Post-Ablation Stroke Despite NOAC Use: Successful Reperfusion Therapy After Dabigatran Reversal with Incidental Discovery of a Large MCA Aneurysm—A Case Report
Abstract
1. Introduction
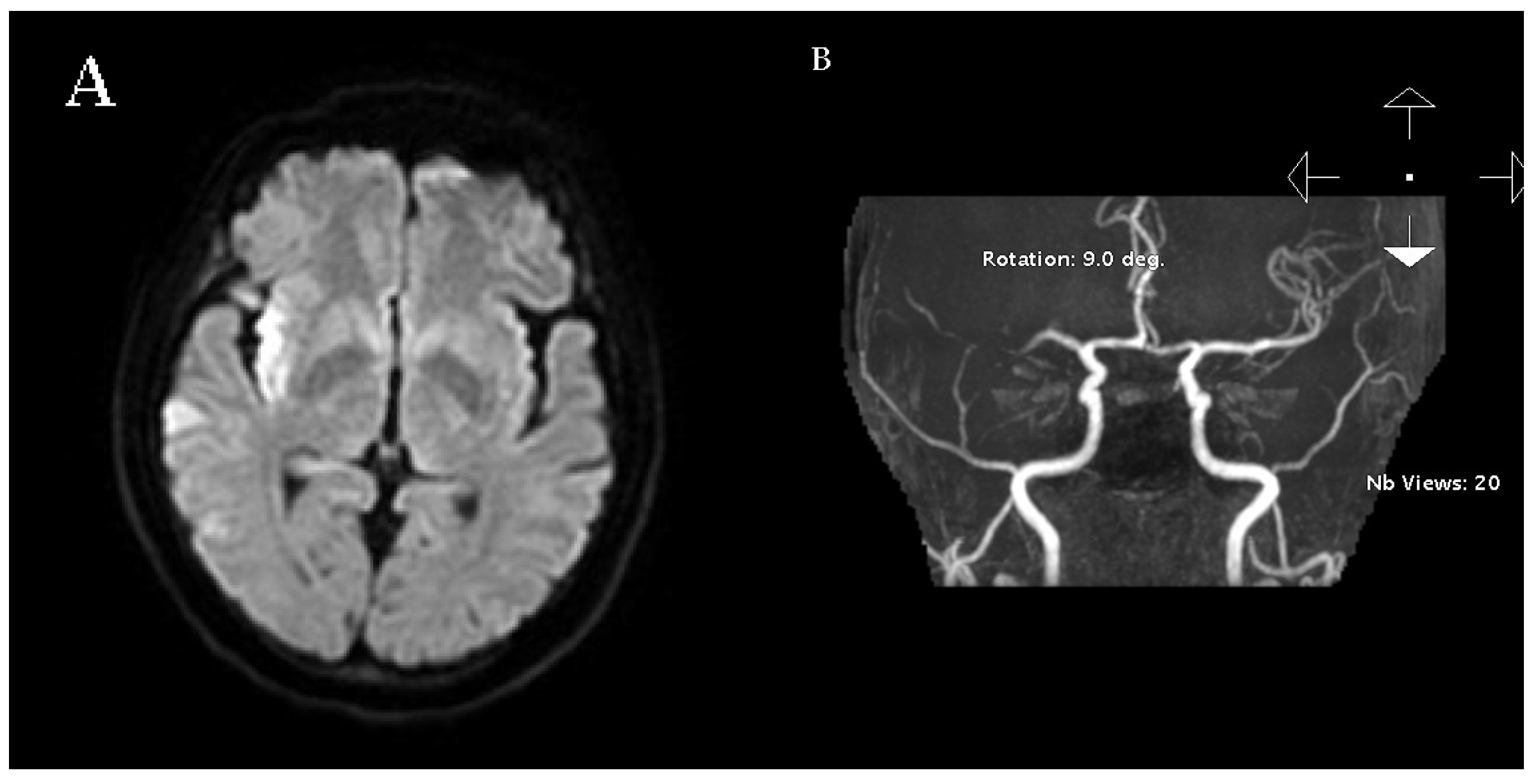
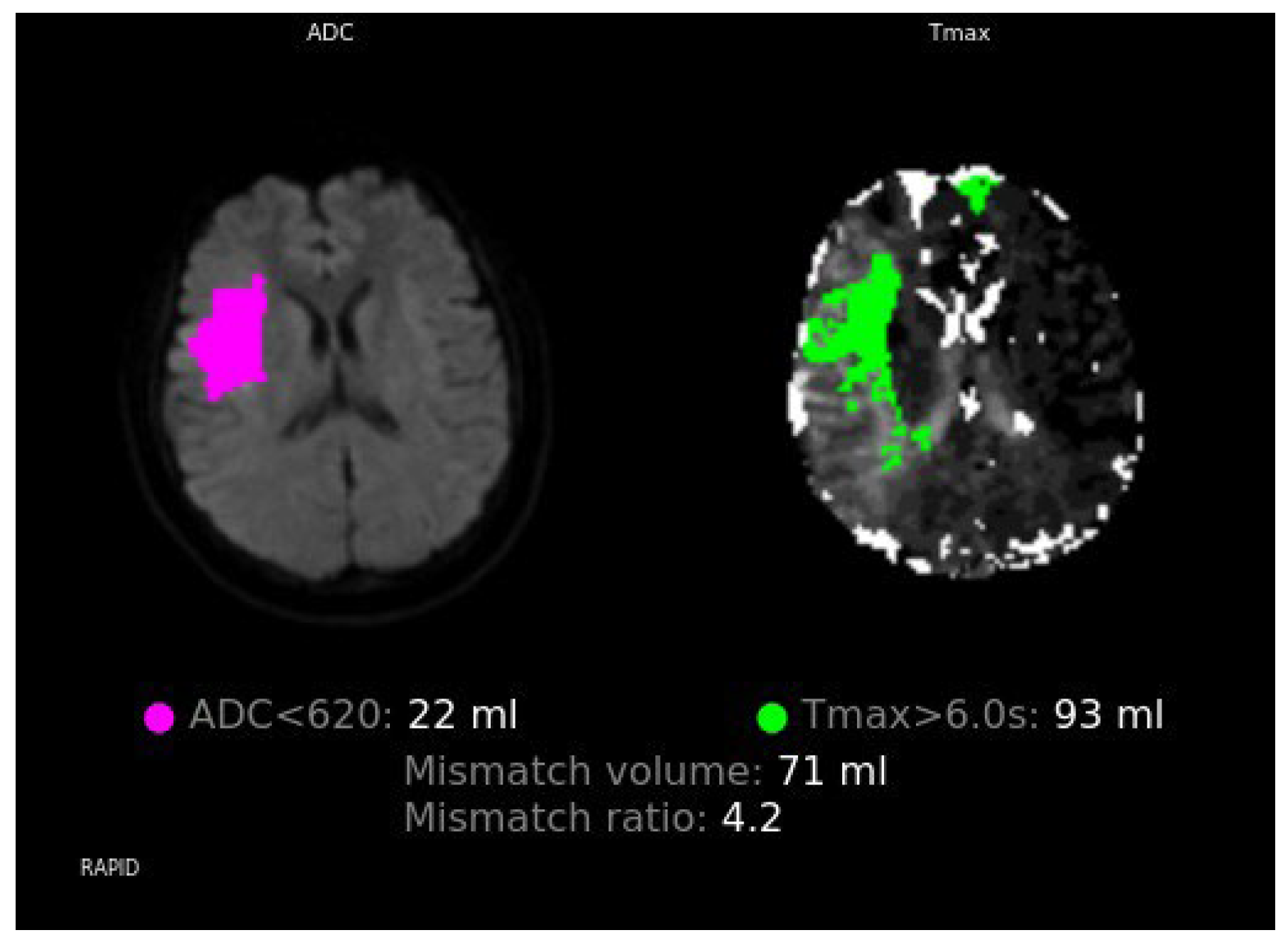
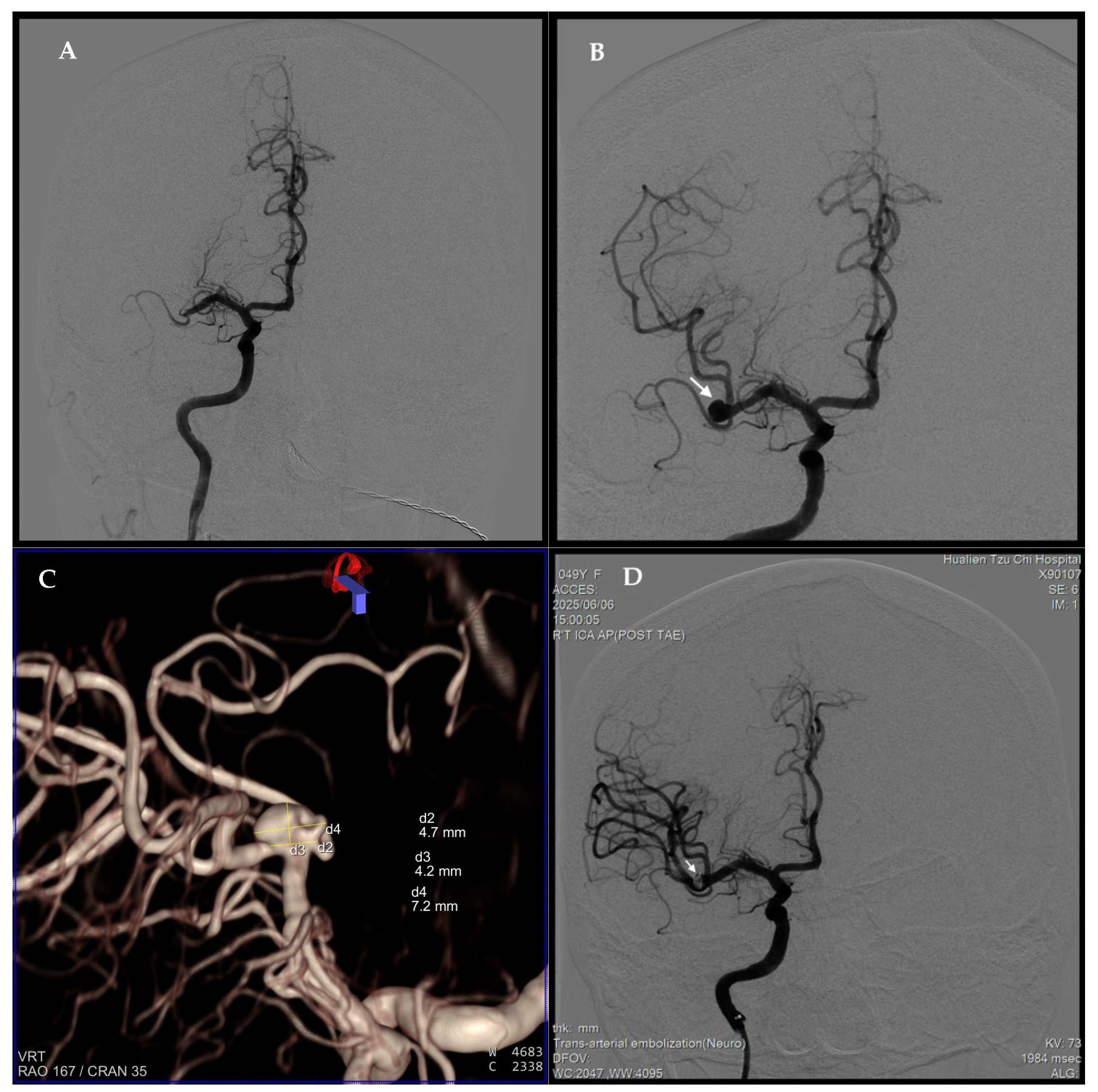
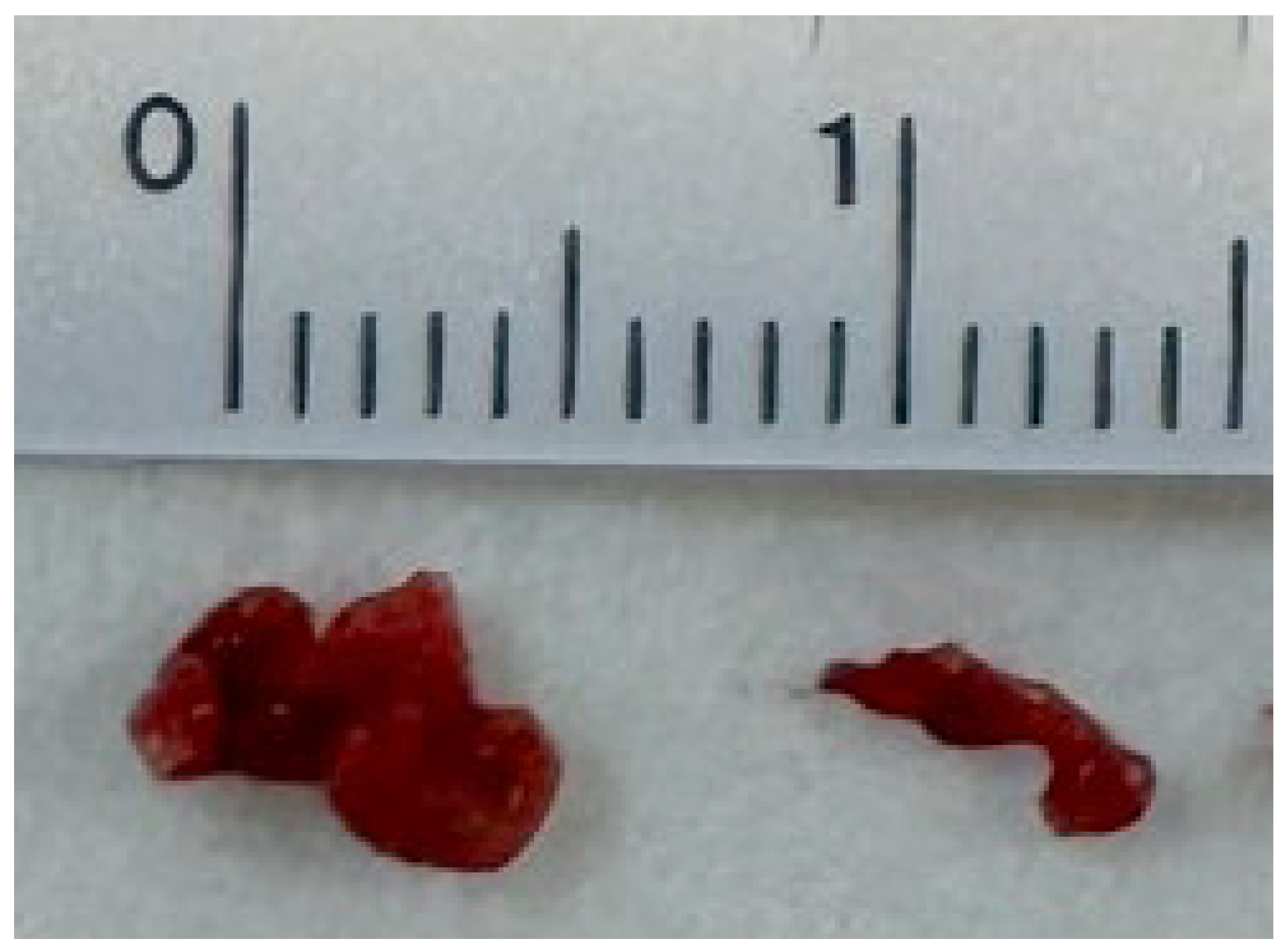
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AF | Atrial fibrillation |
| NOAC | Non-vitamin K oral anticoagulant |
| rt-PA | Recombinant tissue plasminogen activator |
| EVT | Endovascular thrombectomy |
| MCA | Middle cerebral artery |
| NIHSS | National Institutes of Health Stroke Scale |
| CT | Computed tomography |
| MRI | Magnetic resonance imaging |
| DWI | Diffusion-weighted imaging |
| TOF-MRA | Time-of-flight magnetic resonance angiography |
| NHIRD | Health Insurance Research Database |
| AHA/ASA | American Heart Association/American Stroke Association |
References
- Chugh, S.S.; Havmoeller, R.; Narayanan, K.; Singh, D.; Rienstra, M.; Benjamin, E.J.; Gillum, R.F.; Kim, Y.H.; McAnulty, J.H., Jr.; Zheng, Z.J.; et al. Worldwide epidemiology of atrial fibrillation: A Global Burden of Disease 2010 Study. Circulation 2014, 129, 837–847. [Google Scholar] [CrossRef]
- Wolf, P.A.; Abbott, R.D.; Kannel, W.B. Atrial fibrillation as an independent risk factor for stroke: The Framingham Study. Stroke 1991, 22, 983–988. [Google Scholar] [CrossRef] [PubMed]
- Van Gelder, I.C.; Rienstra, M.; Bunting, K.V.; Casado-Arroyo, R.; Caso, V.; Crijns, H.; De Potter, T.J.R.; Dwight, J.; Guasti, L.; Hanke, T.; et al. 2024 ESC Guidelines for the management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2024, 45, 3314–3414. [Google Scholar] [CrossRef]
