Cognitive, Functional, and Emotional Recovery in Patients with Stroke: A Multidimensional Prospective Analysis
Abstract
1. Background
2. Methods
2.1. Design
2.2. Population
Sample and Inclusion/Exclusion Criteria
2.3. Variables
2.3.1. Independent Variables
2.3.2. Dependent Variables
2.4. Instruments
2.4.1. National Institutes Health Stroke Scale (NIHSS)
2.4.2. Beck Depression Inventory (BDI)
2.4.3. Mini-Mental State Examination
2.4.4. Barthel Index
2.5. Data Management
2.6. Confidentiality of Data and Ethical Considerations
2.7. Data Analysis
3. Results
3.1. Sociodemographic and Clinical
3.1.1. Sex, Age and Stay in Hospital
3.1.2. Age and Ischemic Stroke Group
3.1.3. Stroke Type and Subtype
3.1.4. Stroke Subtype in Stroke Group
3.1.5. Treatment Modality
3.1.6. Pain
3.2. Outcome Measures
3.2.1. Neurological Function
3.2.2. Neurological Function and Ischemic Stroke Group
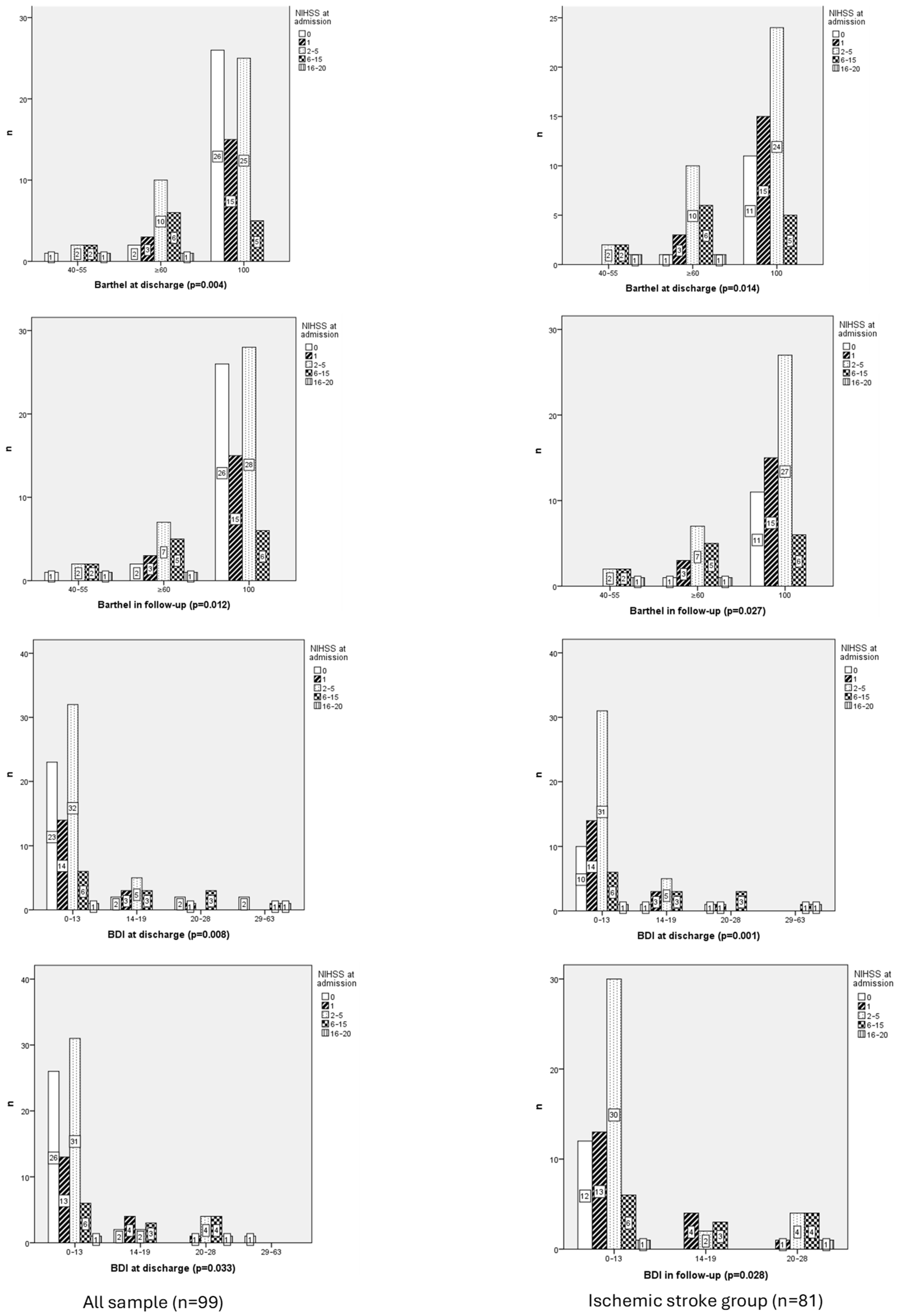
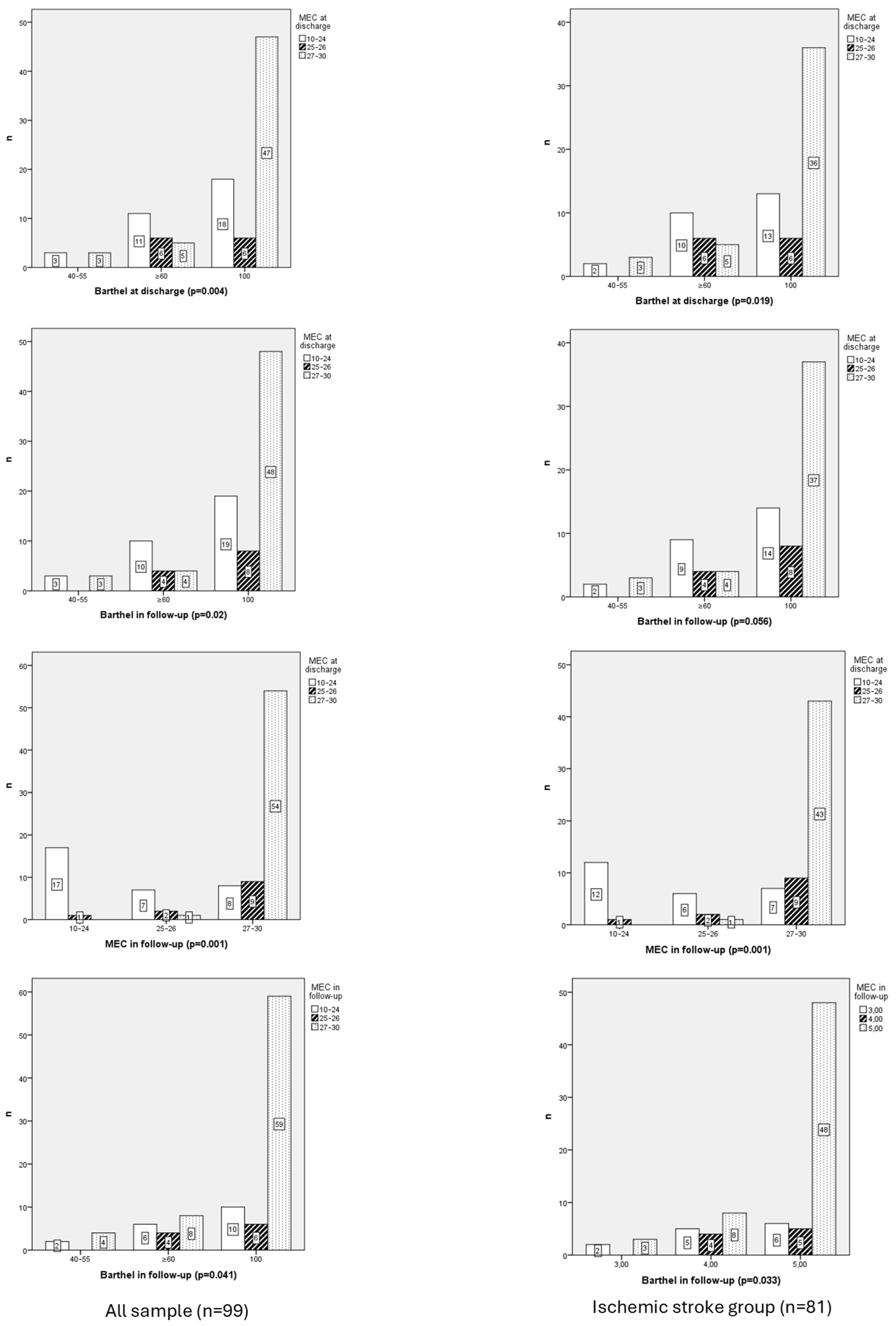
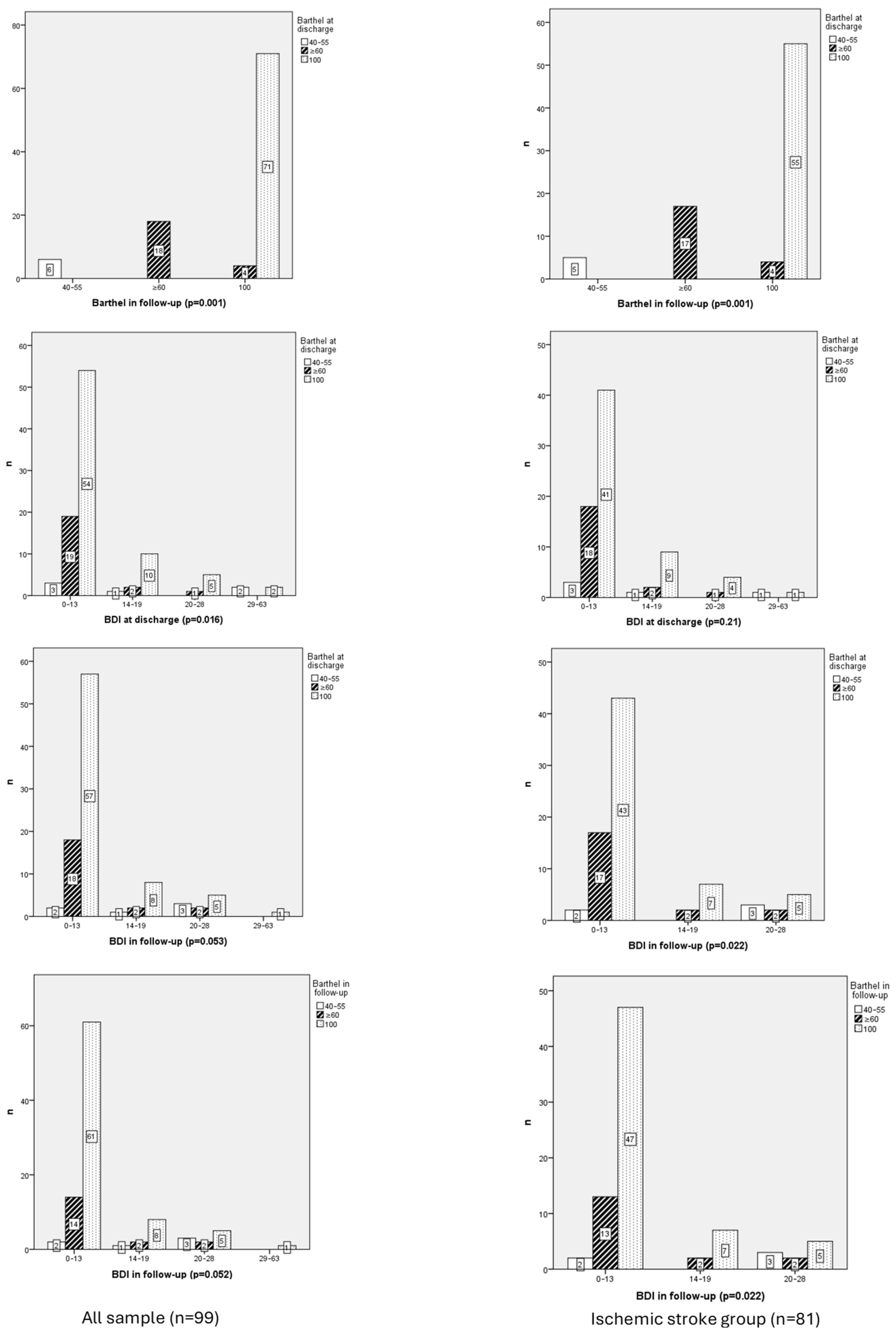
3.2.3. Functional Independence
3.2.4. Functional Independence and Ischemic Stroke Group
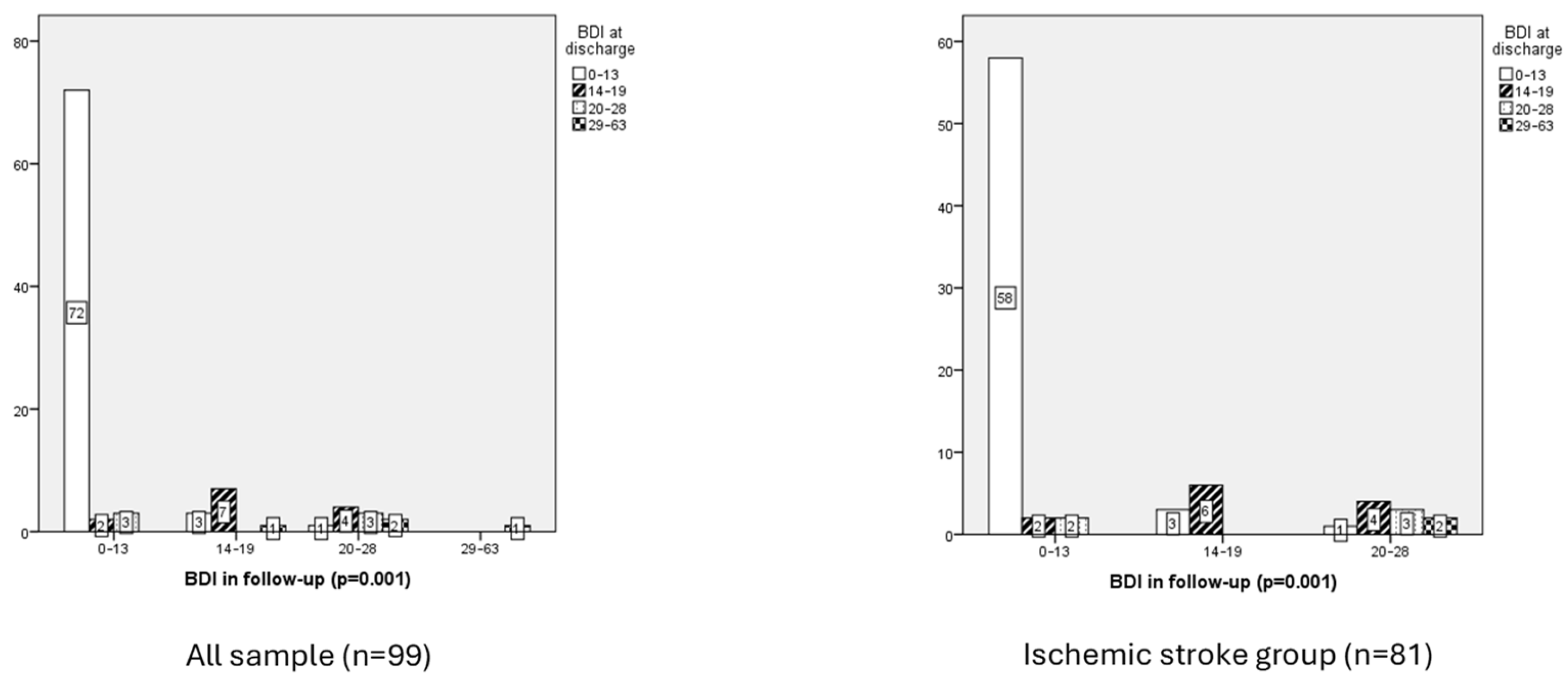
3.2.5. Emotional Function
- Emotional function and ischemic stroke group
