Adult Case of Pontocerebellar Hypoplasia without the Claustrum
Abstract
1. Introduction
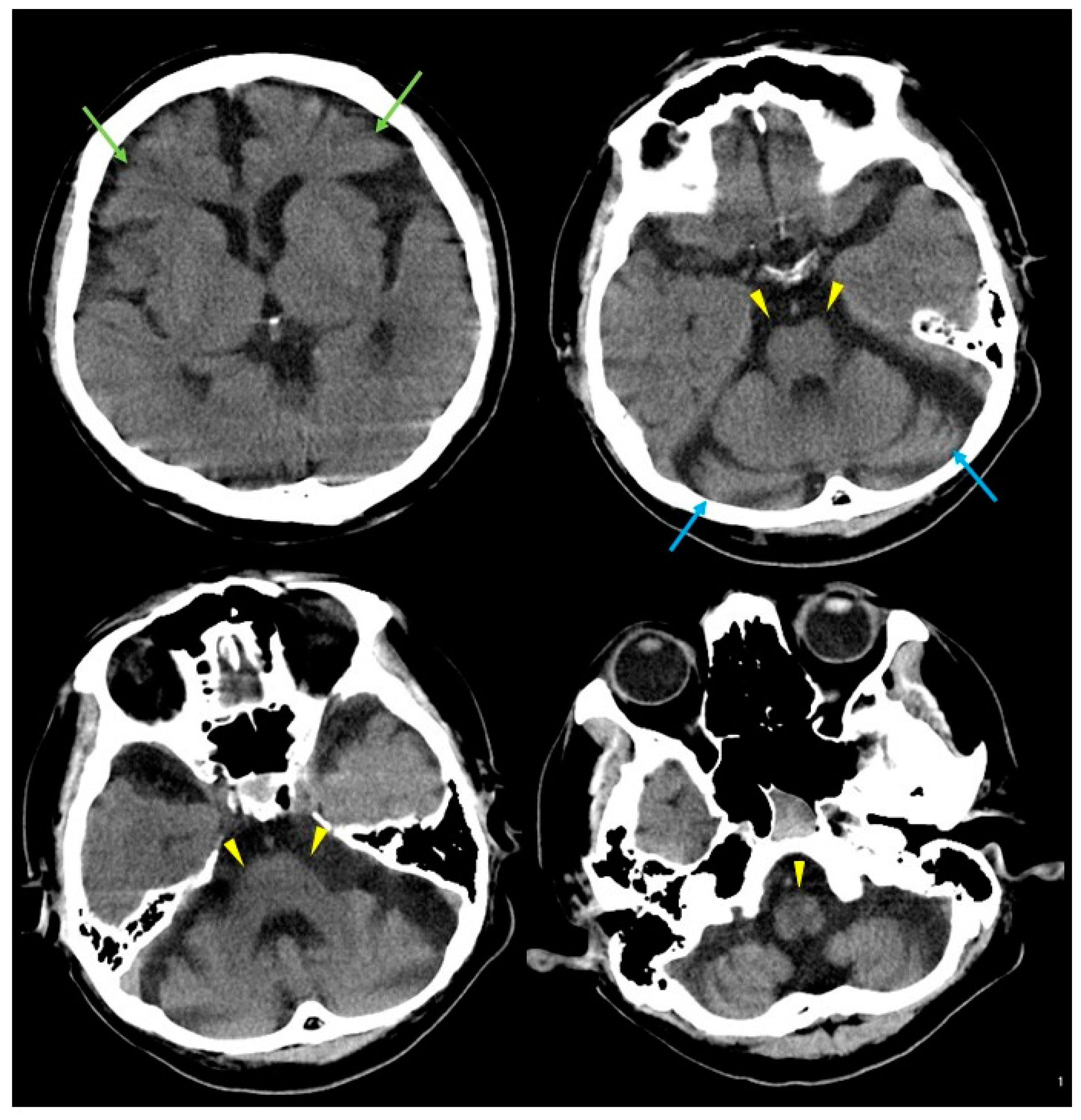
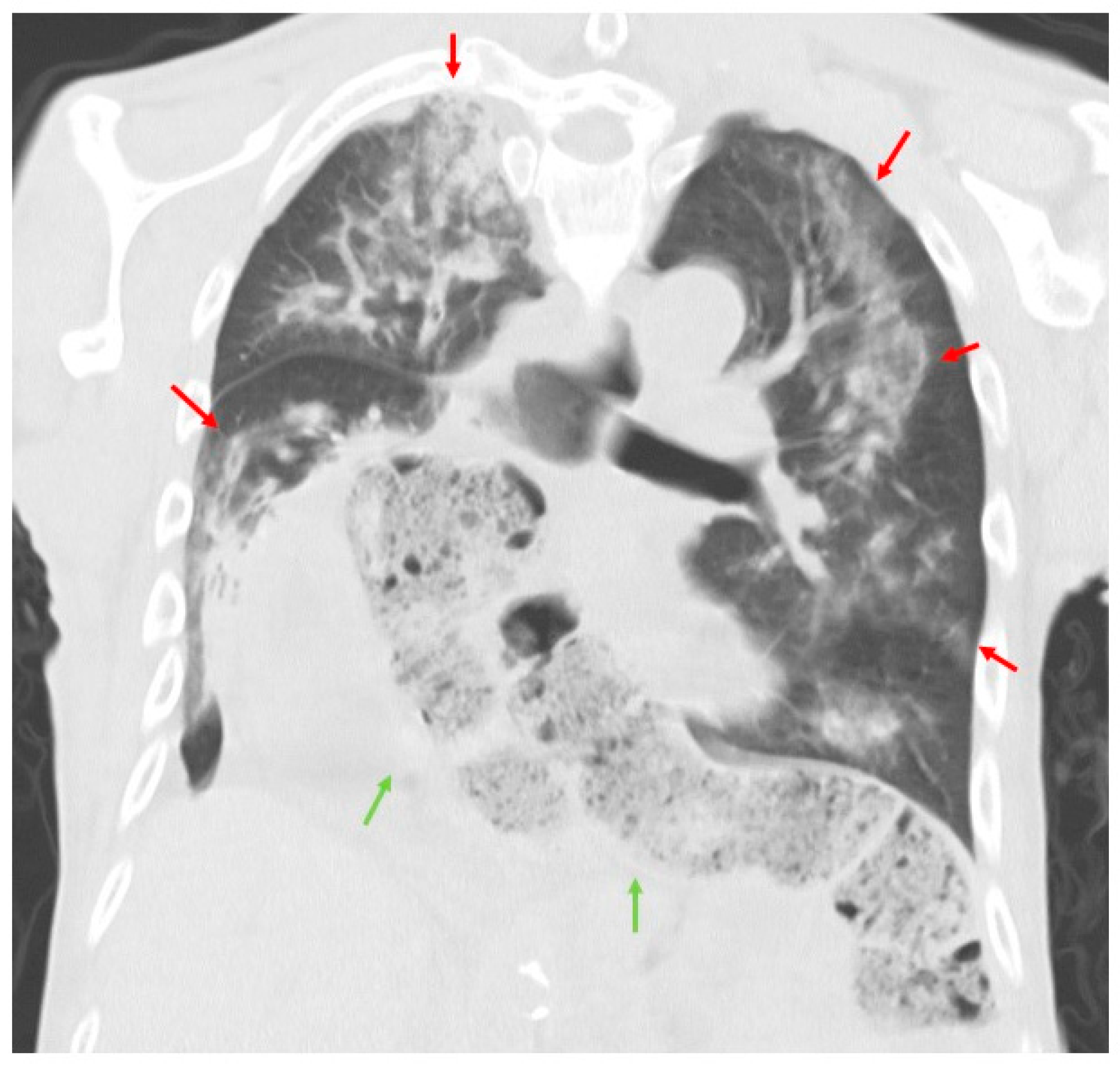
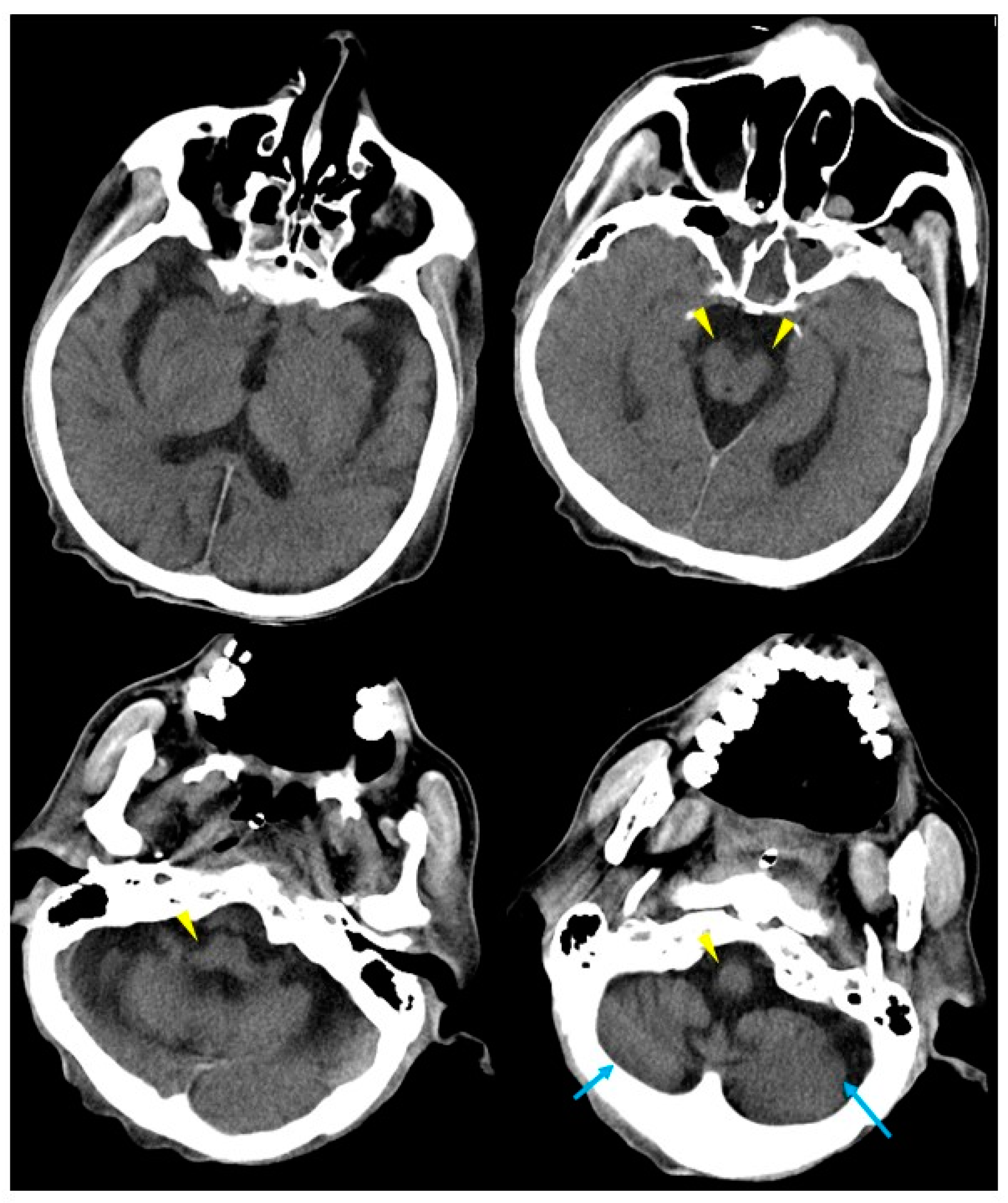
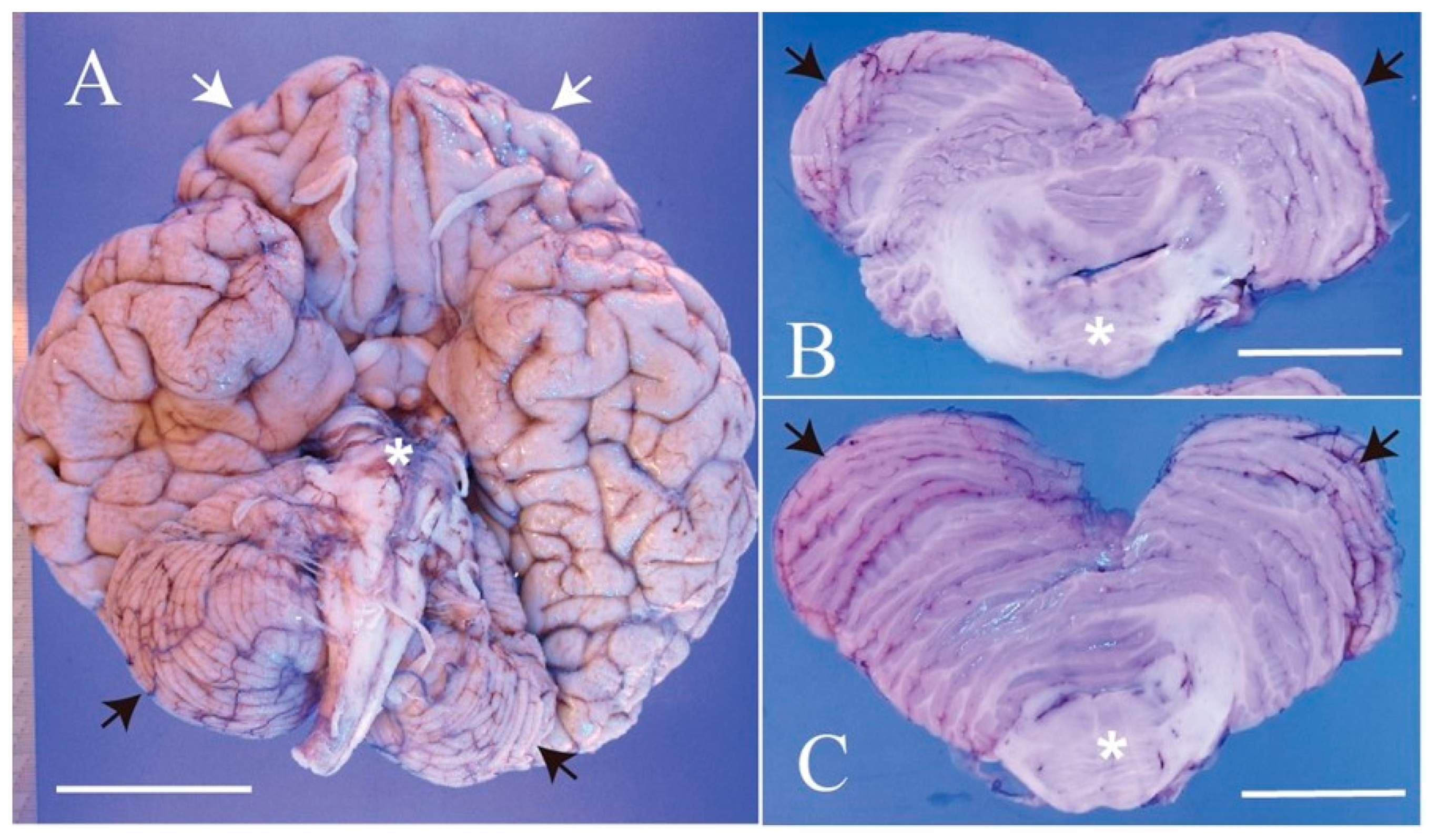
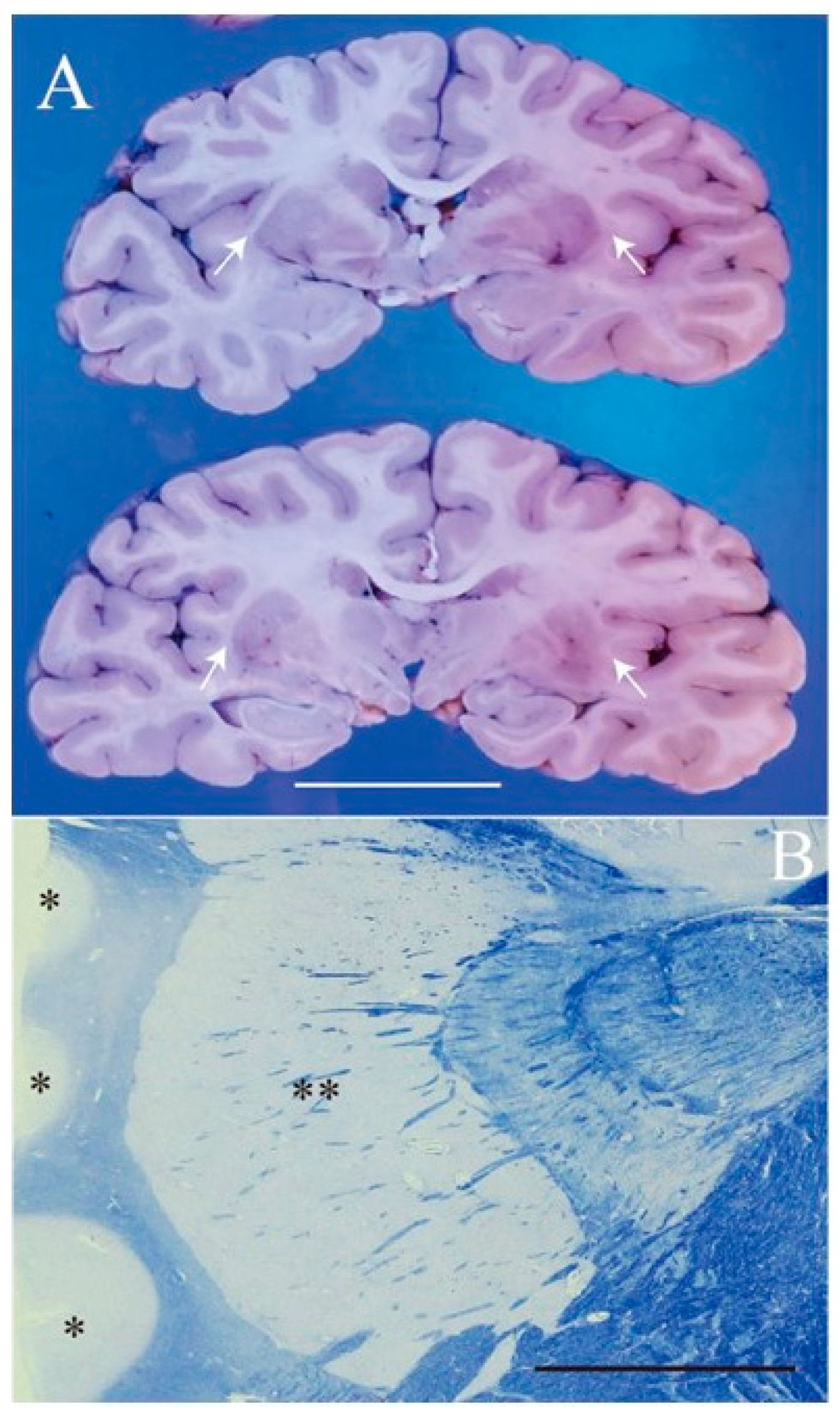
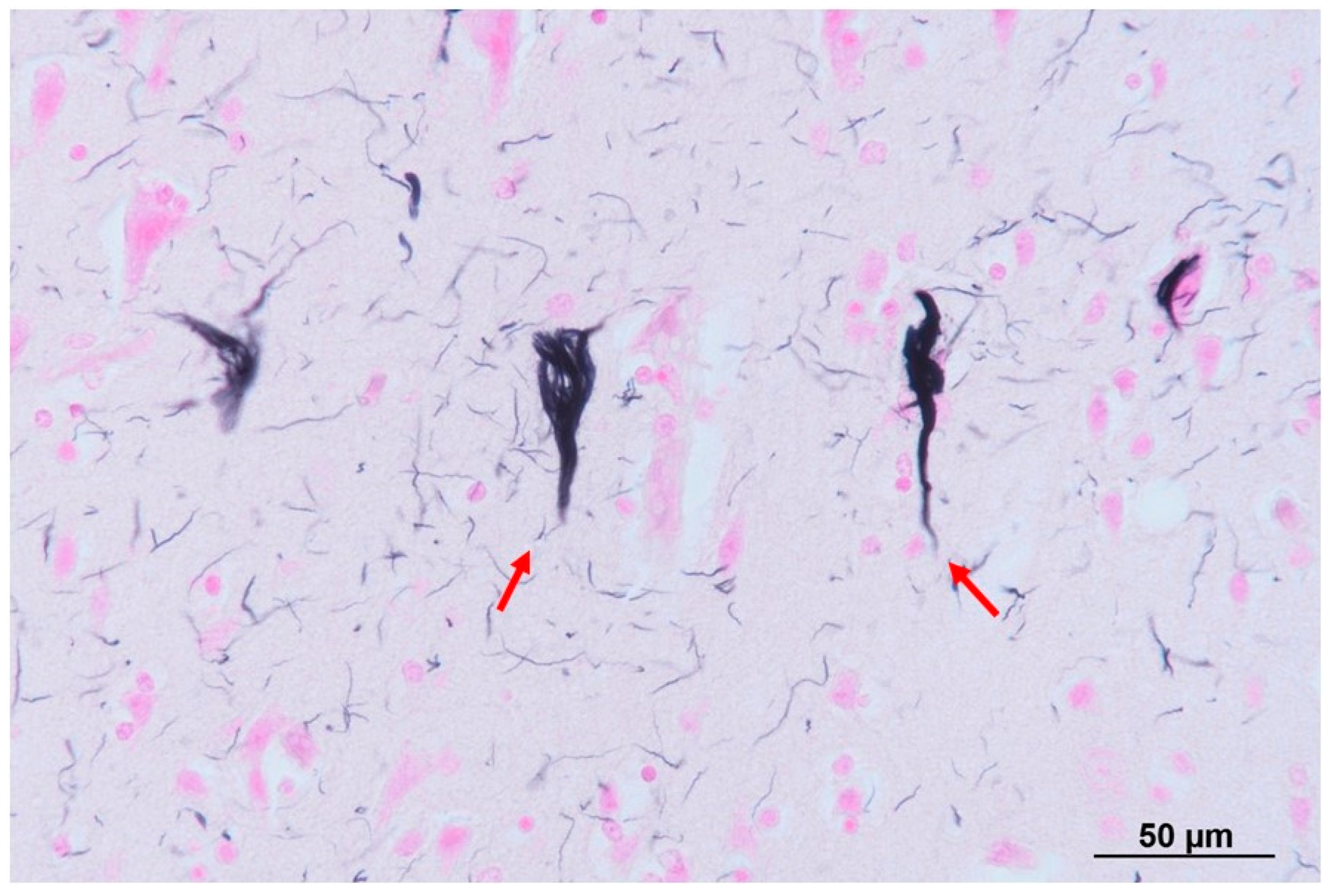
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Narikiyo, K.; Mizuguchi, R.; Ajima, A.; Shiozaki, M.; Hamanaka, H.; Johansen, J.P.; Mori, K.; Yoshihara, Y. The claustrum coordinates cortical slow-wave activity. Nat. Neurosci. 2020, 23, 741–753. [Google Scholar] [CrossRef] [PubMed]
