC Adv. 2022, 12, 19764–19855. [Google Scholar] [CrossRef]
- Reis, J.; Encarnacao, I.; Gaspar, A.; Morales, A.; Milhazes, N.; Borges, F. Parkinson’s Disease Management. Part II- Discovery of MAO-B Inhibitors Based on Nitrogen Heterocycles and Analogues. Curr. Top Med. Chem. 2013, 12, 2116–2130. [Google Scholar] [CrossRef]
- Carradori, S.; Fantacuzzi, M.; Ammazzalorso, A.; Angeli, A.; De Filippis, B.; Galati, S.; Petzer, A.; Petzer, J.P.; Poli, G.; Tuccinardi, T.; et al. Resveratrol Analogues as Dual Inhibitors of Monoamine Oxidase B and Carbonic Anhydrase VII: A New Multi-Target Combination for Neurodegenerative Diseases? Molecules 2022, 27, 7816. [Google Scholar] [CrossRef]
- Pollard, A.; Shephard, F.; Freed, J.; Liddell, S.; Chakrabarti, L. Mitochondrial proteomic profiling reveals increased carbonic anhydrase II in aging and neurodegeneration. Aging 2016, 8, 2425. [Google Scholar] [CrossRef]
- Lemon, N.; Canepa, E.; Ilies, M.A.; Fossati, S. Carbonic Anhydrases as Potential Targets Against Neurovascular Unit Dysfunction in Alzheimer’s Disease and Stroke. Front. Aging Neurosci. 2021, 13, 772278. [Google Scholar] [CrossRef] [PubMed]
- Poggetti, V.; Salerno, S.; Baglini, E.; Barresi, E.; Da Settimo, F.; Taliani, S. Carbonic Anhydrase Activators for Neurodegeneration: An Overview. Molecules 2022, 27, 2544. [Google Scholar] [CrossRef] [PubMed]
- Duarte, P.; Cuadrado, A.; León, R. Monoamine Oxidase Inhibitors: From Classic to New Clinical Approaches. Handb. Exp. Pharmacol. 2020, 264, 229–259. [Google Scholar] [CrossRef]
- Jones, D.N.; Raghanti, M.A. The role of monoamine oxidase enzymes in the pathophysiology of neurological disorders. J. Chem. Neuroanat. 2021, 114, 101957. [Google Scholar] [CrossRef]
- Sharma, P.; Srivastava, P.; Seth, A.; Tripathi, P.N.; Banerjee, A.G.; Shrivastava, S.K. Comprehensive review of mechanisms of pathogenesis involved in Alzheimer’s disease and potential therapeutic strategies. Prog. Neurobiol. 2019, 174, 53–89. [Google Scholar] [CrossRef]
- Behl, T.; Kaur, D.; Sehgal, A.; Singh, S.; Sharma, N.; Zengin, G.; Andronie-Cioara, F.L.; Toma, M.M.; Bungau, S.; Bumbu, A.G. Role of Monoamine Oxidase Activity in Alzheimer’s Disease: An Insight into the Therapeutic Potential of Inhibitors. Molecules 2021, 26, 3724. [Google Scholar] [CrossRef]
- Manzoor, S.; Hoda, N. A comprehensive review of monoamine oxidase inhibitors as Anti-Alzheimer’s disease agents: A review. Eur. J. Med. Chem. 2020, 206, 112787. [Google Scholar] [CrossRef]
- Jayan, J.; Chandran, N.; Thekkantavida, A.C.; Abdelgawad, M.A.; Ghoneim, M.M.; Shaker, M.E.; Prerna, U.; Feba, B.; Zachariah, M.S.; Kumar, S.; et al. Piperidine: A Versatile Heterocyclic Ring for Developing Monoamine Oxidase Inhibitors. ACS Omega 2023, 8, 37731–37751. [Google Scholar] [CrossRef]
- Dhiman, P.; Malik, N.; Khatkar, A. Natural based piperine derivatives as potent monoamine oxidase inhibitors: An in silico ADMET analysis and molecular docking studies. BMC Chem. 2020, 14, 12. [Google Scholar] [CrossRef]
- Chavarria, D.; Fernandes, C.; Silva, V.; Silva, C.; Gil-Martins, E.; Soares, P.; Silva, T.; Silva, R.; Remião, F.; Oliveira, P.J.; et al. Design of novel monoamine oxidase-B inhibitors based on piperine scaffold: Structure-activity-toxicity, drug-likeness and efflux transport studies. Eur. J. Med. Chem. 2020, 185, 111770. [Google Scholar] [CrossRef]
- Dauvilliers, Y.; Tafti, M.; Landolt, H.P. Catechol-O-methyltransferase, dopamine, and sleep-wake regulation. Sleep. Med. Rev. 2015, 22, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Perkovic, M.N.; Strac, D.S.; Tudor, L.; Konjevod, M.; Erjavec, G.N.; Pivac, N. Catechol-O-methyltransferase, Cognition and Alzheimer’s Disease. Curr. Alzheimer Res. 2018, 15, 408–419. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.J.; Yar, M.S.; Grover, G.; Nath, R. Neurological and psychiatric management using COMT inhibitors: A review. Bioorg. Chem. 2020, 94, 103418. [Google Scholar] [CrossRef] [PubMed]
- Serretti, A.; Olgiati, P. Catechol-o-methyltransferase and Alzheimer’s disease: A review of biological and genetic findings. CNS Neurol. Disord. Drug Targets 2012, 11, 299–305. [Google Scholar] [CrossRef]
- Fabbri, M.; Ferreira, J.J.; Rascol, O. COMT Inhibitors in the Management of Parkinson’s Disease. CNS Drugs 2022, 36, 261–282. [Google Scholar] [CrossRef]