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Puente Castillo, E. Stroke; NPunto: Guatemala City, Guatemala, 2022; Volume 5, pp. 4–19. Available online: https://dialnet.unirioja.es/servlet/articulo?codigo=8947482 (accessed on 22 May 2025).
- Mahía Moros, I.; Lacasta Puyuelo, L.; Gracia Iñiguez, C.; Pina Valeg, L.; Sangüesa Ortin, L.; Mainar Crespo, A. Ictus Isquémico: Cuidados de Enfermería en la Unidad de Ictus; RSI: Taipei, Taiwan, 2024; Volume 5, Available online: https://revistasanitariadeinvestigacion.com/ictus-isquemico-cuidados-de-enfermeria-en-la-unidad-de-ictus/#google_vignette (accessed on 22 May 2025).
- Joquera Zuara, S. Actuación Ante el Código Ictus; NPunto: Guatemala City, Guatemala, 2022; Volume 5, pp. 43–66. Available online: https://www.npunto.es/content/src/pdf-articulo/62bc21b13dafaart3.pdf (accessed on 22 May 2025).
- Gasull, T.; Arboix, A. Molecular mechanisms and pathophysiology of acute stroke: Emphasis on biomarkers in the different stroke subtypes. Int. J. Mol. Sci. 2022, 23, 9476. [Google Scholar] [CrossRef]
- Feigin, V.L.; Brainin, M.; Norrving, B.; Martins, S.; Sacco, R.L.; Hacke, W.; Fisher, M.; Pandian, J.; Lindsay, P. World Stroke Organizatión (WSO): Global Stroke Fact Sheet 2022. Int. J. Stroke 2022, 17, 18–29. [Google Scholar] [CrossRef]