- Crick, F.C.; Koch, C. What is the function of the claustrum? Philos. Trans. R. Soc. B Biol. Sci. 2005, 360, 1271–1279. [Google Scholar] [CrossRef]
- Kowianski, P.; Dziewiatkowski, J.; Kowianska, J.; Morys, J. Comparative anatomy of the claustrum in selected species: A morphometric analysis. Brain Behav. Evol. 1999, 53, 44–54. [Google Scholar] [CrossRef]
- Kurada, L.; Bayat, A.; Joshi, S.; Koubeissi, M.Z. The Claustrum in Relation to Seizures and Electrical Stimulation. Front. Neuroanat. 2019, 13, 8. [Google Scholar] [CrossRef]
- Atlan, G.; Matosevich, N.; Peretz-Rivlin, N.; Marsh-Yvgi, I.; Zelinger, N.; Chen, E.; Kleinman, T.; Bleistein, N.; Sheinbach, E.; Groysman, M.; et al. Claustrum neurons projecting to the anterior cingulate restrict engagement during sleep and behavior. Nat. Commun. 2024, 15, 5415. [Google Scholar] [CrossRef]
- Cascella, N.G.; Gerner, G.J.; Fieldstone, S.C.; Sawa, A.; Schretlen, D.J. The insula-claustrum region and delusions in schizophrenia. Schizophr. Res. 2011, 133, 77–81. [Google Scholar] [CrossRef]
