The Association of Cerebral Blood Flow Measured Using Extracranial Carotid Ultrasound with Functional Outcomes in Patients with Anterior Circulation Large Vessel Occlusion After Endovascular Thrombectomy—A Retrospective Study
Abstract
1. Introduction
2. Methods
2.1. Study Population
2.2. Data and Examination
2.3. Variable Abstraction and Management
2.4. Statistical Analysis
3. Results
3.1. Comparison Between Enrolled and Excluded Patients
3.2. Baseline Variables and Correlation Analysis
3.3. Predictors of Fair Outcomes
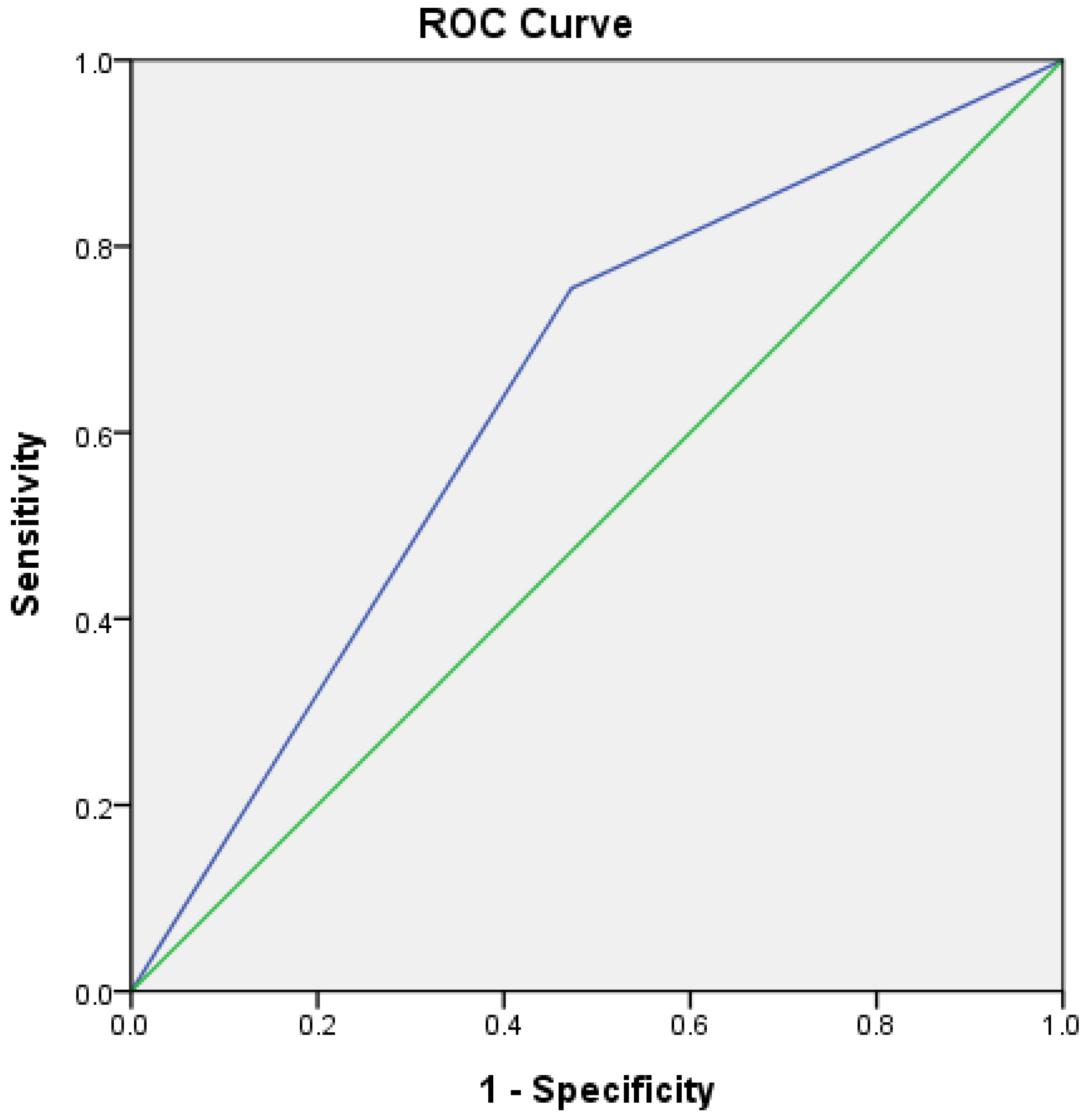
3.4. Validation of Outcome Predictors, Including Sonography Index
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke 2019, 50, e344–e418. [Google Scholar] [CrossRef] [PubMed]
- Turc, G.; Bhogal, P.; Fischer, U.; Khatri, P.; Lobotesis, K.; Mazighi, M.; Schellinger, P.D.; Toni, D.; de Vries, J.; White, P.; et al. European Stroke Organisation (ESO)—European Society for Minimally Invasive Neurological Therapy (ESMINT) Guidelines on Mechanical Thrombectomy in Acute Ischaemic StrokeEndorsed by Stroke Alliance for Europe (SAFE). Eur. Stroke J. 2019, 4, 6–12. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Albers, G.W.; Marks, M.P.; Kemp, S.; Christensen, S.; Tsai, J.P.; Ortega-Gutierrez, S.; McTaggart, R.A.; Torbey, M.T.; Kim-Tenser, M.; Leslie-Mazwi, T.; et al. Thrombectomy for Stroke at 6 to 16 Hours with Selection by Perfusion Imaging. N. Engl. J. Med. 2018, 378, 708–718. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ramos, L.A.; Kappelhof, M.; van Os, H.J.A.; Chalos, V.; Van Kranendonk, K.; Kruyt, N.D.; Roos, Y.; van der Lugt, A.; van Zwam, W.H.; van der Schaaf, I.C.; et al. Predicting Poor Outcome Before Endovascular Treatment in Patients With Acute Ischemic Stroke. Front. Neurol. 2020, 11, 580957. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Haranhalli, N.; Javed, K.; Boyke, A.; Dardick, J.; Naidu, I.; Ryvlin, J.; Kadaba, D.; Fluss, R.; Derby, C.; Altschul, D. A Predictive Model for Functional Outcome in Patients with Acute Ischemic Stroke Undergoing Endovascular Thrombectomy. J. Stroke Cerebrovasc. Dis. 2021, 30, 106054. [Google Scholar] [CrossRef] [PubMed]
- Marks, M.P.; Lansberg, M.G.; Mlynash, M.; Olivot, J.M.; Straka, M.; Kemp, S.; McTaggart, R.; Inoue, M.; Zaharchuk, G.; Bammer, R.; et al. Effect of collateral blood flow on patients undergoing endovascular therapy for acute ischemic stroke. Stroke 2014, 45, 1035–1039. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Flint, A.C.; Cullen, S.P.; Faigeles, B.S.; Rao, V.A. Predicting long-term outcome after endovascular stroke treatment: The totaled health risks in vascular events score. AJNR Am. J. Neuroradiol. 2010, 31, 1192–1196. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hallevi, H.; Barreto, A.D.; Liebeskind, D.S.; Morales, M.M.; Martin-Schild, S.B.; Abraham, A.T.; Gadia, J.; Saver, J.L.; Investigators, U.I.