- Salamon, A.; Zádori, D.; Szpisjak, L.; Klivényi, P.; Vécsei, L. What is the impact of catechol-O-methyltransferase (COMT) on Parkinson’s disease treatment? Expert. Opin. Pharmacother. 2022, 23, 1123–1128. [Google Scholar] [CrossRef]
- Bharat, V.; Durairaj, A.S.; Vanhauwaert, R.; Li, L.; Muir, C.M.; Chandra, S.; Kwak, C.S.; Le Guen, Y.; Nandakishore, P.; Hsieh, C.H.; et al. A mitochondrial inside-out iron-calcium signal reveals drug targets for Parkinson’s disease. Cell Rep. 2023, 42, 113544. [Google Scholar] [CrossRef]
- Jurcau, A. Insights into the Pathogenesis of Neurodegenerative Diseases: Focus on Mitochondrial Dysfunction and Oxidative Stress. Int. J. Mol. Sci. 2021, 22, 11847. [Google Scholar] [CrossRef]
- Lee, K.H.; Cha, M.; Lee, B.H. Neuroprotective Effect of Antioxidants in the Brain. Int. J. Mol. Sci. 2020, 21, 7152. [Google Scholar] [CrossRef]
- Misrani, A.; Tabassum, S.; Yang, L. Mitochondrial Dysfunction and Oxidative Stress in Alzheimer’s Disease. Front. Aging Neurosci. 2021, 13, 617588. [Google Scholar] [CrossRef]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Arch. Toxicol. 2023, 97, 2499. [Google Scholar] [CrossRef] [PubMed]
- Tapias, V.; González-Andrés, P.; Peña, L.F.; Barbero, A.; Núñez, L.; Villalobos, C. Therapeutic Potential of Heterocyclic Compounds Targeting Mitochondrial Calcium Homeostasis and Signaling in Alzheimer’s Disease and Parkinson’s Disease. Antioxidants 2023, 12, 1282. [Google Scholar] [CrossRef] [PubMed]
- Baracaldo-Santamaría, D.; Avendaño-Lopez, S.S.; Ariza-Salamanca, D.F.; Rodriguez-Giraldo, M.; Calderon-Ospina, C.A.; González-Reyes, R.E.; Nava-Mesa, M.O. Role of Calcium Modulation in the Pathophysiology and Treatment of Alzheimer’s Disease. Int. J. Mol. Sci. 2023, 24, 9067. [Google Scholar] [CrossRef] [PubMed]
- Pizarro-Galleguillos, B.M.; Kunert, L.; Brüggemann, N.; Prasuhn, J. Neuroinflammation and Mitochondrial Dysfunction in Parkinson’s Disease: Connecting Neuroimaging with Pathophysiology. Antioxidants 2023, 12, 1411. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.F.; Dang, T.; Wang, H.J.; Zhu, X.Z.; Qiao, C. Mitochondrial homeostasis regulation: A promising therapeutic target for Parkinson’s disease. Behav. Brain Res. 2024, 459, 114811. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, J.; Zhao, K.; Wu, S.; Chen, X.; Hu, W. The Role of Calcium and Iron Homeostasis in Parkinson’s Disease. Brain Sci. 2024, 14, 88. [Google Scholar] [CrossRef]
- Orfali, R.; Alwatban, A.Z.; Orfali, R.S.; Lau, L.; Chea, N.; Alotaibi, A.M.; Nam, Y.W.; Zhang, M. Oxidative stress and ion channels in neurodegenerative diseases. Front. Physiol. 2024, 15, 1320086. [Google Scholar] [CrossRef]
- Heravi, M.M.; Zadsirjan, V. Prescribed drugs containing nitrogen heterocycles: An overview. RSC Adv. 2020, 10, 44247–44311. [Google Scholar] [CrossRef]
- Chauhan, A.; Salahuddin, S.; Mazumder, A.; Kumar, R.; Jawed Ahsan, M.; Shahar Yar, M.; Maqsood, R.; Singh, K. Targeted Development of Pyrazoline Derivatives for Neurological Disorders: A Review. ChemistrySelect 2024, 9, e202400738. [Google Scholar] [CrossRef]
- Martins, P.; Jesus, J.; Santos, S.; Raposo, L.R.; Roma-Rodrigues, C.; Baptista, P.V.; Fernandes, A.R. Heterocyclic Anticancer Compounds: Recent Advances and the Paradigm Shift towards the Use of Nanomedicine’s Tool Box. Molecules 2015, 20, 16852. [Google Scholar] [CrossRef]
- Joubert, J.; Geldenhuys, W.J.; VanderSchyf, C.J.; Oliver, D.W.; Kruger, H.G.; Govender, T.; Malan, S.F. Polycyclic Cage Structures as Lipophilic Scaffolds for Neuroactive Drugs. ChemMedChem 2012, 7, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Gulcin, İ.; Petrova, O.V.; Taslimi, P.; Malysheva, S.F.; Schmidt, E.Y.; Sobenina, L.N.; Gusarova, N.K.; Trofimov, B.A.; Tuzun, B.; Farzaliyev, V.M.; et al. Synthesis, Characterization, Molecular Docking, Acetylcholinesterase and α-Glycosidase Inhibition Profiles of Nitrogen-Based Novel Heterocyclic Compounds. ChemistrySelect 2022, 7, e202200370. [Google Scholar] [CrossRef]
- Pathania, S.; Narang, R.K.; Rawal, R.K. Role of sulphur-heterocycles in medicinal chemistry: An update. Eur. J. Med. Chem. 2019, 180, 486–508. [Google Scholar] [CrossRef] [PubMed]
- Venkatachalam, H.; Kumar, N.V.A.; Venkatachalam, H.; Kumar, N.V.A. The Oxygen-Containing Fused Heterocyclic Compounds. In Heterocycles—Synthesis and Biological Activities; IntechOpen: Geneva, Switzerland, 2019. [Google Scholar] [CrossRef]