- Bai, L.; Di, W.; Xu, Z.; Liu, B.; Lin, N.; Fan, S.; Ren, H.; Lu, Q.; Wang, J.; Guan, H.; et al. Febrile infection-related epilepsy syndrome with claustrum lesion: An underdiagnosed inflammatory encephalopathy. Neurol. Sci. 2024, 45, 3411–3419. [Google Scholar] [CrossRef]
- Chau, A.; Salazar, A.M.; Krueger, F.; Cristofori, I.; Grafman, J. The effect of claustrum lesions on human consciousness and recovery of function. Conscious. Cogn. 2015, 36, 256–264. [Google Scholar] [CrossRef]
- Goll, Y.; Atlan, G.; Citri, A. Attention: The claustrum. Trends Neurosci. 2015, 38, 486–495. [Google Scholar] [CrossRef]
- Joutsa, J.; Horn, A.; Hsu, J.; Fox, M.D. Localizing parkinsonism based on focal brain lesions. Brain 2018, 141, 2445–2456. [Google Scholar] [CrossRef]
- Niu, M.; Kasai, A.; Tanuma, M.; Seiriki, K.; Igarashi, H.; Kuwaki, T.; Nagayasu, K.; Miyaji, K.; Ueno, H.; Tanabe, W.; et al. Claustrum mediates bidirectional and reversible control of stress-induced anxiety responses. Sci. Adv. 2022, 8, eabi6375. [Google Scholar] [CrossRef] [PubMed]
- Oshima, K.; Yoshinaga, S.; Kitazawa, A.; Hirota, Y.; Nakajima, K.; Kubo, K.I. A Unique “Reversed” Migration of Neurons in the Developing Claustrum. J. Neurosci. 2023, 43, 693–708. [Google Scholar] [CrossRef]
- Ogut, E.; Armagan, K.; Tufekci, D. The Guillain-Mollaret triangle: A key player in motor coordination and control with implications for neurological disorders. Neurosurg. Rev. 2023, 46, 181. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, J.; Thada, P.K.; Samanta, D. Spinocerebellar Ataxia; [Updated 15 September 2023]; StatPearls Publishing: Treasure Island, FL, USA, 2024; Available online: https://www.ncbi.nlm.nih.gov/books/NBK557816/ (accessed on 26 September 2024).