-A.T.; Grotta, J.C.; et al. Identifying patients at high risk for poor outcome after intra-arterial therapy for acute ischemic stroke. Stroke 2009, 40, 1780–1785. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lin, M.T.; Wu, H.W.; Chen, H.Y.; Tsai, H.T.; Wu, C.H. Association of clinical factors to functional outcomes in patients with stroke with large-vessel occlusion after endovascular thrombectomy. J. Formos. Med. Assoc. 2023, 122, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Chen, X.Y.; Wang, H.Y.; Shang, Y.F.; Shen, P.P.; Zhang, S.; Guo, S.Y.; Tan, M.M.; Geng, Y. Hemodynamic predictors of early neurological deterioration and clinical outcome after endovascular treatment in large artery occlusion. Heliyon 2024, 10, e24746. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Martin Vicario, C.; Rodriguez Salas, D.; Maier, A.; Hock, S.; Kuramatsu, J.; Kallmuenzer, B.; Thamm, F.; Taubmann, O.; Ditt, H.; Schwab, S.; et al. Uncertainty-aware deep learning for trustworthy prediction of long-term outcome after endovascular thrombectomy. Sci. Rep. 2024, 14, 5544. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Villringer, K.; Zimny, S.; Galinovic, I.; Nolte, C.H.; Fiebach, J.B.; Khalil, A.A. The Association Between Recanalization, Collateral Flow, and Reperfusion in Acute Stroke Patients: A Dynamic Susceptibility Contrast MRI Study. Front. Neurol. 2019, 10, 1147. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chamorro, A.; Meisel, A.; Planas, A.M.; Urra, X.; van de Beek, D.; Veltkamp, R. The immunology of acute stroke. Nat. Rev. Neurol. 2012, 8, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Son, J.P.; Ryoo, S.; Lee, M.J.; Cha, J.; Kim, K.H.; Kim, G.M.; Chung, C.S.; Lee, K.H.; Jeon, P.; et al. A novel magnetic resonance imaging approach to collateral flow imaging in ischemic stroke. Ann. Neurol. 2014, 76, 356–369. [Google Scholar] [CrossRef] [PubMed]
- Cincura, C.; Pontes-Neto, O.M.; Neville, I.S.; Mendes, H.F.; Menezes, D.F.; Mariano, D.C.; Pereira, I.F.; Teixeira, L.A.; Jesus, P.A.; de Queiroz, D.C.; et al. Validation of the National Institutes of Health Stroke Scale, modified Rankin Scale and Barthel Index in Brazil: The role of cultural adaptation and structured interviewing. Cerebrovasc. Dis. 2009, 27, 119–122. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Barber, P.A.; Demchuk, A.M.; Zhang, J.; Buchan, A.M. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. ASPECTS Study Group. Alberta Stroke Programme Early CT Score. Lancet 2000, 355, 1670–1674. [Google Scholar] [CrossRef] [PubMed]