- Cappato, R.; Calkins, H.; Chen, S.A.; Davies, W.; Iesaka, Y.; Kalman, J.; Kim, Y.H.; Klein, G.; Packer, D.; Skanes, A. Worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circulation 2005, 111, 1100–1105. [Google Scholar] [CrossRef]
- Mené, R.; Sousonis, V.; Schmidt, B.; Bordignon, S.; Neven, K.; Reichlin, T.; Blaauw, Y.; Hansen, J.; Ouss, A.; Reinsch, N.; et al. Safety and efficacy of pulsed-field ablation for atrial fibrillation in the elderly: A EU-PORIA sub-analysis. Int. J. Cardiol. 2024, 417, 132522. [Google Scholar] [CrossRef]
- Nagao, T.; Inden, Y.; Shimano, M.; Fujita, M.; Yanagisawa, S.; Kato, H.; Ishikawa, S.; Miyoshi, A.; Okumura, S.; Ohguchi, S.; et al. Feasibility and safety of uninterrupted dabigatran therapy in patients undergoing ablation for atrial fibrillation. Intern. Med. 2015, 54, 1167–1173. [Google Scholar] [CrossRef]
- Elgendy, A.Y.; Mahtta, D.; Barakat, A.F.; Abuzaid, A.; Mahmoud, A.; Mentias, A.; Mahmoud, A.N.; Elgendy, I.Y. Meta-Analysis of Safety and Efficacy of Uninterrupted Non-Vitamin K Antagonist Oral Anticoagulants Versus Vitamin K Antagonists for Catheter Ablation of Atrial Fibrillation. Am. J. Cardiol. 2017, 120, 1830–1836. [Google Scholar] [CrossRef]
- Pollack, C.V., Jr.; Reilly, P.A.; van Ryn, J.; Eikelboom, J.W.; Glund, S.; Bernstein, R.A.; Dubiel, R.; Huisman, M.V.; Hylek, E.M.; Kam, C.W.; et al. Idarucizumab for Dabigatran Reversal—Full Cohort Analysis. N. Engl. J. Med. 2017, 377, 431–441. [Google Scholar] [CrossRef]