- Pego Pérez, E.R. Resultado Funcional de la Trombólisis Mecánica en el Ictus Agudo. Ph.D. Thesis, Universidad de Santiago de Compostela, Santiago, Spain, 2019. Available online: http://hdl.handle.net/10347/20821 (accessed on 20 March 2025).
- Bermello López, M.L.; Pego Pérez, E.R.; Rodríguez Pérez, I. Déficit neurológico y grado de autonomía en pacientes con ictus isquémico, tratados con trombectomía mecánica. Un estudio retrospectivo. Rev. Cient. Soc. Esp. Enferm. Neurol. 2024, 60, 100158. [Google Scholar] [CrossRef]
- Derex, L.; Cho, T.H. Mechanical thrombectomy in acute ischemic stroke. Rev. Neurol. 2017, 173, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Díaz Pérez, J. Trombectomía Mecánica en el Ictus Isquémico Agudo Secundario a Occlusion Carotídea. Ph.D. Thesis, Universidad de Murcia, Murcia, Spain, 2020. Available online: http://hdl.handle.net/10201/98580 (accessed on 20 March 2025).
- Tercero Navarro, M.I.; Catalán Sánchez, C.; Asensio Escolano, M.J.; López Ortiz, S.; Julián Herrero, E.; Lalinde Lidón, B. Cuidados de Enfermería en el Paciente Con Ictus Isquémico Tras Trombectomía Mecánica; RSI: Taipei, Taiwan, 2021; Volume 2, Available online: https://revistasanitariadeinvestigacion.com/cuidados-de-enfermeria-en-el-paciente-con-ictus-isquemico-tras-trombectomia-mecanica/ (accessed on 22 May 2025).