- Goyal, M.; Fargen, K.M.; Turk, A.S.; Mocco, J.; Liebeskind, D.S.; Frei, D.; Demchuk, A.M. 2C or not 2C: Defining an improved revascularization grading scale and the need for standardization of angiography outcomes in stroke trials. J. Neurointerv. Surg. 2014, 6, 83–86. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jovin, T.G.; Chamorro, A.; Cobo, E.; de Miquel, M.A.; Molina, C.A.; Rovira, A.; San Roman, L.; Serena, J.; Abilleira, S.; Ribo, M.; et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N. Engl. J. Med. 2015, 372, 2296–2306. [Google Scholar] [CrossRef] [PubMed]
- Ghozy, S.; Kacimi, S.E.O.; Azzam, A.Y.; Farahat, R.A.; Abdelaal, A.; Kallmes, K.M.; Adusumilli, G.; Heit, J.J.; Kadirvel, R.; Kallmes, D.F. Successful mechanical thrombectomy in acute ischemic stroke: Revascularization grade and functional independence. J. Neurointerv. Surg. 2022, 14, 779–782. [Google Scholar] [CrossRef] [PubMed]
- Berkhemer, O.A.; Fransen, P.S.; Beumer, D.; van den Berg, L.A.; Lingsma, H.F.; Yoo, A.J.; Schonewille, W.J.; Vos, J.A.; Nederkoorn, P.J.; Wermer, M.J.; et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N. Engl. J. Med. 2015, 372, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Goyal, M.; Demchuk, A.M.; Menon, B.K.; Eesa, M.; Rempel, J.L.; Thornton, J.; Roy, D.; Jovin, T.G.; Willinsky, R.A.; Sapkota, B.L.; et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N. Engl. J. Med. 2015, 372, 1019–1030. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Yang, L.; Hang, J.; You, S.; Li, J.; Li, X.; Wang, L.; Jiang, L.; Li, W.; Yu, H. Predictive value of the THRIVE score for outcome in patients with acute basilar artery occlusion treated with thrombectomy. Brain Behav. 2019, 9, e01418. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rangaraju, S.; Aghaebrahim, A.; Streib, C.; Sun, C.H.; Ribo, M.; Muchada, M.; Nogueira, R.; Frankel, M.; Gupta, R.; Jadhav, A.; et al. Pittsburgh Response to Endovascular therapy (PRE) score: Optimizing patient selection for endovascular therapy for large vessel occlusion strokes. J. Neurointerv. Surg. 2015, 7, 783–788. [Google Scholar] [CrossRef] [PubMed]
- Siegler, J.E.; Koneru, M.; Qureshi, M.M.; Doheim, M.; Nogueira, R.G.; Martinez-Majander, N.; Nagel, S.; Penckofer, M.; Demeestere, J.; Puetz, V.; et al. CLEAR Thrombectomy Score: An Index to Estimate the Probability of Good Functional Outcome With or Without Endovascular Treatment in the Late Window for Anterior Circulation Occlusion. J. Am. Heart Assoc. 2024, 13, e034948. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Javed, K.; Qin, J.; Mowery, W.; Kadaba, D.; Altschul, D.; Haranhalli, N. Predicting 90-day Functional Dependency and Death after Endovascular Thrombectomy for Stroke: The BET Score. J. Stroke Cerebrovasc. Dis. 2022, 31, 106342. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Colasurdo, M.; Phipps, M.S.; Miller, T.R.; Cherian, J.; Marino, J.; Cronin, C.A.; Wozniak, M.A.; Gandhi, D.; Chaturvedi, S.; et al. The BAND score: A simple model for upfront prediction of poor outcomes despite successful stroke thrombectomy. J. Stroke Cerebrovasc. Dis. 2024, 33, 107608. [Google Scholar] [CrossRef] [PubMed]