- Singh, K.; Pal, R.; Khan, S.A.; Kumar, B.; Akhtar, M.J. Insights into the structure activity relationship of nitrogen-containing heterocyclics for the development of antidepressant compounds: An updated review. J. Mol. Struct. 2021, 1237, 130369. [Google Scholar] [CrossRef]
- Khan, B.A.; ur Rehman, O.; Alsfouk, A.A.; Ejaz, S.A.; Channar, P.A.; Saeed, A.; Ghafoor, A.; Ujan, R.; Mughal, E.U.; Kumar, R.; et al. Substituted piperidine as a novel lead molecule for the treatment of Parkinson’s disease: Synthesis, crystal structure, hirshfeld surface analysis, and molecular modeling. J. Mol. Struct. 2022, 1265, 133350. [Google Scholar] [CrossRef]
- Al-Baghdadi, O.B.; Prater, N.I.; Van Der Schyf, C.J.; Geldenhuys, W.J. Inhibition of monoamine oxidase by derivatives of piperine, an alkaloid from the pepper plant Piper nigrum, for possible use in Parkinson’s disease. Bioorg. Med. Chem. Lett. 2012, 22, 7183–7188. [Google Scholar] [CrossRef]
- Li, C.Y.; Zhao, L.M.; Shi, X.W.; Zhang, J.D. Lobeline shows protective effects against MPTP-induced dopaminergic neuron death and attenuates behavior deficits in animals. Exp. Ther. Med. 2014, 7, 375–378. [Google Scholar] [CrossRef]
- Jones, C.B.; Eltit, J.M.; Dukat, M. Do 2-(Benzoyl)piperidines Represent a Novel Class of hDAT Reuptake Inhibitors? ACS Chem. Neurosci. 2023, 14, 741–748. [Google Scholar] [CrossRef]
- Hritcu, L.; Noumedem, J.A.; Cioanca, O.; Hancianu, M.; Kuete, V.; Mihasan, M. Methanolic extract of Piper nigrum fruits improves memory impairment by decreasing brain oxidative stress in amyloid beta(1–42) rat model of Alzheimer’s disease. Cell Mol. Neurobiol. 2014, 34, 437–449. [Google Scholar] [CrossRef]
- Zhou, L.C.; Liang, Y.F.; Huang, Y.; Yang, G.X.; Zheng, L.L.; Sun, J.M.; Li, Y.; Zhu, F.L.; Qian, H.W.; Wang, R.; et al. Design, synthesis, and biological evaluation of diosgenin-indole derivatives as dual-functional agents for the treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2021, 219, 113426. [Google Scholar] [CrossRef]
- Azmy, E.M.; Nassar, I.F.; Hagras, M.; Fawzy, I.M.; Hegazy, M.; Mokhtar, M.M.; Yehia, A.M.; Ismail, N.S.; Lashin, W.H. New Indole Derivatives As Multitarget anti-Alzheimer’s Agents: Synthesis, Biological Evaluation and Molecular Dynamics. Future Med. Chem. 2023, 15, 473–495. [Google Scholar] [CrossRef] [PubMed]
- Carrieri, A.; Barbarossa, A.; de Candia, M.; Samarelli, F.; Altomare, C.D.; Czarnota-Łydka, K.; Sudoł-Tałaj, S.; Latacz, G.; Handzlik, J.; Brunetti, L.; et al. Chiral pyrrolidines as multipotent agents in Alzheimer and neurodegenerative diseases. Bioorg. Med. Chem. 2024, 110, 117829. [Google Scholar] [CrossRef] [PubMed]
- Li Petri, G.; Raimondi, M.V.; Spanò, V.; Holl, R.; Barraja, P.; Montalbano, A. Pyrrolidine in Drug Discovery: A Versatile Scaffold for Novel Biologically Active Compounds. Top. Curr. Chem. 2021, 379, 34. [Google Scholar] [CrossRef] [PubMed]
- Poyraz, S.; Döndaş, H.A.; Sansano, J.M.; Belveren, S.; Yamali, C.; Ülger, M.; Döndaş, N.Y.; Sağlık, B.N.; Pask, C.M. N-Benzoylthiourea-pyrrolidine carboxylic acid derivatives bearing an imidazole moiety: Synthesis, characterization, crystal structure, in vitro ChEs inhibition, and antituberculosis, antibacterial, antifungal studies. J. Mol. Struct. 2023, 1273, 134303. [Google Scholar] [CrossRef]
- Lv, Y.; Fan, M.; He, J.; Song, X.; Guo, J.; Gao, B.; Zhang, J.; Zhang, C.; Xie, Y. Discovery of novel benzimidazole derivatives as selective and reversible monoamine oxidase B inhibitors for Parkinson’s disease treatment. Eur. J. Med. Chem. 2024, 274, 116566. [Google Scholar] [CrossRef]
- Ahsan, N.; Mishra, S.; Jain, M.K.; Surolia, A.; Gupta, S. Curcumin Pyrazole and its derivative (N-(3-Nitrophenylpyrazole) Curcumin inhibit aggregation, disrupt fibrils and modulate toxicity of Wild type and Mutant α-Synuclein. Sci. Rep. 2015, 5, 9862. [Google Scholar] [CrossRef]
- Patel, D.V.; Patel, N.R.; Kanhed, A.M.; Teli, D.M.; Patel, K.B.; Joshi, P.D.; Patel, S.P.; Gandhi, P.M.; Chaudhary, B.N.; Prajapati, N.K.; et al. Novel carbazole-stilbene hybrids as multifunctional anti-Alzheimer agents. Bioorg. Chem. 2020, 101, 103977. [Google Scholar] [CrossRef]
- Chiu, Y.J.; Lin, C.H.; Lin, C.Y.; Yang, P.N.; Lo, Y.S.; Chen, Y.C.; Chen, C.M.; Wu, Y.R.; Yao, C.F.; Chang, K.H.; et al. Investigating Therapeutic Effects of Indole Derivatives Targeting Inflammation and Oxidative Stress in Neurotoxin-Induced Cell and Mouse Models of Parkinson’s Disease. Int. J. Mol. Sci. 2023, 24, 2642. [Google Scholar] [CrossRef]