- Dodgson, M.C. A congenital malformation of insular cortex in man, involving the claustrum and certain subcortical centers. J. Comp. Neurol. 1955, 102, 341–364. [Google Scholar] [CrossRef]
- Leroy, J.G.; Lyon, G.; Fallet, C.; Amiel, J.; De Praeter, C.; Van Den Broecke, C.; Vanhaesebrouck, P. Congenital pontocerebellar atrophy and telencephalic defects in three siblings: A new subtype. Acta Neuropathol. 2007, 114, 387–399. [Google Scholar] [CrossRef]
- Kuchelmeister, K.; Bergmann, M.; Gullotta, F. Neuropathology of lissencephalies. Childs Nerv. Syst. 1993, 9, 394–399. [Google Scholar] [CrossRef]
- Korinthenberg, R.; Palm, D.; Schlake, W.; Klein, J. Congenital muscular dystrophy, brain malformation and ocular problems (muscle, eye and brain disease) in two German families. Eur. J. Pediatr. 1984, 142, 64–68. [Google Scholar] [CrossRef]
- Pavone, L.; Gullotta, F.; Grasso, S.; Vannucchi, C. Hydrocephalus, lissencephaly, ocular abnormalities and congenital muscular dystrophy: A Warburg syndrome variant? Neuropediatrics 1986, 17, 206–211. [Google Scholar] [CrossRef]
- Fatemi, S.H. Reelin mutations in mouse and man: From reeler mouse to schizophrenia, mood disorders, autism and lissencephaly. Mol. Psychiatry 2001, 6, 129–133. [Google Scholar] [CrossRef]
- Krawinkel, M.; Steen, H.J.; Terwey, B. Magnetic resonance imaging in lissencephaly. Eur. J. Pediatr. 1987, 146, 205–208. [Google Scholar] [CrossRef]
- Engelkamp, D.; Rashbass, P.; Seawright, A.; van Heyningen, V. Role of Pax6 in development of the cerebellar system. Development 1999, 126, 3585–3596. [Google Scholar] [CrossRef]
- Stoykova, A.; Treichel, D.; Hallonet, M.; Gruss, P. Pax6 modulates the dorsoventral patterning of the mammalian telencephalon. J. Neurosci. 2000, 20, 8042–8050. [Google Scholar] [CrossRef] [PubMed]
- Reardon, S. A giant neuron found wrapped around entire mouse brain. Nature 2017, 543, 14–15. [Google Scholar] [CrossRef]
- Laufs, H.; Richardson, M.P.; Salek-Haddadi, A.; Vollmar, C.; Duncan, J.S.; Gale, K.; Lemieux, L.; Löscher, W.; Koepp, M.J. Converging PET and fMRI evidence for a common area involved in human focal epilepsies. Neurology 2011, 77, 904–910. [Google Scholar] [CrossRef]
- Fahoum, F.; Lopes, R.; Pittau, F.; Dubeau, F.; Gotman, J. Widespread epileptic networks in focal epilepsies: EEG-fMRI study. Epilepsia 2012, 53, 1618–1627. [Google Scholar] [CrossRef]
- Flanagan, D.; Badawy, R.A.; Jackson, G.D. EEG-fMRI in focal epilepsy: Local activation and regional networks. Clin. Neurophysiol. 2014, 125, 21–31. [Google Scholar] [CrossRef]
- Vaughan, D.N.; Jackson, G.D. The piriform cortex and human focal epilepsy. Front. Neurol. 2014, 5, 259. [Google Scholar] [CrossRef]
- Smith, J.B.; Liang, Z.; Watson, G.D.R.; Alloway, K.D.; Zhang, N. Interhemispheric resting-state functional connectivity of the claustrum in the awake and anesthetized states. Brain Struct. Funct. 2017, 222, 2041–2058. [Google Scholar] [CrossRef]
- Koubeissi, M.Z.; Bartolomei, F.; Beltagy, A.; Picard, F. Electrical stimulation of a small brain area reversibly disrupts consciousness. Epilepsy Behav. 2014, 37, 32–35. [Google Scholar] [CrossRef]
- Meletti, S.; Slonkova, J.; Mareckova, I.; Monti, G.; Specchio, N.; Hon, P.; Giovannini, G.; Marcian, V.; Chiari, A.; Krupa, P.; et al. Claustrum damage and refractory status epilepticus following febrile illness. Neurology 2015, 85, 1224–1232. [Google Scholar] [CrossRef]
- Wada, J.A.; Tsuchimochi, H. Role of the claustrum in convulsive evolution of visual afferent and partial nonconvulsive seizure in primates. Epilepsia 1997, 38, 897–906. [Google Scholar] [CrossRef] [PubMed]
- Mohapel, P.; Zhang, X.; Gillespie, G.W.; Chlan-Fourney, J.; Hannesson, D.K.; Corley, S.M.; Li, X.-M.; Corcoran, M.E. Kindling of claustrum and insular cortex: Comparison to perirhinal cortex in the rat. Eur. J. Neurosci. 2001, 13, 1501–1519. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Hannesson, D.K.; Saucier, D.M.; Wallace, A.E.; Howland, J.; Corcoran, M.E. Susceptibility to kindling and neuronal connections of the anterior claustrum. J. Neurosci. 2001, 21, 3674–3687. [Google Scholar] [CrossRef]
- Sheerin, A.H.; Nylen, K.; Zhang, X.; Saucier, D.M.; Corcoran, M.E. Further evidence for a role of the anterior claustrum in epileptogenesis. Neuroscience 2004, 125, 57–62. [Google Scholar] [CrossRef]
- Ishii, K.; Tsuji, H.; Tamaoka, A. Mumps virus encephalitis with symmetric claustrum lesions. AJNR Am. J. Neuroradiol. 2011, 32, E139. [Google Scholar] [CrossRef]