- Nogueira, R.G.; Jadhav, A.P.; Haussen, D.C.; Bonafe, A.; Budzik, R.F.; Bhuva, P.; Yavagal, D.R.; Ribo, M.; Cognard, C.; Hanel, R.A.; et al. Thrombectomy 6 to 24 Hours after Stroke with a Mismatch between Deficit and Infarct. N. Engl. J. Med. 2018, 378, 11–21. [Google Scholar] [CrossRef]
- Alam, K.; Khan, A.N.; Fatima, A.; Haseeb, A.; Jaffar, D.; Mussarat, A.; Amir, M.; Rana, M.O.; Saeed, H.; Asmar, A. Assessing mortality and safety of IV thrombolysis in ischemic stroke patients on direct oral anticoagulants (DOACs): A systematic review and meta-analysis. Clin. Neurol. Neurosurg. 2024, 246, 108523. [Google Scholar] [CrossRef]
- Calkins, H.; Willems, S.; Gerstenfeld, E.P.; Verma, A.; Schilling, R.; Hohnloser, S.H.; Okumura, K.; Serota, H.; Nordaby, M.; Guiver, K.; et al. Uninterrupted Dabigatran versus Warfarin for Ablation in Atrial Fibrillation. N. Engl. J. Med. 2017, 376, 1627–1636. [Google Scholar] [CrossRef]
- Boeckh-Behrens, T.; Kleine, J.F.; Zimmer, C.; Neff, F.; Scheipl, F.; Pelisek, J.; Schirmer, L.; Nguyen, K.; Karatas, D.; Poppert, H. Thrombus Histology Suggests Cardioembolic Cause in Cryptogenic Stroke. Stroke 2016, 47, 1864–1871. [Google Scholar] [CrossRef]
- Anselmino, M.; Matta, M.; Toso, E.; Ferraris, F.; Castagno, D.; Scaglione, M.; Cesarani, F.; Faletti, R.; Gaita, F. Silent Cerebral Embolism during Atrial Fibrillation Ablation:Pathophysiology, Prevention and Management. J. Atr. Fibrillation 2013, 6, 796. [Google Scholar]
- Calkins, H.; Kuck, K.H.; Cappato, R.; Brugada, J.; Camm, A.J.; Chen, S.A.; Crijns, H.J.; Damiano, R.J., Jr.; Davies, D.W.; DiMarco, J.; et al. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: Recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design: A report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. Developed in partnership with the European Heart Rhythm Association (EHRA), a registered branch of the European Society of Cardiology (ESC) and the European Cardiac Arrhythmia Society (ECAS); and in collaboration with the American College of Cardiology (ACC), American Heart Association (AHA), the Asia Pacific Heart Rhythm Society (APHRS), and the Society of Thoracic Surgeons (STS). Endorsed by the governing bodies of the American College of Cardiology Foundation, the American Heart Association, the European Cardiac Arrhythmia Society, the European Heart Rhythm Association, the Society of Thoracic Surgeons, the Asia Pacific Heart Rhythm Society, and the Heart Rhythm Society. Heart Rhythm 2012, 9, 632–696.e621. [Google Scholar] [CrossRef]
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2019, 50, e344–e418. [Google Scholar] [CrossRef]
- van der Horst, S.F.B.; Martens, E.S.L.; den Exter, P.L.; Bos, M.H.A.; van Mens, T.E.; Huisman, M.V.; Klok, F.A. Idarucizumab for dabigatran reversal: A systematic review and meta-analysis of indications and outcomes. Thromb. Res. 2023, 228, 21–32. [Google Scholar] [CrossRef]
- Strbian, D.; Tsivgoulis, G.; Ospel, J.; Räty, S.; Cimflova, P.; Georgiopoulos, G.; Ullberg, T.; Arquizan, C.; Gralla, J.; Zeleňák, K.; et al. European Stroke Organisation and European Society for Minimally Invasive Neurological Therapy guideline on acute management of basilar artery occlusion. Eur. Stroke J. 2024, 9, 835–884. [Google Scholar] [CrossRef]