- Saini, N.; Akhtar, A.; Chauhan, M.; Dhingra, N.; Pilkhwal Sah, S. Protective effect of Indole-3-carbinol, an NF-κB inhibitor in experimental paradigm of Parkinson’s disease: In silico and in vivo studies. Brain Behav. Immun. 2020, 90, 108–137. [Google Scholar] [CrossRef]
- Elbatrawy, A.A.; Ademoye, T.A.; Alnakhala, H.; Tripathi, A.; Zami, A.; Ostafe, R.; Dettmer, U.; Fortin, J.S. Discovery of small molecule benzothiazole and indole derivatives tackling tau 2N4R and α-synuclein fibrils. Bioorg Med. Chem. 2024, 100, 117613. [Google Scholar] [CrossRef]
- Bansal, R.; Singh, R.; Rana, P. Synthesis, in silico Studies and Pharmacological Evaluation of a New Series of Indanone Derivatives as Anti-Parkinsonian and Anti-Alzheimer’s Agents. Curr. Comput. Aided Drug Des. 2023, 19, 94–107. [Google Scholar] [CrossRef]
- Michalska, P.; Mayo, P.; Fernández-Mendívil, C.; Tenti, G.; Duarte, P.; Buendia, I.; Ramos, M.T.; López, M.G.; Menéndez, J.C.; León, R. Antioxidant, Anti-inflammatory and Neuroprotective Profiles of Novel 1,4-Dihydropyridine Derivatives for the Treatment of Alzheimer’s Disease. Antioxidants 2020, 9, 650. [Google Scholar] [CrossRef] [PubMed]
- Gutti, G.; Kumar, D.; Paliwal, P.; Ganeshpurkar, A.; Lahre, K.; Kumar, A.; Krishnamurthy, S.; Singh, S.K. Development of pyrazole and spiropyrazoline analogs as multifunctional agents for treatment of Alzheimer’s disease. Bioorg. Chem. 2019, 90, 103080. [Google Scholar] [CrossRef]
- Jayaraj, R.L.; Elangovan, N.; Dhanalakshmi, C.; Manivasagam, T.; Essa, M.M. CNB-001, a novel pyrazole derivative mitigates motor impairments associated with neurodegeneration via suppression of neuroinflammatory and apoptotic response in experimental Parkinson’s disease mice. Chem. Biol. Interact. 2014, 220, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.; Chioua, M.; Samadi, A.; Carmo Carreiras, M.; Jimeno, M.L.; Mendes, E.; Ríos Cde, L.; Romero, A.; Villarroya, M.; López, M.G.; et al. Synthesis and pharmacological assessment of diversely substituted pyrazolo[3,4-b]quinoline, and benzo[b]pyrazolo[4,3-g][1,8]naphthyridine derivatives. Eur. J. Med. Chem. 2011, 46, 4676–4681. [Google Scholar] [CrossRef] [PubMed]
- Pisani, L.; Farina, R.; Catto, M.; Iacobazzi, R.M.; Nicolotti, O.; Cellamare, S.; Mangiatordi, G.F.; Denora, N.; Soto-Otero, R.; Siragusa, L.; et al. Exploring Basic Tail Modifications of Coumarin-Based Dual Acetylcholinesterase-Monoamine Oxidase B Inhibitors: Identification of Water-Soluble, Brain-Permeant Neuroprotective Multitarget Agents. J. Med. Chem. 2016, 59, 6791–6806. [Google Scholar] [CrossRef]
- González-Muñoz, G.C.; Arce, M.P.; Pérez, C.; Romero, A.; Villarroya, M.; López, M.G.; Conde, S.; Rodríguez-Franco, M.I. Dibenzo[1,4,5]thiadiazepine: A hardly-known heterocyclic system with neuroprotective properties of potential usefulness in the treatment of neurodegenerative diseases. Eur. J. Med. Chem. 2014, 81, 350–358. [Google Scholar] [CrossRef]
- Lincoln, K.M.; Richardson, T.E.; Rutter, L.; Gonzalez, P.; Simpkins, J.W.; Green, K.N. An N-heterocyclic amine chelate capable of antioxidant capacity and amyloid disaggregation. ACS Chem. Neurosci. 2012, 3, 919–927. [Google Scholar] [CrossRef]
- Matos, M.J.; Rodríguez-Enríquez, F.; Borges, F.; Santana, L.; Uriarte, E.; Estrada, M.; Rodríguez-Franco, M.I.; Laguna, R.; Viña, D. 3-Amidocoumarins as Potential Multifunctional Agents against Neurodegenerative Diseases. ChemMedChem 2015, 10, 2071–2079. [Google Scholar] [CrossRef]
- Papagiouvannis, G.; Theodosis-Nobelos, P.; Rekka, E.A. Nipecotic Acid Derivatives as Potent Agents against Neurodegeneration: A Preliminary Study. Molecules 2022, 27, 6984. [Google Scholar] [CrossRef]
- Sirakanyan, S.N.; Spinelli, D.; Petrou, A.; Geronikaki, A.; Kartsev, V.G.; Hakobyan, E.K.; Yegoryan, H.A.; Zuppiroli, L.; Zuppiroli, R.; Ayvazyan, A.G.; et al. New Bicyclic Pyridine-Based Hybrids Linked to the 1,2,3-Triazole Unit: Synthesis via Click Reaction and Evaluation of Neurotropic Activity and Molecular Docking. Molecules 2023, 28, 921. [Google Scholar] [CrossRef] [PubMed]