- Amin, S.D.; Chen, H.; Rewinkel, S.E.; Lockwood, D.A.; Kim, D.; Priest, R.A.; Nesbit, G.M.; Liu, J.J.; Horikawa, M.; Clark, W.M.; et al. The PANDA score: External validation and modification of a simple upfront prediction tool for poor outcomes despite successful stroke thrombectomy. Clin. Neurol. Neurosurg. 2025, 249, 108722. [Google Scholar] [CrossRef] [PubMed]
- Shahripour, R.B.; Azarpazhooh, M.R.; Akhuanzada, H.; Labin, E.; Borhani-Haghighi, A.; Agrawal, K.; Meyer, D.; Meyer, B.; Hemmen, T. Transcranial Doppler to evaluate postreperfusion therapy following acute ischemic stroke: A literature review. J. Neuroimaging 2021, 31, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Kneihsl, M.; Niederkorn, K.; Deutschmann, H.; Enzinger, C.; Poltrum, B.; Horner, S.; Thaler, D.; Kraner, J.; Fandler, S.; Colonna, I.; et al. Abnormal Blood Flow on Transcranial Duplex Sonography Predicts Poor Outcome After Stroke Thrombectomy. Stroke 2018, 49, 2780–2782. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Su, Y.; He, Y.; Zhang, Y.; Sun, Y.; Fan, L.; Liu, G.; Chen, Z. Controlling Blood Pressure Under Transcranial Doppler Guidance after Endovascular Treatment in Patients with Acute Ischemic Stroke. Cerebrovasc. Dis. 2020, 49, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Baracchini, C.; Farina, F.; Palmieri, A.; Kulyk, C.; Pieroni, A.; Viaro, F.; Cester, G.; Causin, F.; Manara, R. Early hemodynamic predictors of good outcome and reperfusion injury after endovascular treatment. Neurology 2019, 92, e2774–e2783. [Google Scholar] [CrossRef] [PubMed]
- Anadani, M.; Orabi, M.Y.; Alawieh, A.; Goyal, N.; Alexandrov, A.V.; Petersen, N.; Kodali, S.; Maier, I.L.; Psychogios, M.N.; Swisher, C.B.; et al. Blood Pressure and Outcome After Mechanical Thrombectomy With Successful Revascularization. Stroke 2019, 50, 2448–2454. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, R.C.; Aries, M.; Minhas, J.S.; Petersen, N.H.; Xiong, L.; Kainerstorfer, J.M.; Castro, P. Review of studies on dynamic cerebral autoregulation in the acute phase of stroke and the relationship with clinical outcome. J. Cereb. Blood Flow. Metab. 2022, 42, 430–453. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xu, J.; Liu, Y.; Wang, A.; Gao, Y.; Wang, Y.; Wang, Y. Blood pressure fluctuation pattern and stroke outcomes in acute ischemic stroke. Hypertens. Res. 2019, 42, 1776–1782. [Google Scholar] [CrossRef] [PubMed]