- Park, G.-Y.; Im, S.; Lee, S.-J.; Pae, C.-U. The Association between Post-Stroke Depression and the Activities of Daily Living/Gait Balance in Patients with First-Onset Stroke Patients. Psychiatry Investig. 2016, 13, 659–664. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, G.C.; Roy, D.; Kontos, N.; Beach, S.R. Post- Stroke depression; A 2020 updated review. Gen. Hosp. Psychiatry 2020, 66, 70–80. [Google Scholar] [CrossRef]
- Herrera, M.; Lama, J.; De-La-Cruz, J. Factores de reisgo de depression post ictus isquémico. Rev. Méd. Hered. 2020, 31, 181–189. [Google Scholar] [CrossRef]
- Kong, D.; Zou, W. Association between atherogenic index of plasma and post-stroke depression: A cross sectional study. Eur. J. Psychotraumatol. 2024, 15, 2429266. [Google Scholar] [CrossRef]
- Villatoro González, P.; De Catro, F.J.; García-Culebras, A.; Cuartero, M.I.; Moro, M.Á. Caracterización de la neurogénesis maladaptativa y el deterioro cognitive post-ictus en un modelo experimental de isquemia cerebral inducida mediante cloruro férrico en ratón. Dianas 2022, 11, 4. [Google Scholar]
- Registered Nurses Association of Ontario. Guía de Buenas Prácticas en Enfermería. Cómo Enfocar el Futuro de la Enfermería. Valoración del Ictus Mediante Atención Continuada, 1st ed; Registered Nurses’ Association of Ontario (RNAO): Toronto, ON, Canada, 2005. [Google Scholar]
- Sociedad Española de Neurología. El Atlas Del Ictus; Sociedad Española de Neurología: Madrid, Spain, 2019; Available online: https://www.sen.es/images/2020/atlas/Atlas_del_Ictus_de_Espana_version_web.pdf (accessed on 30 May 2025).
- Williams, Z.J.; Everaert, J.; Gotham, K.O. Measuring Depression in Autistic Adults: Psychometric Validation of the Beck Depression Inventory-II. Assessment 2021, 28, 858–876. [Google Scholar] [CrossRef]
- Macchi, C.; Favero, C.; Ceresa, A.; Vigna, L.; Conti, D.M.; Pesatori, A.C.; Racagni, G.; Corsini, A.; Ferri, N.; Sirtori, C.R.; et al. Depression and cardiovascular risk- association among Beck Depression Inventory, PCSK9 levels and insulin resistance. Cardiovasc. Diabetol. 2020, 19, 187. [Google Scholar] [CrossRef] [PubMed]
- Calle Calle, C.E.; Abreu Rubiño, M.R.; Lashetas Ginés, A.C.; Fleta Cubero, P.; Coscolín, L. Valoración Cognitiva en Geriatría: Test Mini-Mental (Folstein); RSI: Taipei, Taiwan, 2024; Volume 5, Available online: https://revistasanitariadeinvestigacion.com/valoraciones-cognitivas-en-geriatria-test-mini-mental-folstein/ (accessed on 24 May 2025).
- Mateos-Arroyo, J.A.; Zaragoza-García, I.; Sánchez-Gómez, R.; Posada-Moreno, P.; Ortuño-Soriano, I. Validation of the Barthel Index as a Predictor of In-Hospital Mortality among COVID-19 Patients. Healthcare 2023, 11, 1338. [Google Scholar] [CrossRef] [PubMed]
- Escobedo Romero, R.; Izquierdo Fernández, M. El Índice BARTHEL Como Predictor del Riesgo en el Anciano Frágil en Urgencias; ENE: Brockton, MA, USA, 2023; Volume 17, Available online: https://scielo.isciii.es/pdf/ene/v17n1/1988-348X-ene-17-01-1666.pdf (accessed on 24 May 2025).
- Winstein, C.J.; Stein, J.; Arena, R.; Bates, B.; Cherney, L.R.; Cramer, S.C.; Deruyter, F.; Eng, J.J.; Fisher, B.; Harvey, R.L.; et al. Guidelines for adult stroke rehabilitation and recovery: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2016, 47, e98–e169. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.-J.; Stewart, R.; Park, M.-S.; Bae, K.-Y.; Kim, S.-W.; Kim, J.-M.; Shin, I.-S.; Cho, K.-H.; Yoon, J.-S. White matter hyperintensities and functional outcomes at 2 weeks and 1 year after stroke. Cerebrovasc. Dis. 2013, 35, 138–145. [Google Scholar] [CrossRef]
- Dolgova, I.; Santikova, L.; Chipova, D. Relationship between stroke-associated pneumonia and long-term outcomes of ischemic stroke. Zh. Nevrol. Psikhiatr. Im. S. S. Korsakova 2025, 125, 57–61. [Google Scholar] [CrossRef]
- Grau-Olivares, M.; Bartrés-Faz, D.; Arboix, A.; Soliva, J.-C.; Rovira, M.; Targa, C.; Junqué, C. Mild cognitive impairment after lacunar infarction: Voxel-based morphometry and neuropsychological assessment. Cerebrovasc. Dis. 2007, 23, 353–361. [Google Scholar] [CrossRef]
- Shi, Y.; Lenze, E.J.; Mohr, D.C.; Lee, J.-M.; Hu, L.; Metts, C.L.; Fong, M.W.; Wong, A.W. Post-stroke depressive symptoms and cognitive performances: A network analysis. Arch. Phys. Med. Rehabil. 2024, 105, 892–900. [Google Scholar] [CrossRef]