- Porcal, W.; Hernández, P.; González, M.; Ferreira, A.; Olea-Azar, C.; Cerecetto, H.; Castro, A. Heteroarylnitrones as drugs for neurodegenerative diseases: Synthesis, neuroprotective properties, and free radical scavenger properties. J. Med. Chem. 2008, 51, 6150–6159. [Google Scholar] [CrossRef] [PubMed]
- Escribano, L.; Simón, A.M.; Gimeno, E.; Cuadrado-Tejedor, M.; De Maturana, R.L.; García-Osta, A.; Pérez-Mediavilla, A.; Del Río, J.; Frechilla, D. Rosiglitazone Rescues Memory Impairment in Alzheimer’s Transgenic Mice: Mechanisms Involving a Reduced Amyloid and Tau Pathology. Neuropsychopharmacology 2010, 35, 1593–1604. [Google Scholar] [CrossRef] [PubMed]
- Mokhtari, Z.; Baluchnejadmojarad, T.; Nikbakht, F.; Mansouri, M.; Roghani, M. Riluzole ameliorates learning and memory deficits in Aβ25-35-induced rat model of Alzheimer’s disease and is independent of cholinoceptor activation. Biomed. Pharmacother. 2017, 87, 135–144. [Google Scholar] [CrossRef]
- Vanda, D.; Zajdel, P.; Soural, M. Imidazopyridine-based selective and multifunctional ligands of biological targets associated with psychiatric and neurodegenerative diseases. Eur. J. Med. Chem. 2019, 181, 111569. [Google Scholar] [CrossRef]
- Sridhar, P.; Alagumuthu, M.; Ram, B.; Arumugam, S.; Reddy, S.R. Drugs Against Neurodegenerative Diseases: Design and Synthesis of 6-Amino–substituted Imidazo[1,2-b]pyridazines as Acetylcholinesterase Inhibitors. ChemistrySelect 2017, 2, 842–847. [Google Scholar] [CrossRef]
- Işık, M.; Demir, Y.; Durgun, M.; Türkeş, C.; Necip, A.; Beydemir, Ş. Molecular docking and investigation of 4-(benzylideneamino)- and 4-(benzylamino)-benzenesulfonamide derivatives as potent AChE inhibitors. Chem. Pap. 2020, 74, 1395–1405. [Google Scholar] [CrossRef]
- Sever, B.; Türkeş, C.; Altıntop, M.D.; Demir, Y.; Beydemir, Ş. Thiazolyl-pyrazoline derivatives: In vitro and in silico evaluation as potential acetylcholinesterase and carbonic anhydrase inhibitors. Int. J. Biol. Macromol. 2020, 163, 1970–1988. [Google Scholar] [CrossRef]
- Gündoğdu, S.; Türkeş, C.; Arslan, M.; Demir, Y.; Beydemir, Ş. New Isoindole-1,3-dione Substituted Sulfonamides as Potent Inhibitors of Carbonic Anhydrase and Acetylcholinesterase: Design, Synthesis, and Biological Evaluation. ChemistrySelect 2019, 4, 13347–13355. [Google Scholar] [CrossRef]
- Andrade-Jorge, E.; Sánchez-Labastida, L.A.; Soriano-Ursúa, M.A.; Guevara-Salazar, J.A.; Trujillo-Ferrara, J.G. Isoindolines/isoindoline-1,3-diones as AChE inhibitors against Alzheimer’s disease, evaluated by an improved ultra-micro assay. Med. Chem. Res. 2018, 27, 2187–2198. [Google Scholar] [CrossRef]
- Azimi, S.; Firuzi, O.; Iraji, A.; Zonouzi, A.; Khoshneviszadeh, M.; Mahdavi, M.; Edraki, N. Synthesis and In Vitro Biological Activity Evaluation of Novel Imidazo [2,1-B][1,3,4] Thiadiazole as Anti-Alzheimer Agents. Lett. Drug Des. Discov. 2018, 17, 610–617. [Google Scholar] [CrossRef]
- Bergin, J.C.J.; Tan, K.K.; Nelson, A.K.; Amarandei, C.A.; Hubscher-Bruder, V.; Brandel, J.; Voinarovska, V.; Dejaegere, A.; Stote, R.H.; Tétard, D. 1-Hydroxy-2(1H)-pyridinone-Based Chelators with Potential Catechol O-Methyl Transferase Inhibition and Neurorescue Dual Action against Parkinson’s Disease. Molecules 2022, 27, 2816. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, S.; Dhavan, P.; Pavale, G.; Ramana, M.M.V.; Jadhav, B.L. Design, synthesis and evaluation of pyrazole bearing α-aminophosphonate derivatives as potential acetylcholinesterase inhibitors against Alzheimer’s disease. Bioorg Chem. 2020, 96, 103589. [Google Scholar] [CrossRef] [PubMed]
- Engelbrecht, I.; Petzer, J.P.; Petzer, A. Nitrocatechol Derivatives of Chalcone as Inhibitors of Monoamine Oxidase and Catechol-O-Methyltransferase. Cent. Nerv. Syst. Agents Med. Chem. 2018, 18, 115–127. [Google Scholar] [CrossRef]
- Hussain, R.; Rahim, F.; Rehman, W.; Khan, S.; Rasheed, L.; Maalik, A.; Taha, M.; Alanazi, M.M.; Alanazi, A.S.; Khan, I. Synthesis, in vitro analysis and molecular docking study of novel benzoxazole-based oxazole derivatives for the treatment of Alzheimer’s disease. Arab. J. Chem. 2023, 16, 105244. [Google Scholar] [CrossRef]
- García Marín, I.D.; Camarillo López, R.H.; Martínez, O.A.; Padilla-Martínez, I.I.; Correa-Basurto, J.; Rosales-Hernández, M.C. New compounds from heterocyclic amines scaffold with multitarget inhibitory activity on Aβ aggregation, AChE, and BACE1 in the Alzheimer disease. PLoS ONE 2022, 17, e0269129. [Google Scholar] [CrossRef]