- Jurcau, A.; Ardelean, A.I. Oxidative Stress in Ischemia/Reperfusion Injuries following Acute Ischemic Stroke. Biomedicines 2022, 10, 574. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lin, Y.H.; Liu, H.M. Update on cerebral hyperperfusion syndrome. J. Neurointerv. Surg. 2020, 12, 788–793. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Makris, N.; Chamard, L.; Mikkelsen, I.K.; Hermier, M.; Derex, L.; Pedraza, S.; Thomalla, G.; Ostergaard, L.; Baron, J.C.; Nighoghossian, N.; et al. Acute reperfusion without recanalization: Serial assessment of collaterals within 6 h of using perfusion-weighted magnetic resonance imaging. J. Cereb. Blood Flow. Metab. 2019, 39, 251–259. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Naito, H.; Hosomi, N.; Kuzume, D.; Nezu, T.; Aoki, S.; Morimoto, Y.; Kinboshi, M.; Yoshida, T.; Shiga, Y.; Kinoshita, N.; et al. Increased blood pressure variability during the subacute phase of ischemic stroke is associated with poor functional outcomes at 3 months. Sci. Rep. 2020, 10, 811. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Harper, G.; Castleden, C.M.; Potter, J.F. Factors affecting changes in blood pressure after acute stroke. Stroke 1994, 25, 1726–1729. [Google Scholar] [CrossRef] [PubMed]
- Britton, M.; Carlsson, A.; de Faire, U. Blood pressure course in patients with acute stroke and matched controls. Stroke 1986, 17, 861–864. [Google Scholar] [CrossRef] [PubMed]
- Scheel, P.; Ruge, C.; Schoning, M. Flow velocity and flow volume measurements in the extracranial carotid and vertebral arteries in healthy adults: Reference data and the effects of age. Ultrasound Med. Biol. 2000, 26, 1261–1266. [Google Scholar] [CrossRef] [PubMed]
- Schoning, M.; Walter, J.; Scheel, P. Estimation of cerebral blood flow through color duplex sonography of the carotid and vertebral arteries in healthy adults. Stroke 1994, 25, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Campbell, B.C.; Christensen, S.; Tress, B.M.; Churilov, L.; Desmond, P.M.; Parsons, M.W.; Barber, P.A.; Levi, C.R.; Bladin, C.; Donnan, G.A.; et al. Failure of collateral blood flow is associated with infarct growth in ischemic stroke. J. Cereb. Blood Flow. Metab. 2013, 33, 1168–1172. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, J.S.; Bang, O.Y. Collateral Status and Outcomes after Thrombectomy. Transl. Stroke Res. 2023, 14, 22–37. [Google Scholar] [CrossRef] [PubMed]
- Hassler, E.; Kneihsl, M.; Deutschmann, H.; Hinteregger, N.; Magyar, M.; Wiesspeiner, U.; Haidegger, M.; Fandler-Hofler, S.; Eppinger, S.; Niederkorn, K.; et al. Relationship between stroke etiology and collateral status in anterior circulation large vessel occlusion. J. Neurol. 2020, 267, 3362–3370. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]

| Variables | Enrolled Patients N: 89 | Excluded Patients N: 29 | p |
|---|---|---|---|
| Age (years) | 69.45 ± 13.59 (24–95) | 72 ± 12.59 (47–89) | p: 0.73 |
| Sex (n) | M: 51/F: 38 | M: 13/F: 16 | p: 0.286 |
| Diabetes mellitus (n, %) | 17 (19.1%) | 9 (31%) | p: 0.308 |
