Cerebrovascular Reactivity Assessed by Breath-Hold Functional MRI in Patients with Neurological Post-COVID-19 Syndrome—A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.1.1. Patients with PCS
2.1.2. Control Persons Previously Infected with SARS-CoV-2 without PCS
2.2. MRI Data Acquisition
2.3. Bh-fMRI Data Processing and Analysis
2.4. Statistical Analysis
3. Results
3.1. Patients
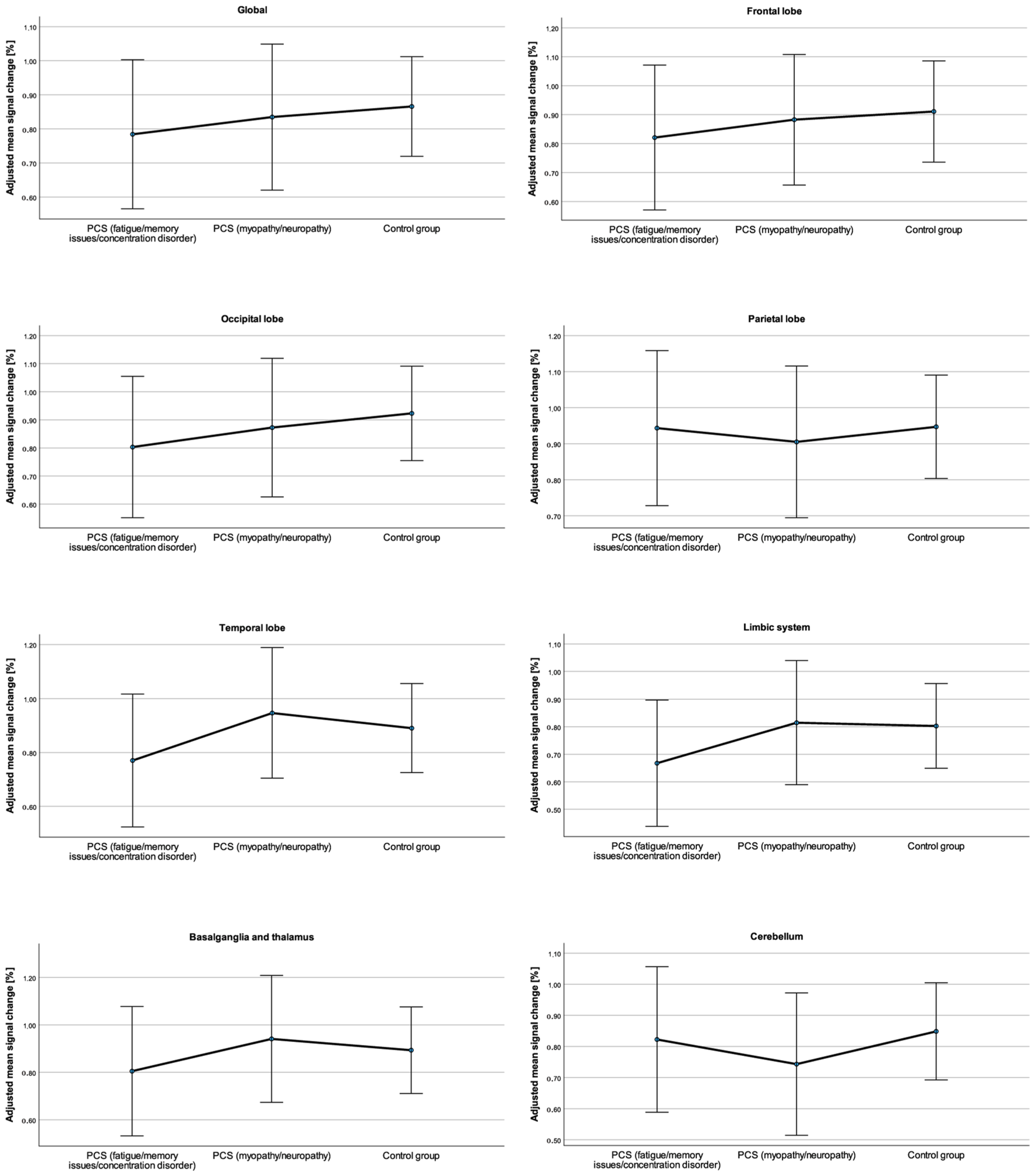
3.2. Regional Differences in the Percentage Signal Change
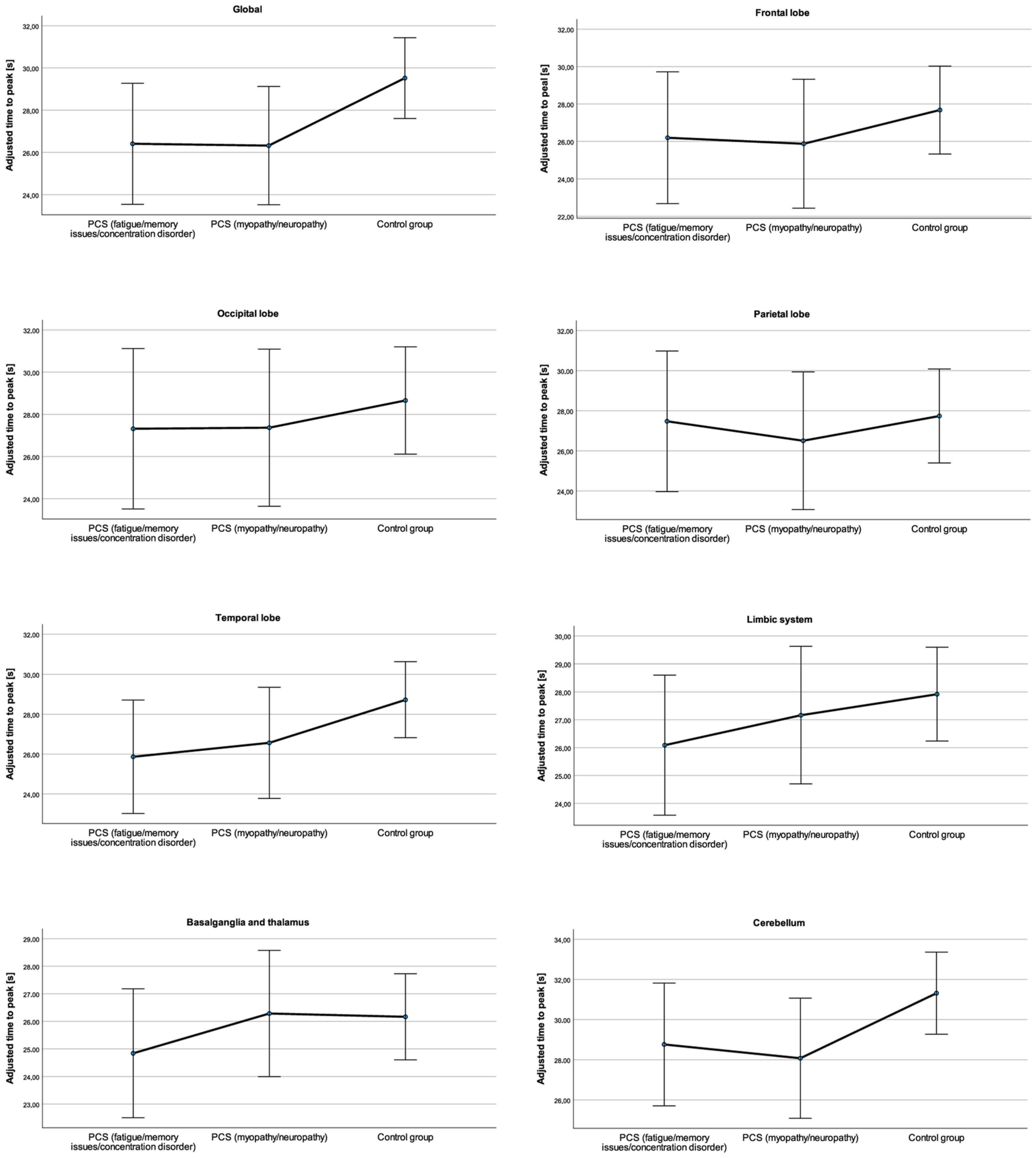
3.3. Regional Differences in the TTP
3.4. Correlation between CVR and White Matter Changes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Soriano, J.B.; Murthy, S.; Marshall, J.C.; Relan, P.; Diaz, J.V. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect. Dis. 2022, 22, e102–e107. [Google Scholar] [CrossRef] [PubMed]
- Hallek, M.; Adorjan, K.; Behrends, U.; Ertl, G.; Suttorp, N.; Lehmann, C. Post-COVID Syndrome. Dtsch. Arztebl. Int. 2023, 120, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Peter, R.S.; Nieters, A.; Krausslich, H.G.; Brockmann, S.O.; Gopel, S.; Kindle, G.; Merle, U.; Steinacker, J.M.; Rothenbacher, D.; Kern, W.V.; et al. Post-acute sequelae of COVID-19 six to 12 months after infection: Population based study. BMJ 2022, 379, e071050. [Google Scholar] [CrossRef] [PubMed]
- Premraj, L.; Kannapadi, N.V.; Briggs, J.; Seal, S.M.; Battaglini, D.; Fanning, J.; Suen, J.; Robba, C.; Fraser, J.; Cho, S.M. Mid and long-term neurological and neuropsychiatric manifestations of post-COVID-19 syndrome: A meta-analysis. J. Neurol. Sci. 2022, 434, 120162. [Google Scholar] [CrossRef]
- Wesselingh, R. Prevalence, pathogenesis and spectrum of neurological symptoms in COVID-19 and post-COVID-19 syndrome: A narrative review. Med. J. Aust. 2023, 219, 230–236. [Google Scholar] [CrossRef]
- Ghotbi, Z.; Estakhr, M.; Hosseini, M.; Shahripour, R.B. Cerebral Vasomotor Reactivity in COVID-19: A Narrative Review. Life 2023, 13, 1614. [Google Scholar] [CrossRef]