- Benek, O.; Hroch, L.; Aitken, L.; Dolezal, R.; Guest, P.; Benkova, M.; Soukup, O.; Musil, K.; Kuca, K.; Smith, T.K.; et al. 6-benzothiazolyl ureas, thioureas and guanidines are potent inhibitors of ABAD/17β-HSD10 and potential drugs for Alzheimer’s disease treatment: Design, synthesis and in vitro evaluation. Med. Chem. 2017, 13, 345–358. [Google Scholar] [CrossRef]
- Patil, S.B. Recent medicinal approaches of novel pyrimidine analogs: A review. Heliyon 2023, 9, e16773. [Google Scholar] [CrossRef]
- Pant, S.; Kapri, A.; Nain, S. Pyrimidine analogues for the management of neurodegenerative diseases. Eur. J. Med. Chem. Rep. 2022, 6, 100095. [Google Scholar] [CrossRef]
- Costa, R.F.; Turones, L.C.; Cavalcante, K.V.N.; Rosa Júnior, I.A.; Xavier, C.H.; Rosseto, L.P.; Napolitano, H.B.; Castro, P.F.D.S.; Neto, M.L.F.; Galvão, G.M.; et al. Heterocyclic Compounds: Pharmacology of Pyrazole Analogs From Rational Structural Considerations. Front. Pharmacol. 2021, 12, 666725. [Google Scholar] [CrossRef]
- Li, X.; Yu, Y.; Tu, Z. Pyrazole Scaffold Synthesis, Functionalization, and Applications in Alzheimer’s Disease and Parkinson’s Disease Treatment (2011–2020). Molecules 2021, 26, 1202. [Google Scholar] [CrossRef] [PubMed]
- Turkan, F.; Cetin, A.; Taslimi, P.; Gulçin, İ. Some pyrazoles derivatives: Potent carbonic anhydrase, α-glycosidase, and cholinesterase enzymes inhibitors. Arch. Pharm. 2018, 351, 1800200. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, M.J.; Ali, A.; Ali, A.; Thiriveedhi, A.; Bakht, M.A.; Yusuf, M.; Salahuddin Afzal, O.; Altamimi, A.S.A. Pyrazoline Containing Compounds as Therapeutic Targets for Neurodegenerative Disorders. ACS Omega 2022, 7, 38207–38245. [Google Scholar] [CrossRef] [PubMed]
- Hitge, R.; Smit, S.; Petzer, A.; Petzer, J.P. Evaluation of nitrocatechol chalcone and pyrazoline derivatives as inhibitors of catechol-O-methyltransferase and monoamine oxidase. Bioorg Med. Chem. Lett. 2020, 30, 127188. [Google Scholar] [CrossRef]
- Abdelgawad, M.A.; Oh, J.M.; Parambi, D.G.T.; Kumar, S.; Musa, A.; Ghoneim, M.M.; Nayl, A.A.; El-Ghorab, A.H.; Iqrar Ahmad Patel, H.; Kim, H.; et al. Development of bromo- and fluoro-based α, β-unsaturated ketones as highly potent MAO-B inhibitors for the treatment of Parkinson’s disease. J. Mol. Struct. 2022, 1266, 133545. [Google Scholar] [CrossRef]
- Mathew, B.; Parambi, D.G.T.; Mathew, G.E.; Uddin, M.S.; Inasu, S.T.; Kim, H.; Marathakam, A.; Unnikrishnan, M.K.; Carradori, S. Emerging therapeutic potentials of dual-acting MAO and AChE inhibitors in Alzheimer’s and Parkinson’s diseases. Arch. Pharm. 2019, 352, 1900177. [Google Scholar] [CrossRef]
- Husain, A.; Balushi, K.A.; Akhtar, M.J.; Khan, S.A. Coumarin linked heterocyclic hybrids: A promising approach to develop multi target drugs for Alzheimer’s disease. J. Mol. Struct. 2021, 1241, 130618. [Google Scholar] [CrossRef]
- Petrou, A.; Fesatidou, M.; Geronikaki, A. Thiazole Ring—A Biologically Active Scaffold. Molecules 2021, 26, 3166. [Google Scholar] [CrossRef]
- Mishra, C.B.; Kumari, S.; Tiwari, M. Thiazole: A promising heterocycle for the development of potent CNS active agents. Eur. J. Med. Chem. 2015, 92, 1–34. [Google Scholar] [CrossRef]
- Askin, S.; Tahtaci, H.; Türkeş, C.; Demir, Y.; Ece, A.; Akalın Çiftçi, G.; Beydemir, Ş. Design, synthesis, characterization, in vitro and in silico evaluation of novel imidazo[2,1-b][1,3,4]thiadiazoles as highly potent acetylcholinesterase and non-classical carbonic anhydrase inhibitors. Bioorg. Chem. 2021, 113, 105009. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.; Xu, H.; Zheng, Z.; Meng, Z.; Xu, Z.; Li, J.; Xue, M. Design and synthesis of five-membered heterocyclic derivatives of istradefylline with comparable pharmacological activity. Chem. Biol. Drug Des. 2022, 100, 534–552. [Google Scholar] [CrossRef] [PubMed]
- Oz, M.; Lorke, D.E.; Hasan, M.; Petroianu, G.A. Cellular and molecular actions of Methylene Blue in the nervous system. Med. Res. Rev. 2011, 31, 93–117. [Google Scholar] [CrossRef] [PubMed]
- Akoury, E.; Pickhardt, M.; Gajda, M.; Biernat, J.; Mandelkow, E.; Zweckstetter, M. Mechanistic basis of phenothiazine-driven inhibition of Tau aggregation. Angew. Chem. Int. Ed. Engl. 2013, 52, 3511–3515. [Google Scholar] [CrossRef]