- Ghannam, M.; AlMajali, M.; Galecio-Castillo, M.; Al Qudah, A.; Khasiyev, F.; Dibas, M.; Ghazaleh, D.; Vivanco-Suarez, J.; Morán-Mariños, C.; Farooqui, M.; et al. Intravenous Thrombolysis for Acute Ischemic Stroke in Patients With Recent Direct Oral Anticoagulant Use: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2023, 12, e031669. [Google Scholar] [CrossRef]
- Lin, Y.T.; Lai, Y.J.; Lai, T.H. Idarucizumab for Intravenous Thrombolysis and Endovascular Thrombectomy in Acute Stroke: A Case Report. J. Emerg. Med. 2020, 58, e113–e116. [Google Scholar] [CrossRef]
- Anadani, M.; Marnat, G.; Consoli, A.; Papanagiotou, P.; Nogueira, R.G.; Siddiqui, A.; Ribo, M.; Spiotta, A.M.; Bourcier, R.; Kyheng, M.; et al. Endovascular Therapy of Anterior Circulation Tandem Occlusions: Pooled Analysis From the TITAN and ETIS Registries. Stroke 2021, 52, 3097–3105. [Google Scholar] [CrossRef]
- Schirmer, C.M.; Bulsara, K.R.; Al-Mufti, F.; Haranhalli, N.; Thibault, L.; Hetts, S.W. Antiplatelets and antithrombotics in neurointerventional procedures: Guideline update. J. Neurointerv. Surg. 2023, 15, 1155–1162. [Google Scholar] [CrossRef] [PubMed]
- Wiebers, D.O.; Whisnant, J.P.; Huston, J., 3rd; Meissner, I.; Brown, R.D., Jr.; Piepgras, D.G.; Forbes, G.S.; Thielen, K.; Nichols, D.; O’Fallon, W.M.; et al. Unruptured intracranial aneurysms: Natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet 2003, 362, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Virta, J.J.; Strbian, D.; Putaala, J.; Kaprio, J.; Korja, M. Characteristics and Outcomes of Thrombolysis-Treated Stroke Patients With and Without Saccular Intracranial Aneurysms. Stroke 2022, 53, 3616–3621. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Duan, Z.; Liu, Y.; Zhou, C.; Jiao, Z.; Zhao, Y.; Tang, T. Incidental Unruptured Intracranial Aneurysms Do Not Impact Outcome in Patients With Acute Cerebral Infarction. Front. Neurol. 2021, 12, 613027. [Google Scholar] [CrossRef]