- Bauer, L.; Laksono, B.M.; de Vrij, F.M.S.; Kushner, S.A.; Harschnitz, O.; van Riel, D. The neuroinvasiveness, neurotropism, and neurovirulence of SARS-CoV-2. Trends Neurosci. 2022, 45, 358–368. [Google Scholar] [CrossRef]
- Bosselmann, C.M.; Kegele, J.; Zerweck, L.; Klose, U.; Ethofer, S.; Roder, C.; Grimm, A.M.; Hauser, T.K. Breath-Hold-Triggered BOLD fMRI in Drug-Resistant Nonlesional Focal Epilepsy—A Pilot Study. Clin. Neuroradiol. 2023, 34, 315–324. [Google Scholar] [CrossRef]
- Frontera, J.A.; Melmed, K.; Fang, T.; Granger, A.; Lin, J.; Yaghi, S.; Zhou, T.; Lewis, A.; Kurz, S.; Kahn, D.E.; et al. Toxic Metabolic Encephalopathy in Hospitalized Patients with COVID-19. Neurocrit Care 2021, 35, 693–706. [Google Scholar] [CrossRef]
- Ostergaard, L. SARS CoV-2 related microvascular damage and symptoms during and after COVID-19: Consequences of capillary transit-time changes, tissue hypoxia and inflammation. Physiol. Rep. 2021, 9, e14726. [Google Scholar] [CrossRef]
- Charfeddine, S.; Ibn Hadj Amor, H.; Jdidi, J.; Torjmen, S.; Kraiem, S.; Hammami, R.; Bahloul, A.; Kallel, N.; Moussa, N.; Touil, I.; et al. Long COVID 19 Syndrome: Is It Related to Microcirculation and Endothelial Dysfunction? Insights From TUN-EndCOV Study. Front. Cardiovasc. Med. 2021, 8, 745758. [Google Scholar] [CrossRef]
- Marcic, M.; Marcic, L.; Marcic, B.; Capkun, V.; Vukojevic, K. Cerebral Vasoreactivity Evaluated by Transcranial Color Doppler and Breath-Holding Test in Patients after SARS-CoV-2 Infection. J. Pers. Med. 2021, 11, 379. [Google Scholar] [CrossRef] [PubMed]
- Urback, A.L.; MacIntosh, B.J.; Goldstein, B.I. Cerebrovascular reactivity measured by functional magnetic resonance imaging during breath-hold challenge: A systematic review. Neurosci. Biobehav. Rev. 2017, 79, 27–47. [Google Scholar] [CrossRef] [PubMed]