- Kassab, A.E.; Gedawy, E.M.; Sayed, A.S. Fused thiophene as a privileged scaffold: A review on anti-Alzheimer’s disease potentials via targeting cholinesterases, monoamine oxidases, glycogen synthase kinase-3, and Aβ aggregation. Int. J. Biol. Macromol. 2024, 265, 131018. [Google Scholar] [CrossRef] [PubMed]
- Kiss, L.E.; Bonifácio, M.J.; Rocha, J.F.; Soares-da-Silva, P. Opicapone, a Novel Catechol-O-Methyltranferase Inhibitor (COMT) to Manage the Symptoms of Parkinson’s Disease. Success. Drug Discov. 2018, 3, 319–340. [Google Scholar] [CrossRef]
- Shen, Z.B.; Meng, H.W.; Meng, X.S.; Lv, Z.K.; Fang, M.Y.; Zhang, L.L.; Lv, Z.L.; Li, M.S.; Liu, A.K.; Han, J.H.; et al. Design, synthesis, and SAR study of novel flavone 1,2,4-oxadiazole derivatives with anti-inflammatory activities for the treatment of Parkinson’s disease. Eur. J. Med. Chem. 2023, 255, 115417. [Google Scholar] [CrossRef]
- Królicka, E.; Kieć-Kononowicz, K.; Łażewska, D. Chalcones as Potential Ligands for the Treatment of Parkinson’s Disease. Pharmaceuticals 2022, 15, 847. [Google Scholar] [CrossRef]
- Ceyhun, İ.; Karaca, Ş.; Osmaniye, D.; Sağlık, B.N.; Levent, S.; Özkay, Y.; Kaplancıklı, Z.A. Design and synthesis of novel chalcone derivatives and evaluation of their inhibitory activities against acetylcholinesterase. Arch. Pharm. 2022, 355, e2100372. [Google Scholar] [CrossRef]
- Pibiri, I. Recent Advances: Heterocycles in Drugs and Drug Discovery. Int. J. Mol. Sci. 2024, 25, 9503. [Google Scholar] [CrossRef]
- Adelusi, T.I.; Oyedele, A.Q.K.; Boyenle, I.D.; Ogunlana, A.T.; Adeyemi, R.O.; Ukachi, C.D.; Idris, M.O.; Olaoba, O.T.; Adedotun, I.O.; Kolawole, O.E.; et al. Molecular modeling in drug discovery. Inf. Med. Unlocked 2022, 29, 100880. [Google Scholar] [CrossRef]
- Lim, H.-S. Evolving role of modeling and simulation in drug development. Transl. Clin. Pharmacol. 2019, 27, 19. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Ye, Z.; Gao, H.; Ouyang, D. Computational pharmaceutics—A new paradigm of drug delivery. J. Control. Release 2021, 338, 119–136. [Google Scholar] [CrossRef] [PubMed]
- AlRawashdeh, S.; Barakat, K.H. Applications of Molecular Dynamics Simulations in Drug Discovery. Methods Mol. Biol. 2024, 2714, 127–141. [Google Scholar] [CrossRef] [PubMed]
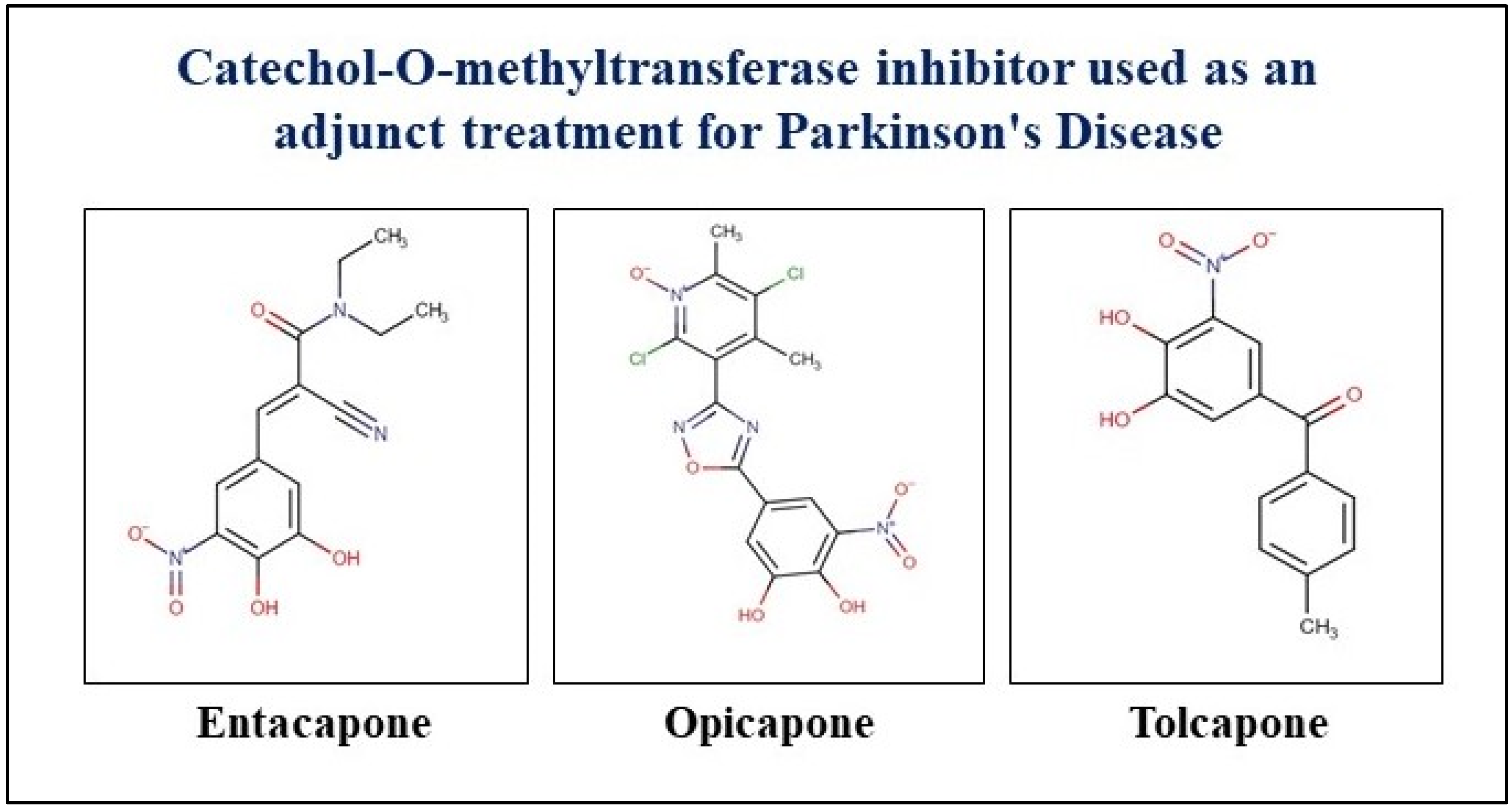
| Drug | Therapeutic Category | Brand Name | Target | FDA Approval | Application |
|---|---|---|---|---|---|
| Brexpiprazole | Atypical antipsychotic | Rexulti | Novel D2 dopamine and serotonin 1A partial agonist | 2015 | Approved for the treatment of depression, schizophrenia, and agitation associated with dementia due to AD |
| Donepezil | Parasympathomemetic | Adlarity, Aricept, Namzaric | AChE inhibitor | 1996 | Used to treat the behavioral and cognitive effects of AD and other types of dementia |
| Rivastigmine | Parasympathomemetic | Exelon, Nimvastid, Prometax | Inhibits both BChE and AChE | 2000 | Used to treat mild to moderate dementia in AD and PD |
| Galantamine | Parasympathomemetic | Razadyne | Competitive inhibitor of AChE | 2001 | Reduces the severity of dementia in patients with AD |
| Memantine | NAMD antagonist | Axura, Ebixa, Marixino, Namenda, Namzaric | NMDA receptor antagonist | 2013 | Blocks the effects of Glu in the brain that lead to neuronal excitability and excessive stimulation in AD |
| Safinamide | MAO-B inhibitor | Xadago | Selective and reversible inhibition of MAO-B with blockade of voltage-dependent Na+ and Ca2+ channels and inhibition of Glu release | 2017 | Neuroprotective and neuro-rescuing effects in PD |
| Istradefylline | - | Nourianz | Targets adenosine A2A receptors in the basal ganglia | 2019 | Used to treat reduced GABAergic action and motor control in PD patients |
| Pimavanserin | Atypical antipsychotic | Nuplazid | Interacts with the serotonin receptors, particularly the 5-HT2A and HT2C receptors | 2016 | Used to treat PD- associated psychotic symptoms without causing extrapyramidal or worsening motor symptoms |
| Amantadine | Influenza A M2 protein inhibitor | Gocovri, Osmolex | Releasing dopamine from the nerve endings of the brain cells, and stimulation of norepinephrine response; it also has NMDA receptor antagonistic effects | 1973 (for PD) | Used to treat dyskinesia in Parkinson’s patients |
| Benzatropine | Anticholinergic agent and histamine antagonist | Cogentin | Selective inhibition of dopamine transporters | 1996 | Used as an adjunct in the therapy of all forms of parkinsonism |