- Moniz-Garcia, D.; Ravindran, K.; Wessell, A.; Muneer, M.S.; Ahmed, E.; Perez Vega, C.; Kashyap, S.; Vibhute, P.; Gupta, V.; Freeman, W.D.; et al. Intracranial aneurysms in patients with acute ischemic stroke prevalence and influence on mechanical thrombectomy over 14 years in a tertiary-care center. J. Clin. Neurosci. 2024, 124, 109–114. [Google Scholar] [CrossRef]
- Zeymer, U.; Leiva, O.; Hohnloser, S.H.; Steg, P.G.; Oldgren, J.; Nickenig, G.; Gabor Kiss, R.; Ongen, Z.; Navarro Estrada, J.; Oude Ophuis, T.; et al. Dual antithrombotic therapy with dabigatran in patients with atrial fibrillation after percutaneous coronary intervention for ST-segment elevation myocardial infarction: A post hoc analysis of the randomised RE-DUAL PCI trial. EuroIntervention 2021, 17, 474–480. [Google Scholar] [CrossRef]
- Oldgren, J.; Steg, P.G.; Hohnloser, S.H.; Lip, G.Y.H.; Kimura, T.; Nordaby, M.; Brueckmann, M.; Kleine, E.; Ten Berg, J.M.; Bhatt, D.L.; et al. Dabigatran dual therapy with ticagrelor or clopidogrel after percutaneous coronary intervention in atrial fibrillation patients with or without acute coronary syndrome: A subgroup analysis from the RE-DUAL PCI trial. Eur. Heart J. 2019, 40, 1553–1562. [Google Scholar] [CrossRef]
- Lopes, R.D.; Heizer, G.; Aronson, R.; Vora, A.N.; Massaro, T.; Mehran, R.; Goodman, S.G.; Windecker, S.; Darius, H.; Li, J.; et al. Antithrombotic Therapy after Acute Coronary Syndrome or PCI in Atrial Fibrillation. N. Engl. J. Med. 2019, 380, 1509–1524. [Google Scholar] [CrossRef]