- Pego Pérez, E.R.; Bermello López, L.; Marín Arnés, M.d.R.; Gómez Fernández, E.; Núñez Hernández, M.I.; Rodríguez Pérez, I. Autonomy Outcomes Measured with Barthel Index in Patients with Ischaemic Stroke during SARS-CoV-2 Pandemic. A Descriptive Study. Neurol. Neurosurg. 2022, 17, 555961. [Google Scholar] [CrossRef]
- Sánchez Silverio, V.; Abuín Porras, V.; Rodríguez Costa, I. Análisis del estado cognitivo y su relación con la dependencia en las actividades de la vida diaria: Un estudio transversal en pacientes con accidente cerebrovascular. Rev. Cient. Soc. Esp. Enferm. Neurol. 2022, 56, 4–10. [Google Scholar] [CrossRef]
- El Husseini, N.; Katzan, I.L.; Rost, N.S.; Blake, M.L.; Byun, E.; Pendlebury, S.T.; Aparicio, H.J.; Marquine, M.J.; Gottesman, R.F.; Smith, E.E.; et al. Cognitive impairment after ischemic and hemorrhagic stroke: A scientific statement from the American Heart Association/American Stroke Association. Stroke 2023, 54, e272–e291. [Google Scholar] [CrossRef]
- Pego Pérez, E.R.; Jacobo Vázquez, S.; Bermello López, L.; Rodríguez Pérez, I.; Marín Arnés, M.D.R.; Gómez Fernández, E. Good nursing practice guide results aimed at the care of patients with ischemic stroke or transit ischemic attack. A cohort study. Res. Sq. 2025. [Google Scholar] [CrossRef]
- Carnés Vendrell, A.; Deus Yela, J.; Molina Seguin, J.; Pifarré-Paradero, J.; Purroy, F. Actualización de la depresión postictus: Nuevos retos en pacientes con ictus minor o ataque isquémico transitorio. Rev. Neurol. 2016, 62, 460–467. [Google Scholar] [CrossRef]
| n | % | Mean | SD | P25 | P50 | P75 | IL | SL | |
|---|---|---|---|---|---|---|---|---|---|
| Age | 72.43 | 11.72 | 65 | 64 | 82 | 41 | 95 | ||
| <65 | 23 | 23.2 | |||||||
| 65–80 | 46 | 46.5 | |||||||
| >80 | 30 | 30.3 | |||||||
| Sex | |||||||||
| Man | 65 | 65.7 | |||||||
| Women | 34 | 34.3 | |||||||
| Type of stroke | |||||||||
| Ischemic | 81 | 81.8 | |||||||
| TIA | 18 | 18.2 | |||||||
| Ischemic stroke subtypes | |||||||||
| Atherothrombotic infarct | 12 | 12.1 | |||||||
| Cardioembolic stroke | 24 | 24.2 | |||||||
| Essential cerebral infarct | 44 | 44.4 | |||||||
| Infarct of unusual etiology | 7 | 7.1 | |||||||
| Lacunar infarct | 12 | 12.1 | |||||||
| rt-PA | |||||||||
| No | 76 | 76.8 | |||||||
| Yes | 23 | 23.2 | |||||||
| Thrombectomy | |||||||||
| No | 88 | 88.9 | |||||||
| Yes | 11 | 11.1 | |||||||
| PU | |||||||||
| No | 99 | 100 | |||||||
| Yes | 0 | 0 | |||||||
| Dysphagia Test | |||||||||
| No | 0 | 0 | |||||||
| Yes | 99 | 100 | |||||||
| Dysphagia | |||||||||
| No | 98 | 99 | |||||||
| Yes | 1 | 1 | |||||||
| Aspiration Pneumonia | |||||||||
| No | 98 | 99 | |||||||
| Yes | 1 | 1 | |||||||
| Pain | 0.36 | 1.26 | 0 | 7 | |||||
| 0 | 89 | 89.9 | |||||||
| 1 | 1 | 1.0 | |||||||
| 2 | 3 | 3.0 | |||||||
| 3 | 1 | 1.0 | |||||||
| 4 | 1 | 1.0 | |||||||
| 5 | 3 | 3.0 | |||||||
| 6 | 0 | 0 | |||||||
| 7 | 1 | 1.0 | |||||||
| 8 | 0 | 0 | |||||||
| 9 | 0 | 0 | |||||||
| 10 | 0 | 0 | |||||||
| NIHSS | 2.72 | 3.56 | 0 | 2 | 3 | 0 | 20 | ||
| 0 | 29 | 29.3 | |||||||
| 1 | 18 | 18.2 | |||||||
| 2–5 | 37 | 37.4 | |||||||
| 6–15 | 13 | 13.1 | |||||||
| 16–20 | 2 | 2.0 | |||||||
| >20 | 0 | 0 | |||||||
| Basal Barthel | 98.5 | 7.2 | 100 | 100 | 100 | 55 | 100 | ||
| 100 | 93 | 93.9 | |||||||
| ≥60 | 4 | 4.1 | |||||||
| 40–55 | 2 | 2 | |||||||
| 20–35 | 0 | 0 | |||||||
| <20 | 0 | 0 | |||||||
| Barthel at discharge | 91.92 | 15.60 | 95 | 100 | 100 | 40 | 100 | ||
| 100 | 71 | 71.7 | |||||||
| ≥60 | 22 | 22.2 | |||||||
| 40–55 | 6 | 6.1 | |||||||
| 20–35 | 0 | 0 | |||||||
| <20 | 0 | 0 | |||||||
| Barthel Index in Follow-Up | 92.17 | 15.67 | 100 | 100 | 100 | 40 | 100 | ||
| 100 | 75 | 75.8 | |||||||
| ≥60 | 18 | 18.2 | |||||||
| 40–55 | 6 | 6.1 | |||||||
| 20–35 | 0 | 0 | |||||||
| <20 | 0 | 0 | |||||||
| BDI at discharge | 9.05 | 8.56 | 3 | 7 | 13 | 0 | 43 | ||
| 0–13 | 76 | 76.8 | |||||||
| 14–19 | 13 | 13.1 | |||||||
| 20–28 | 6 | 6.1 | |||||||
| 29–63 | 4 | 4.0 | |||||||
| BDI in follow-up | 8.27 | 7.56 | 1 | 7 | 13 | 0 | 34 | ||
| 0–13 | 77 | 77.8 | |||||||
| 14–19 | 11 | 11.1 | |||||||
| 20–28 | 10 | 10.1 | |||||||
| 29–63 | 1 | 1.0 | |||||||
| Mini-Mental at discharge | 25.38 | 4.57 | 23 | 27 | 29 | 10 | 30 | ||