| Biperiden | Anticholinergic agent | NA | Competitive antagonism of ACh at cholinergic receptors in the corpus striatum | 1959 | An adjunct in the therapy of all forms of parkinsonism and control of extrapyramidal disorders secondary to neuroleptic drug therapy |
| Trihexyphenidyl | Anticholinergic agent and muscarinic antagonist | NA | ACh receptor (M1 subtype) antagonist | 1949 | Anticholinergic activity useful in the treatment of symptoms associated with PD |
| Carbidopa | AADC inhibitor | Crexont, Dhivy, Duodopa, Duopa, Lodosyn, Parcopa, Rytary, Sinemet, Stalevo | Dopa decarboxylase inhibitor used in combination with levodopa | 2014 | Symptomatic treatment of idiopathic PD and other conditions associated with parkinsonian symptoms |
| Entacapone | COMT inhibitor | Comtan, Comtess, Stalevo | Administered concomittantly with levodopa and carbidopa; increased and more sustained plasma levodopa concentrations are reached as compared to the administration of levodopa and a decarboxylase inhibitor | 1999 | Symptomatic treatment of patients with idiopathic PD |
| Tolcapone | COMT inhibitor | Tasmar | Inhibits the enzyme COMT used as an adjunct | 1998 | Helps to improve the symptoms of PD such as trembling, difficulty with movement, stiffness, and other symptoms |
| Opicapone | COMT inhibitor | Ongentys | COMT inhibitor used as an adjunct | 2020 | Managing motor and some nonmotor symptoms associated with PD |
| Drug | IUPAC Name | Compound and Class | Structure | Diseases | Mechanism of Action | Clinical Trial No | Clinical Phase | Sponsor Company |
|---|---|---|---|---|---|---|---|---|
| Semagacestat (LY-450139) | (2S)-2-hydroxy-3-methyl-N-[(2S)-1-[[(1S)-3-methyl-2-oxo-4,5-dihydro-1H-3-benzazepin-1-yl]amino]-1-oxopropan-2-yl]butanamide | Organic acids and derivatives Class: carboxylic acids and derivatives | 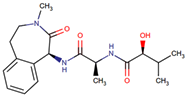 | AD | Small-molecule γ-secretase inhibitor | NCT01035138 | Phase 3 | Eli Lilly and Company |
| ANAVEX2-73 (Blarcamesine) | [(2,2-diphenyloxolan-3-yl)methyl]dimethylamine | NA | 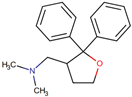 | AD | Cognition and function | NCT03790709 | Phase 2b/3 | Anavex Life Sciences Corp. |
| Dimebon (Latrepirdine) | 5-(2-{2,8-dimethyl-1H,2H,3H,4H,5H-pyrido[4,3-b]indol-5-yl}ethyl)-2-methylpyridine | Organoheterocyclic compounds Class: indoles and derivatives | 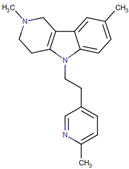 | AD | Improves cognition in models of AD | NCT00912288 | Phase 3 | Pfizer |
| ALZ- 801 (Valiltramiprosate) | 3-[[(2S)-2-amino-3-methylbutanoyl]amino]propane-1-sulfonic acid | NA | 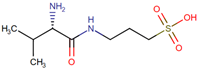 | AD | Prevents Aβ42 from forming oligomers | NCT06304883 | Phase 3 | Alzheon Inc. |
| Isradipine | 3-methyl 5-propan-2-yl 4-(2,1,3-benzoxadiazol-4-yl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate | Organoheterocyclic compounds Class: benzoxadiazoles | 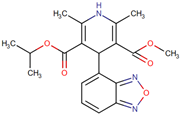 | PD | Calcium channel blocker | NCT02168842 | Phase 3 | University of Rochester |
| Nilvadipine | 3-O-methyl 5-O-propan-2-yl 2-cyano-6-methyl-4-(3-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylate | Organoheterocyclic compounds Class: pyridines and derivatives | 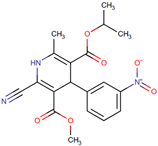 | Calcium channel blocker | NCT02017340 | Phase 3 | Prof Brian Lawlor, St. James’s Hospital, Ireland |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puranik, N.; Song, M. Therapeutic Role of Heterocyclic Compounds in Neurodegenerative Diseases: Insights from Alzheimer’s and Parkinson’s Diseases. Neurol. Int. 2025, 17, 26. https://doi.org/10.3390/neurolint17020026
Puranik N, Song M. Therapeutic Role of Heterocyclic Compounds in Neurodegenerative Diseases: Insights from Alzheimer’s and Parkinson’s Diseases. Neurology International. 2025; 17(2):26. https://doi.org/10.3390/neurolint17020026
Chicago/Turabian StylePuranik, Nidhi, and Minseok Song. 2025. "Therapeutic Role of Heterocyclic Compounds in Neurodegenerative Diseases: Insights from Alzheimer’s and Parkinson’s Diseases" Neurology International 17, no. 2: 26. https://doi.org/10.3390/neurolint17020026
APA StylePuranik, N., & Song, M. (2025). Therapeutic Role of Heterocyclic Compounds in Neurodegenerative Diseases: Insights from Alzheimer’s and Parkinson’s Diseases. Neurology International, 17(2), 26. https://doi.org/10.3390/neurolint17020026