- Kirschenbaum, D.; Imbach, L.L.; Rushing, E.J.; Frauenknecht, K.B.M.; Gascho, D.; Ineichen, B.V.; Keller, E.; Kohler, S.; Lichtblau, M.; Reimann, R.R.; et al. Intracerebral endotheliitis and microbleeds are neuropathological features of COVID-19. Neuropathol. Appl. Neurobiol. 2021, 47, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Callen, A.L.; Tanabe, J.; Thaker, A.A.; Pollard, R.; Sauer, B.; Jones, W.; Pattee, J.; Steach, B.; Timpone, V. Evaluation of Cerebrovascular Reactivity and Vessel-Wall Imaging in Patients With Prior COVID-19: A Prospective Case-Control MRI Study. Am. J. Roentgenol. 2022; ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Fierstra, J.; Sobczyk, O.; Battisti-Charbonney, A.; Mandell, D.M.; Poublanc, J.; Crawley, A.P.; Mikulis, D.J.; Duffin, J.; Fisher, J.A. Measuring cerebrovascular reactivity: What stimulus to use? J. Physiol. 2013, 591, 5809–5821. [Google Scholar] [CrossRef]
- Zerweck, L.; Roder, C.; Hauser, T.-K.; Thurow, J.; Mengel, A.; Tatagiba, M.; Khan, N.; Meyer, P.T.; Ernemann, U.; Klose, U. Hemodynamic evaluation of patients with Moyamoya Angiopathy: Comparison of resting-state fMRI to breath-hold fMRI and [15O]water PET. Neuroradiology 2022, 64, 553–563. [Google Scholar] [CrossRef]
- Marcic, M.; Marcic, L.; Lovric Kojundzic, S.; Marinovic Guic, M.; Marcic, B.; Caljkusic, K. Chronic Endothelial Dysfunction after COVID-19 Infection Shown by Transcranial Color-Coded Doppler: A Cross-Sectional Study. Biomedicines 2022, 10, 2550. [Google Scholar] [CrossRef]
- Brasil, S.; Taccone, F.S.; Wayhs, S.Y.; Tomazini, B.M.; Annoni, F.; Fonseca, S.; Bassi, E.; Lucena, B.; Nogueira, R.C.; De-Lima-Oliveira, M.; et al. Cerebral Hemodynamics and Intracranial Compliance Impairment in Critically Ill COVID-19 Patients: A Pilot Study. Brain Sci. 2021, 11, 874. [Google Scholar] [CrossRef]
- Abdo-Cuza, A.A.; Hall-Smith, C.; Suarez-Lopez, J.; Castellanos-Gutierrez, R.; Blanco-Gonzalez, M.A.; Machado-Martinez, R.; Pi-Avila, J.; Gomez-Peire, F.; Espinosa-Nodarse, N.; Lopez-Gonzalez, J.C. Cerebral Hemodynamic Reserve Abnormalities Detected Via Transcranial Doppler Ultrasound in Recovered COVID-19 Patients. MEDICC Rev. 2022, 24, 28–31. [Google Scholar] [CrossRef]