| 27–30 | 55 | 55.6 | |||||||
| 25–26 | 12 | 12.1 | |||||||
| 10–24 | 32 | 32.3 | |||||||
| 6–9 | 0 | 0 | |||||||
| <6 | 0 | 0 | |||||||
| Mini-Mental in follow-up | 27.03 | 4.02 | 26 | 28 | 30 | 12 | 30 | ||
| 27–30 | 71 | 71.7 | |||||||
| 25–26 | 10 | 10.1 | |||||||
| 10–24 | 18 | 18.2 | |||||||
| 6–9 | 0 | 0 | |||||||
| <6 | 0 | 0 | |||||||
| Falls | |||||||||
| No | 97 | ||||||||
| Yes | 2 | ||||||||
| Temperature | 36.29 | 0.44 | 36 | 36.3 | 36.6 | 35 | 37.3 | ||
| Systolic blood pressure | 146.25 | 25.15 | 129 | 148 | 162 | 96 | 218 | ||
| Diastolic blood pressure | 78.01 | 12.56 | 70 | 78 | 85 | 48 | 114 | ||
| Mean arterial pressure (MAP) | 112.13 | 16.48 | 101 | 111 | 123.5 | 73 | 154 | ||
| Heart rate | 69.59 | 12.8 | 60 | 68 | 78 | 45 | 110 | ||
| Glucose | 112.61 | 38.71 | 93 | 103 | 120 | 72 | 349 | ||
| Stay in hospital (days) | 7.1 | 5.2 | 3 | 6 | 9 | 1 | 32 | ||
| 0–3 | 25 | 25.3 | |||||||
| 4–6 | 30 | 30.3 | |||||||
| 7–8 | 17 | 17.2 | |||||||
| ≥9 | 27 | 27.3 |
| (A) | |||||||
| Item number | Symptom | Severity | BDI at discharge, n (%) | ||||
| Minimal | Mild | Moderate | Severe | ||||
| 1 | Pessimism | Minimal | 56 (86.2) | 6 (9.2) | 2 (3.1) | 1 (1.5) | |
| Mild | 18 (66.7) | 6 (22.2) | 2 (7.4) | 1 (3.7) | |||
| Moderate | 0 (0) | 1 (33.3) | 1 (33.3) | 1 (33.3) | |||
| Severe | 2 (50) | 0 (0) | 1 (25) | 1 (25) | |||
| 2 | Mood | Minimal | 63 (88.7) | 5 (7) | 2 (2.8) | 1 (1.4) | |
| Mild | 10 (41.7) | 8 (33.3) | 4 (16.7) | 2 (8.3) | |||
| Moderate | 2 (100) | 0 (0) | 0 (0) | 0 (0) | |||
| Severe | 1 (50) | 0 (0) | 0 (0) | 1 (50) | |||
| 3 | Failure | Minimal | 71 (82.6) | 11 (12.8) | 3 (3.5) | 1 (1.2) | |
| Mild | 5 (55.6) | 1 (11.1) | 2 (22.2) | 1 (11.1) | |||
| Moderate | 0 (0) | 1 (33.3) | 1 (33.3) | 1 (33.3) | |||
| Severe | 0 (0) | 0 (0) | 0 (0) | 1 (100) | |||
| 4 | Dissatisfaction | Minimal | 57 (87.7) | 7 (10.8) | 1 (1.5) | 0 (0) | |
| Mild | 17 (54.8) | 6 (19.4) | 5 (16.1) | 3 (9.7) | |||
| Moderate | 2 (66.7) | 0 (0) | 0 (0) | 1 (33.3) | |||
| Severe | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||
| 5 | Feelings of guilt | Minimal | 66 (85.7) | 9 (11.7) | 2 (2.6) | 0 (0) | |
| Mild | 9 (45) | 4 (20) | 4 (20) | 3 (15) | |||
| Moderate | 1 (50) | 0 (0) | 0 (0) | 1 (50) | |||
| Severe | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||
| 6 | Feelings of punishment | Minimal | 71 (84.5) | 10 (11.9) | 3 (3.6) | 0 (0) | |
| Mild | 5 (55.6) | 1 (11.1) | 2 (22.2) | 1 (11.1) | |||
| Moderate | 0 (0) | 1 (100) | 0 (0) | 0 (0) | |||
| Severe | 0 (0) | 1 (20) | 1 (20) | 3 (60) | |||
| 7 | Self-disapproval | Minimal | 73 (84.9) | 8 (9.3) | 5 (5.8) | 0 (0) | |
| Mild | 3 (42.9) | 3 (42.9) | 1 (14.3) | 0 (0) | |||
| Moderate | 0 (0) | 1 (20) | 0 (0) | 4 (80) | |||
| Severe | 0 (0) | 1 (100) | 0 (0) | 0 (0) | |||
| 8 | Self-criticism | Minimal | 68 (89.5) | 6 (7.9) | 2 (2.6) | 0 (0) | |
| Mild | 7 (50) | 5 (35.7) | 2 (14.3) | 0 (0) | |||
| Moderate | 1 (12.5) | 2 (25) | 2 (25) | 3 (37.5) | |||
| Severe | 0 (0) | 0 (0) | 0 (0) | 1 (100) | |||
| 9 | Suicidal thoughts or ideas | Minimal | 73 (81.1) | 9 (10) | 5 (5.6) | 3 (3.3) | |
| Mild | 3 (33.3) | 4 (44.4) | 1 (11.1) | 1 (11.1) | |||
| Moderate | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||
| Severe | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||
| 10 | Crying | Minimal | 66 (84.6) | 9 (11.5) | 2 (2.6) | 1 (1.3) | |
| Mild | 7 (50) | 4 (28.6) | 3 (21.4) | 0 (0) | |||
| Moderate | 3 (75) | 0 (0) | 1 (25) | 0 (0) | |||
| Severe | 0 (0) | 0 (0) | 0 (0) | 3 (100) | |||
| 11 | Fatigability | Minimal | 58 (92.1) | 5 (7.9) | 0 (0) | 0 (0) | |
| Mild | 13 (52) | 7 (28) | 5 (20) | 0 (0) | |||
| Moderate | 2 (50) | 1 (25) | 0 (0) | 1 (25) | |||
| Severe | 3 (42.9) | 0 (0) | 1 (14.3) | 3 (42.9) | |||
| 12 | Social withdrawal | Minimal | 65 (82.3) | 10 (12.7) | 2 (2.5) | 2 (2.5) | |
| Mild | 11 (64.7) | 2 (11.8) | 3 (17.6) | 1 (5.9) | |||
| Moderate | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||
| Severe | 0 (0) | 1 (33.3) | 1 (33.3) | 1 (33.3) | |||
| 13 | Indecisiveness | Minimal | 65 (91.5) | 6 (8.5) | 0 (0) | 0 (0) | |
| Mild | 10 (50) | 6 (30) | 3 (15) | 1 (5) | |||
| Moderate | 1 (16.7) | 1 (16.7) | 2 (33.3) | 2 (33.3) | |||
| Severe | 0 (0) | 0 (0) | 1 (50) | 1 (50) | |||