| Hypertension (n, %) | 58 (65.2%) | 19 (65.5%) | p > 0.999 |
| Dyslipidemia (n, %) | 23 (25.8%) | 7 (24.1%) | p > 0.999 |
| Af (n, %) | 50 (56.2%) | 10 (34.5%) | p: 0.053 |
| Smoking (n, %) | 26 (29.7%) | 6 (20.7%) | p: 0.474 |
| Previous stroke (n, %) | 15 (16.9%) | 7 (24.1%) | p: 0.415 |
| Cancer (n, %) | 12 (13.5%) | 7 (24.1%) | p: 0.243 |
| Alcohol (n, %) | 16 (18%) | 4 (13.8%) | p: 0.778 |
| NOACs (n, %) | 36 (40.4%) | 6 (20.7%) | * p: 0.048 |
| Initial NIHSS median (IQR) | 14 (10.5–17) | 17 (14.5–21) | * p: 0.017 |
| Pre-stroke mRS median (IQR) | 0 (0–0) | 0 (0–1) | p: 0.241 |
| ASPECTS median (IQR) | 8 (3–10.3) | 6 (4.5–7) | * p: 0.004 |
| r-tPA (n, %) | 28 (31.5%) | 8 (27.6%) | p: 0.818 |
| TICI of 2b to 3 (n, %) | 71 (79.81%) | 19 (65.5%) | * p: 0.002 |
| Variables | Correlation with mRS Score at 3 Months | |
|---|---|---|
| Age (years) (mean ± SD, range) | 69.45 ± 13.59 (24–95) | 0.296; p: 0.005 |
| Sex (n, %) | M: 51 (57.3%)/F: 38 (42.7%) | −0.106; p: 0.265 |
| Diabetes mellitus (n, %) | 17 (19.1%) | −0.080; p: 0.459 |
| Hypertension (n, %) | 58 (65.2%) | 0.044; p: 0.687 |
| Dyslipidemia (n, %) | 23 (25.8%) | 0.001; p: 0.992 |
| Af (n, %) | 50 (56.2%) | 0.066; p: 0.542 |
| Smoking (n, %) | 26 (29.7%) | −0.105; p: 0.334 |
| Previous stroke (n, %) | 15 (16.9%) | 0.111; p: 0.306 |
| Cancer (n, %) | 12 (13.5%) | 0.113; p: 0.290 |
| Alcohol (n, %) | 16 (18%) | −0.083; p: 0.445 |
| NOACs (n, %) | 36 (40.4%) | −0.026; p: 0.814 |
| Initial NIHSS median (IQR) | 14 (10.5–17) | 0.226; p: 0.035 |
| Pre-stroke mRS median (IQR) | 0 (0–0) | 0.200; p: 0.064 |
| ASPECTS median (IQR) | 8 (6–9) | −0.453; p < 0.001 |
| r-tPA (n, %) | 28 (31.5%) | −0.342; p: 0.001 |
| TICI of 2b to 3 (n, %) | 71 (79.81%) | −0.353; p: 0.001 |
| QTI/non-QTI median (IQR) | 0.99 (0.77–1.17) | −0.247; p: 0.021 |
| QTI/QTC ratio (mean ± SD) | 0.63 ± 0.16 | −0.221; p: 0.04 |
| Variables | mRS: 0–3 n: 53 | mRS: 4–6 n: 36 | p | Univariate Analysis OR (95% CI), p | Multivariate Analysis OR (95% CI), p |
|---|---|---|---|---|---|
| Age (years) (mean ± SD) | 67.19 ± 14.21 | 72.78 ± 12.07 | 0.057 | 0.97 (0.93–1.00), p: 0.061 | 0.96 (0.92–1.00), p: 0.08 |
| female (n, %) | 19 (35.84%) | 19 (52.77%) | 0.131 | 2 (0.84–4.73), p: 0.115 | 1.38 (0.44–4.36), p: 0.58 |
| Diabetes Mellitus (n, %) | 10 (18.87%) | 7 (19.44%) | >0.999 | 0.96 (0.33–2.82), p: 0.946 | |
| Hypertension (n, %) | 33 (62.26%) | 25 (69.44%) | 0.507 | 0.73 (0.29–1.79), p: 0.486 | |
| Dyslipidemia (n, %) | 12 (22.64%) | 11 (30.55%) | 0.464 | 0.67 (0.25–1.73), p: 0.404 | |
| Af (n, %) | 30 (56.60%) | 20 (55.55%) | >0.999 | 1.04 (0.44–2.45), p: 0.922 | |
| Smoking (n, %) | 8 (15.09%) | 8 (22.22%) | 0.342 | 1.80 (0.68–4.75), p: 0.235 | |
| Previous Stroke (n, %) | 7 (13.21%) | 8 (22.22%) | 0.387 | 0.53 (0.17–1.63), p: 0.269 | |