- Sperner, J.; Sander, B.; Lau, S.; Krude, H.; Scheffner, D. Severe transitory encephalopathy with reversible lesions of the claustrum. Pediatr. Radiol. 1996, 26, 769–771. [Google Scholar] [CrossRef]
- Matsuzono, K.; Kurata, T.; Deguchi, S.; Yamashita, T.; Deguchi, K.; Ikeda, Y.; Abe, K. Two unique cases with anti-Glur antibody-positive encephalitis. Clin. Med. Insights Case Rep. 2013, 6, 113–117. [Google Scholar] [CrossRef]
| Patient (No.) | Ref. | Gender (M/F) | Premature Birth (Y/N) | Symptoms by Nature | Malformation by Nature | Clinical Diagnosis | The Cause of Death | Age of Death | Brain Malformation | CL |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | [15] | F | N | cyanosis and abnormal respiration | micrognathos, bilateral equino-varus deformities of the feet, whilst the hands were flexed and held in ulnar deviation | arthrogryposis multiplex congenita | purulent bronchitis and bronchopneumonia | 16 months | cerebral malformation | Absent, but a claustral rudiment lay was noted |
| 2 | [16] | F | N | respiratory failure, no suction reflex, increasing generalized hypertonia and seizures | severe reduction in the cerebral volume and much widened subarachnoidal spaces | pontocerebellar hypoplasia | status epilepticus led to intractable desaturation | 5 days | small neocerebellar hemispheres, volumetric reduction in the cerebral cortex and white matter (pallium), thickened the meninges, small and simplified cortical convolutions | Absent |
| 3 | [16] | F | N | no suction reflex, difficulty of oral feeding, tonic seizures, apnea, bradycardia and O2-desaturation, exaggerated startle response (hyperacousis) | severe reduction in the cerebral volume and much widened subarachnoidal spaces | pontocerebellar hypoplasia | convulsions | 22 days | small neocerebellar hemispheres, volumetric reduction in the cerebral cortex and white matter (pallium), thickened the meninges, small and simplified cortical convolutions | Absent |
| 4 | [17] | F | N.D. | seizure and hypertonia of limbs | N.D. | lissencephaly, type I | infection | 11.5 months | microcephalic and agyria | Absent |
| 5 | [17] | F | N | growth deficiency, severe psychomotor retardation followed by seizure | microcephaly and facial dysmorphism | Miller–Dicker syndrome, lissencephaly, type I | acute biventricular heart failure | 9 months | microcephalic and agyria | Absent |
| 6 | [17,18] | F | N | severe muscular hypotonia | N.D. | muscular dystrophy, lissencephaly, type II | cerebral dysregulation | 9 months | agyria, polymicrogyria, pachygyria, absent of cerebral peduncles, Dandy–Walker malformation | Absent |
| 7 | [17,19] | M | N | severe asphyxia, muscular hypotonia, poor sucking | N.D. | muscular dystrophy, lissencephaly, type II | bronchopneumonia | 14 months | argyria, polymicrogyria, pachygyria, absence of olfactory nerve, cerebral peduncles and inferior vermis, hypoplasia of the superior cerebellar vermis, Dandy–Walker malformation | Absent |
| 8 | [17] | F | asphyxia and hydrocephalus, with little spontaneous motility and poor reaction to painful stimuli | N.D. | lissencephaly, type II | cerebral dysregulation | 4 days | agyria, polymicrogyria, pachygyria, absent of cerebral peduncles, Dandy–Walker malformation | Absent | |
| 9 | This report | M | N.D. | intelligent disability and seizures | cerebellar atrophy | cerebral palsy, cerebellar atrophy | aspiration pneumonia | 63 years | atrophy of the frontal lobe, cerebellum and pons | Absent |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hayashi, K.; Mitsuhashi, S.; Kawahara, E.; Suzuki, A.; Nakaya, Y.; Sato, M.; Kobayashi, Y. Adult Case of Pontocerebellar Hypoplasia without the Claustrum. Neurol. Int. 2024, 16, 1132-1142. https://doi.org/10.3390/neurolint16050085
Hayashi K, Mitsuhashi S, Kawahara E, Suzuki A, Nakaya Y, Sato M, Kobayashi Y. Adult Case of Pontocerebellar Hypoplasia without the Claustrum. Neurology International. 2024; 16(5):1132-1142. https://doi.org/10.3390/neurolint16050085
Chicago/Turabian StyleHayashi, Koji, Shiho Mitsuhashi, Ei Kawahara, Asuka Suzuki, Yuka Nakaya, Mamiko Sato, and Yasutaka Kobayashi. 2024. "Adult Case of Pontocerebellar Hypoplasia without the Claustrum" Neurology International 16, no. 5: 1132-1142. https://doi.org/10.3390/neurolint16050085
APA StyleHayashi, K., Mitsuhashi, S., Kawahara, E., Suzuki, A., Nakaya, Y., Sato, M., & Kobayashi, Y. (2024). Adult Case of Pontocerebellar Hypoplasia without the Claustrum. Neurology International, 16(5), 1132-1142. https://doi.org/10.3390/neurolint16050085