| 14 | Changes in physical appearance | Minimal | 70 (90.9) | 5 (6.5) | 2 (2.6) | 0 (0) | |
| Mild | 6 (35.3) | 7 (41.2) | 3 (17.6) | 1 (5.9) | |||
| Moderate | 0 (0) | 0 (0) | 1 (33.3) | 2 (66.7) | |||
| Severe | 0 (0) | 1 (50) | 0 (0) | 1 (50) | |||
| 15 | Loss of energy | Minimal | 30 (96.8) | 1 (3.2) | 0 (0) | 0 (0) | |
| Mild | 41 (80.4) | 8 (15.7) | 2 (3.9) | 0 (0) | |||
| Moderate | 5 (33.3) | 4 (26.7) | 4 (26.7) | 2 (13.3) | |||
| Severe | 0 (0) | 0 (0) | 0 (0) | 2 (100) | |||
| 16 | Changes in sleep patterns | Minimal | 41 (91.1) | 4 (8.9) | 0 (0) | 0 (0) | |
| Mild | 27 (79.4) | 4 (11.8) | 1 (2.9) | 2 (5.9) | |||
| Moderate | 8 (57.1) | 2 (14.3) | 3 (21.4) | 1 (7.1) | |||
| Severe | 0 (0) | 3 (50) | 2 (33.3) | 1 (16.7) | |||
| 17 | Irritability | Minimal | 64 (88.9) | 6 (8.3) | 2 (2.8) | 0 (0) | |
| Mild | 11 (52.4) | 5 (23.8) | 2 (9.5) | 3 (14.3) | |||
| Moderate | 0 (0) | 0 (0) | 2 (100) | 0 (0) | |||
| Severe | 1 (25) | 2 (50) | 0 (0) | 1 (25) | |||
| 18 | Loss of appetite | Minimal | 62 (88.6) | 4 (5.7) | 2 (4.3) | 1 (1.4) | |
| Mild | 13 (56.5) | 6 (26.1) | 2 (8.7) | 2 (8.7) | |||
| Moderate | 1 (20) | 2 (40) | 1 (20) | 1 (20) | |||
| Severe | 0 (0) | 1 (100) | 0 (0) | 0 (0) | |||
| 19 | Difficulty concentrating | Minimal | 52 (100) | 0 (0) | 0 (0) | 0 (0) | |
| Mild | 22 (61.1) | 10 (27.8) | 4 (11.1) | 0 (0) | |||
| Moderate | 2 (22.2) | 3 (33.3) | 2 (22.2) | 2 (22.2) | |||
| Severe | 0 (0) | 0 (0) | 0 (0) | 2 (100) | |||
| 20 | Fatigue or tiredness | Minimal | 36 (97.3) | 1 (2.7) | 0 (0) | 0 (0) | |
| Mild | 31 (70.5) | 10 (22.7) | 2 (4.5) | 1 (2.3) | |||
| Moderate | 8 (61.5) | 2 (15.4) | 2 (15.4) | 1 (7.7) | |||
| Severe | 1 (20) | 0 (0) | 2 (40) | 2 (40) | |||
| 21 | Loss of libido | Minimal | 50 (94.3) | 2 (3.8) | 1 (1.9) | 0 (0) | |
| Mild | 17 (81) | 4 (19) | 0 (0) | 0 (0) | |||
| Moderate | 4 (44.4) | 1 (11.1) | 2 (22.2) | 2 (22.2) | |||
| Severe | 5 (31.3) | 6 (37.5) | 3 (18.8) | 2 (12.5) | |||
| (B) | BDI in follow-up, n (%) | ||||||
| Minimal | Mild | Moderate | Severe | ||||
| 1 | Pessimism | Minimal | 61 (89.7) | 4 (5.9) | 2 (2.9) | 1 (1.5) | |
| Mild | 16 (57.1) | 7 (25) | 5 (17.9) | 0 (0) | |||
| Moderate | 0 (0) | 0 (0) | 2 (100) | 0 (0) | |||
| Severe | 0 (0) | 0 (0) | 1 (100) | 0 (0) | |||
| 2 | Mood | Minimal | 68 (90.7) | 4 (5.3) | 2 (7.7) | 1 (1.3) | |
| Mild | 7 (33.3) | 6 (28.6) | 8 (38.1) | 0 (0) | |||
| Moderate | 1 (50) | 1 (50) | 0 (0) | 0 (0) | |||
| Severe | 1 (100) | 0 (0) | 0 (0) | 0 (0) | |||
| 3 | Failure | Minimal | 74 (85.1) | 8 (9.2) | 4 (4.6) | 1 (1.1) | |
| Mild | 3 (30) | 3 (30) | 4 (40) | 0 (0) | |||
| Moderate | 0 (0) | 0 (0) | 1 (100) | 0 (0) | |||
| Severe | 0 (0) | 0 (0) | 1 (100) | 0 (0) | |||
| 4 | Dissatisfaction | Minimal | 60 (92.3) | 2 (3.1) | 3 (4.6) | 0 (0) | |
| Mild | 16 (51.6) | 9 (29) | 5 (16.1) | 1 (3.2) | |||
| Moderate | 1 (33.3) | 0 (0) | 2 (66.7) | 0 (0) | |||
| Severe | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||
| 5 | Feelings of guilt | Minimal | 70 (84.3) | 6 (7.2) | 7 (8,4) | 0 (0) | |
| Mild | 7 (46.7) | 5 (33.3) | 3 (20) | 0 (0) | |||
| Moderate | 0 (0) | 0 (0) | 0 (0) | 1 (100) | |||
| Severe | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||
| 6 | Feelings of punishment | Minimal | 75 (83.3) | 8 (8.9) | 7 (7.8) | 0 (0) | |
| Mild | 2 (40) | 2 (40) | 1 (20) | 0 (0) | |||
| Moderate | 0 (0) | 1 (50) | 0 (0) | 1 (50) | |||
| Severe | 0 (0) | 0 (0) | 2 (100) | 0 (0) | |||
| 7 | Self-disapproval | Minimal | 72 (83.7) | 8 (9.3) | 6 (7) | 0 (0) | |
| Mild | 4 (44.4) | 3 (33.3) | 2 (22.2) | 0 (0) | |||
| Moderate | 1 (25) | 0 (0) | 2 (50) | 1 (25) | |||
| Severe | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||
| 8 | Self-criticism | Minimal | 71 (85.5) | 7 (8.4) | 5 (6) | 0 (0) | |
| Mild | 6 (54.5) | 3 (27.3) | 2 (18.2) | 0 (0) | |||
| Moderate | 0 (0) | 1 (25) | 3 (75) | 0 (0) | |||
| Severe | 0 (0) | 0 (0) | 0 (0) | 1 (100) | |||
| 9 | Suicidal thoughts or ideas | Minimal | 74 (80.4) | 9 (9.8) | 8 (8.7) | 1 (1.1) | |
| Mild | 3 (42.9) | 2 (28.6) | 2 (28.6) | 0 (0) | |||
| Moderate | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||
| Severe | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||
| 10 | Crying | Minimal | 64 (94.1) | 3 (4.4) | 1 (1.5) | 0 (0) | |