| Cancer (n, %) | 5 (9.43%) | 7 (19.44%) | 0.214 | 0.43 (0.13–1.49), * p: 0.183 | 1.04 (0.22–4.86), p: 0.96 |
| Alcohol (n, %) | 11 (20.75) | 5 (13.89) | 0.575 | 1.62 (0.51–5.15), p: 0.411 | |
| r-tPA (n, %) | 22 (41.51) | 6 (16.67) | * 0.019 | 3.55 (1.26–9.97), * p: 0.016 | 2.89 (0.85–9.89), p: 0.08 |
| NOACs (n, %) | 22 (41.51) | 14 (38.89) | 0.829 | 1.1 (0.47–2.56), p: 0.805 | |
| Initial NIHSS median (IQR) | 14 (10–17) | 14 (11–20) | 0.152 | 0.94 (0.86 -1.03), * p: 0.154 | 0.98 (0.87–1.10), p: 0.75 |
| ASPECTS median (IQR) | 8 (7–9) | 7 (6–8) | * <0.001 | 1.93 (1.38–2.72), * p < 0.001 | 1.79 (1.16–2.78), p: 0.009 |
| TICI of 2b to 3 (n, %) | 48 (90.57) | 23 (63.89) | * 0.003 | 5.43 (1.73–17.05), * p: 0.004 | 4.91 (1.10–21.89), p: 0.037 |
| QTI/QTC (mean ± SD) | 0.66 ± 0.13 | 0.57 ± 0.19 | * 0.009 | * 49.51 (1.99–1232.59), p: 0.017 | 45.35 (1.11–1847.51) p: 0.04 |
| Variables | Odds Ratio | 95% CI | p-Value |
|---|---|---|---|
| TICI: 2b to 3 | 3.72 | 0.97–14.27 | 0.056 |
| Age < 70 y/o | 2.58 | 0.87–7.71 | 0.089 |
| ASPECTS > 8 | 72.89 | 0.94–8.91 | 0.064 |
| Initial NIHSS < 15 | 2.00 | 0.65–6.15 | 0.225 |
| Applying r-tPA | 4.25 | 1.25–14.46 | * 0.002 |
| QTI/QTC > 0.6 | 3.83 | 1.28–11.48 | * 0.016 |
| Constant | 0.002 | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, X.-H.; Chen, K.-W.; Hsu, C.-F.; Chang, T.-W.; Shen, C.-Y.; Chi, H.-Y. The Association of Cerebral Blood Flow Measured Using Extracranial Carotid Ultrasound with Functional Outcomes in Patients with Anterior Circulation Large Vessel Occlusion After Endovascular Thrombectomy—A Retrospective Study. Neurol. Int. 2025, 17, 67. https://doi.org/10.3390/neurolint17050067
Lin X-H, Chen K-W, Hsu C-F, Chang T-W, Shen C-Y, Chi H-Y. The Association of Cerebral Blood Flow Measured Using Extracranial Carotid Ultrasound with Functional Outcomes in Patients with Anterior Circulation Large Vessel Occlusion After Endovascular Thrombectomy—A Retrospective Study. Neurology International. 2025; 17(5):67. https://doi.org/10.3390/neurolint17050067
Chicago/Turabian StyleLin, Xin-Hong, Kuan-Wen Chen, Chung-Fu Hsu, Ting-Wei Chang, Chao-Yu Shen, and Hsin-Yi Chi. 2025. "The Association of Cerebral Blood Flow Measured Using Extracranial Carotid Ultrasound with Functional Outcomes in Patients with Anterior Circulation Large Vessel Occlusion After Endovascular Thrombectomy—A Retrospective Study" Neurology International 17, no. 5: 67. https://doi.org/10.3390/neurolint17050067
APA StyleLin, X.-H., Chen, K.-W., Hsu, C.-F., Chang, T.-W., Shen, C.-Y., & Chi, H.-Y. (2025). The Association of Cerebral Blood Flow Measured Using Extracranial Carotid Ultrasound with Functional Outcomes in Patients with Anterior Circulation Large Vessel Occlusion After Endovascular Thrombectomy—A Retrospective Study. Neurology International, 17(5), 67. https://doi.org/10.3390/neurolint17050067