- Nandadeva, D.; Young, B.E.; Stephens, B.Y.; Grotle, A.K.; Skow, R.J.; Middleton, A.J.; Haseltine, F.P.; Fadel, P.J. Blunted peripheral but not cerebral vasodilator function in young otherwise healthy adults with persistent symptoms following COVID-19. Am. J. Physiol. Heart Circ. Physiol. 2021, 321, H479–H484. [Google Scholar] [CrossRef]
- Sebok, M.; van der Wouden, F.; Mader, C.; Pangalu, A.; Treyer, V.; Fisher, J.A.; Mikulis, D.J.; Hullner, M.; Regli, L.; Fierstra, J.; et al. Hemodynamic Failure Staging with Blood Oxygenation Level-Dependent Cerebrovascular Reactivity and Acetazolamide-Challenged (15O-)H2O-Positron Emission Tomography Across Individual Cerebrovascular Territories. J. Am. Heart Assoc. 2023, 12, e029491. [Google Scholar] [CrossRef]
- Zerweck, L.; Hauser, T.K.; Roder, C.; Blazhenets, G.; Khan, N.; Ernemann, U.; Meyer, P.T.; Klose, U. Evaluation of the cerebrovascular reactivity in patients with Moyamoya Angiopathy by use of breath-hold fMRI: Investigation of voxel-wise hemodynamic delay correction in comparison to [15O]water PET. Neuroradiology 2023, 65, 539–550. [Google Scholar] [CrossRef] [PubMed]
- Hauser, T.-K.; Seeger, A.; Bender, B.; Klose, U.; Thurow, J.; Ernemann, U.; Tatagiba, M.; Meyer, P.T.; Khan, N.; Roder, C. Hypercapnic BOLD MRI compared to H215O PET/CT for the hemodynamic evaluation of patients with Moyamoya Disease. NeuroImage Clin. 2019, 22, 101713. [Google Scholar] [CrossRef] [PubMed]
- Zerweck, L.; Hauser, T.-K.; Roder, C.; Klose, U. Investigation of the BOLD-Based MRI Signal Time Course During Short Breath-Hold Periods for Estimation of the Cerebrovascular Reactivity. SN Compr. Clin. Med. 2020, 2, 1551–1562. [Google Scholar] [CrossRef]
- Baum, E.; Lindner, N.; Andreas, S.; Behrends, U.; Scheibenbogen, C.; Christmann, T.; Horneber, M.; Klassen, O.; Geisler, P.; Maisel, P.; et al. Müdigkeit S3-Leitlinie AWMF-Register-Nr. 053-002 DEGAM-Leitlinie Nr. 2. Available online: https://register.awmf.org/assets/guidelines/053-002l_S3_Muedigkeit_2023-01_01.pdf (accessed on 3 May 2023).
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Smith, A. Symbol Digit Modalities Test; Western Psychological Services: Los Angeles, CA, USA, 1973. [Google Scholar]