- Huang, Y.C.; Huang, Y.C.; Cheng, Y.C.; Chen, M. Choice of antithrombotic therapy for patients with atrial fibrillation undergoing carotid angioplasty and stenting: A nationwide population-based study. Sci. Rep. 2022, 12, 1417. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sari, S.M.; Chen, W.-T.; Lee, C.-H.; Huang, N.-H.; Thu, P.-W.; Teoh, L.-L.; Wu, Y.-M.; Liu, A.-B. Post-Ablation Stroke Despite NOAC Use: Successful Reperfusion Therapy After Dabigatran Reversal with Incidental Discovery of a Large MCA Aneurysm—A Case Report. Neurol. Int. 2025, 17, 190. https://doi.org/10.3390/neurolint17120190
Sari SM, Chen W-T, Lee C-H, Huang N-H, Thu P-W, Teoh L-L, Wu Y-M, Liu A-B. Post-Ablation Stroke Despite NOAC Use: Successful Reperfusion Therapy After Dabigatran Reversal with Incidental Discovery of a Large MCA Aneurysm—A Case Report. Neurology International. 2025; 17(12):190. https://doi.org/10.3390/neurolint17120190
Chicago/Turabian StyleSari, Santi Mitra, Wei-Tso Chen, Chien-Hui Lee, Nai-Hsin Huang, Phyo-Wai Thu, Ling-Lun Teoh, Yu-Mei Wu, and An-Bang Liu. 2025. "Post-Ablation Stroke Despite NOAC Use: Successful Reperfusion Therapy After Dabigatran Reversal with Incidental Discovery of a Large MCA Aneurysm—A Case Report" Neurology International 17, no. 12: 190. https://doi.org/10.3390/neurolint17120190
APA StyleSari, S. M., Chen, W.-T., Lee, C.-H., Huang, N.-H., Thu, P.-W., Teoh, L.-L., Wu, Y.-M., & Liu, A.-B. (2025). Post-Ablation Stroke Despite NOAC Use: Successful Reperfusion Therapy After Dabigatran Reversal with Incidental Discovery of a Large MCA Aneurysm—A Case Report. Neurology International, 17(12), 190. https://doi.org/10.3390/neurolint17120190