| Mild | 11 (39.3) | 8 (28.6) | 9 (32.1) | 0 (0) | |||
| Moderate | 2 (100) | 0 (0) | 0 (0) | 0 (0) | |||
| Severe | 0 (0) | 0 (0) | 0 (0) | 1 (100) | |||
| 11 | Fatigability | Minimal | 64 (94.1) | 3 (4.4) | 1 (1.5) | 0 (0) | |
| Mild | 11 (39.3) | 8 (28.6) | 9 (32.1) | 0 (0) | |||
| Moderate | 2 (100) | 0 (0) | 0 (0) | 0 (0) | |||
| Severe | 0 (0) | 0 (0) | 0 (0) | 1 (100) | |||
| 12 | Social withdrawal | Minimal | 64 (90.1) | 4 (5.6) | 2 (2.8) | 1 (1.4) | |
| Mild | 13 (54.2) | 6 (25) | 5 (20.8) | 0 (0) | |||
| Moderate | 0 (0) | 1 (33.3) | 2 (66.7) | 0 (0) | |||
| Severe | 0 (0) | 0 (0) | 1 (100) | 0 (0) | |||
| 13 | Indecisiveness | Minimal | 65 (94.2) | 4 (5.8) | 0 (0) | 0 (0) | |
| Mild | 12 (50) | 6 (25) | 5 (20.8) | 1 (4.2) | |||
| Moderate | 0 (0) | 1 (20) | 4 (80) | 0 (0) | |||
| Severe | 0 (0) | 0 (0) | 1 (100) | 0 (0) | |||
| 14 | Changes in physical appearance | Minimal | 65 (90.3) | 3 (4.2) | 4 (5.6) | 0 (0) | |
| Mild | 10 (47.6) | 7 (33.3) | 4 (19) | 0 (0) | |||
| Moderate | 2 (40) | 1 (20) | 2 (40) | 0 (0) | |||
| Severe | 0 (0) | 0 (0) | 0 (0) | 1 (100) | |||
| 15 | Loss of energy | Minimal | 35 (100) | 0 (0) | 0 (0) | 0 (0) | |
| Mild | 39 (81.3) | 7 (14.6) | 2 (4.2) | 0 (0) | |||
| Moderate | 3 (23.1) | 4 (30.8) | 6 (46.2) | 0 (0) | |||
| Severe | 0 (0) | 0 (0) | 2 (66.7) | 1 (33.3) | |||
| 16 | Changes in sleep patterns | Minimal | 48 (90.6) | 3 (5.7) | 2 (3.8) | 0 (0) | |
| Mild | 21 (77.8) | 4 (14.8) | 2 (7.4) | 0 (0) | |||
| Moderate | 7 (46.7) | 3 (20) | 5 (33.3) | 0 (0) | |||
| Severe | 1 (25) | 1 (25) | 1 (25) | 1 (25) | |||
| 17 | Irritability | Minimal | 58 (89.2) | 3 (4.6) | 4 (6.2) | 0 (0) | |
| Mild | 18 (58.1) | 8 (25.8) | 5 (16.1) | 0 (0) | |||
| Moderate | 1 (100) | 0 (0) | 0 (0) | 0 (0) | |||
| Severe | 0 (0) | 0 (0) | 1 (50) | 1 (50) | |||
| 18 | Loss of appetite | Minimal | 63 (88.7) | 4 (5.6) | 4 (5.6) | 0 (0) | |
| Mild | 14 (63.6) | 4 (18.2) | 3 (13.6) | 1 (4.5) | |||
| Moderate | 0 (0) | 3 (50) | 3 (50) | 0 (0) | |||
| Severe | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||
| 19 | Difficulty concentrating | Minimal | 49 (94.2) | 1 (1.9) | 2 (3.8) | 0 (0) | |
| Mild | 26 (68.4) | 8 (21.1) | 4 (10.5) | 0 (0) | |||
| Moderate | 2 (22.2) | 2 (22.2) | 4 (44.4) | 1 (11.1) | |||
| Severe | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||
| 20 | Fatigue or tiredness | Minimal | 36 (97.3) | 0 (0) | 1 (2.7) | 0 (0) | |
| Mild | 37 (80.4) | 8 (17.4) | 1 (2.2) | 0 (0) | |||
| Moderate | 3 (25) | 3 (25) | 6 (50) | 0 (0) | |||
| Severe | 1 (25) | 0 (0) | 2 (52) | 1 (50) | |||
| 21 | Loss of libido | Minimal | 49 (90.7) | 2 (3.7) | 3 (5.6) | 0 (0) | |
| Mild | 15 (75) | 5 (25) | 0 (0) | 0 (0) | |||
| Moderate | 3 (42.9) | 2 (28.6) | 1 (14.3) | 1 (14.3) | |||
| Severe | 10 (55.6) | 2 (11.1) | 6 (33.3) | 0 (0) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pego Pérez, E.R.; Bermello López, L.; Gómez Fernández, E.; Marín Arnés, M.d.R.; Fernández Vázquez, M.; Touza González, C.; Núñez Hernández, M.I. Cognitive, Functional, and Emotional Recovery in Patients with Stroke: A Multidimensional Prospective Analysis. Neurol. Int. 2025, 17, 164. https://doi.org/10.3390/neurolint17100164
Pego Pérez ER, Bermello López L, Gómez Fernández E, Marín Arnés MdR, Fernández Vázquez M, Touza González C, Núñez Hernández MI. Cognitive, Functional, and Emotional Recovery in Patients with Stroke: A Multidimensional Prospective Analysis. Neurology International. 2025; 17(10):164. https://doi.org/10.3390/neurolint17100164
Chicago/Turabian StylePego Pérez, Emilio Rubén, Lourdes Bermello López, Eva Gómez Fernández, María del Rosario Marín Arnés, Mercedes Fernández Vázquez, Carlota Touza González, and María Irene Núñez Hernández. 2025. "Cognitive, Functional, and Emotional Recovery in Patients with Stroke: A Multidimensional Prospective Analysis" Neurology International 17, no. 10: 164. https://doi.org/10.3390/neurolint17100164
APA StylePego Pérez, E. R., Bermello López, L., Gómez Fernández, E., Marín Arnés, M. d. R., Fernández Vázquez, M., Touza González, C., & Núñez Hernández, M. I. (2025). Cognitive, Functional, and Emotional Recovery in Patients with Stroke: A Multidimensional Prospective Analysis. Neurology International, 17(10), 164. https://doi.org/10.3390/neurolint17100164