- Rodewald, K.; Bartolovic, M.; Debelak, R.; Aschenbrenner, S.; Weisbrod, M.; Roesch-Ely, D. Eine Normierungsstudie eines modifizierten Trail Making Tests im deutschsprachigen Raum. Z. Für Neuropsychol. 2012, 23, 37–48. [Google Scholar] [CrossRef]
- Heuß, D. Diagnostik bei Polyneuropathien, S1-Leitlinie. 2019. Available online: https://www.springermedizin.de/polyneuropathie/neurologie/s1-leitlinie-diagnostik-bei-polyneuropathien/16924714 (accessed on 3 May 2023).
- Lauria, G.; Bakkers, M.; Schmitz, C.; Lombardi, R.; Penza, P.; Devigili, G.; Smith, A.G.; Hsieh, S.T.; Mellgren, S.I.; Umapathi, T.; et al. Intraepidermal nerve fiber density at the distal leg: A worldwide normative reference study. J. Peripher. Nerv. Syst. 2010, 15, 202–207. [Google Scholar] [CrossRef]
- Klehmet, J.; Beutner, S.; Hoffmann, S.; Dornauer, M.; Paul, F.; Reilmann, R.; Brandt, A.U.; Meisel, A. Quantitative grip force assessment of muscular weakness in chronic inflammatory demyelinating polyneuropathy. BMC Neurol. 2019, 19, 118. [Google Scholar] [CrossRef]
- Wei, C.; Yu, X.; Chen, Y.; Yang, T.; Li, S.; Li, J.; Chen, X. Can Patients with Asymptomatic/Mild Illness and Moderate Illness COVID-19 Have White Matter Damage? Int. J. Gen. Med. 2023, 16, 4585–4593. [Google Scholar] [CrossRef]
- Vasilev, Y.; Blokhin, I.; Khoruzhaya, A.; Kodenko, M.; Kolyshenkov, V.; Nanova, O.; Shumskaya, Y.; Omelyanskaya, O.; Vladzymyrskyy, A.; Reshetnikov, R. Routine Brain MRI Findings on the Long-Term Effects of COVID-19: A Scoping Review. Diagnostics 2023, 13, 2533. [Google Scholar] [CrossRef] [PubMed]
- Bungenberg, J.; Humkamp, K.; Hohenfeld, C.; Rust, M.I.; Ermis, U.; Dreher, M.; Hartmann, N.K.; Marx, G.; Binkofski, F.; Finke, C.; et al. Long COVID-19: Objectifying most self-reported neurological symptoms. Ann. Clin. Transl. Neurol. 2022, 9, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Zerweck, L.; Roder, C.; Blazhenets, G.; Martus, P.; Thurow, J.; Haas, P.; Estler, A.; Gohla, G.; Ruff, C.; Selo, N.; et al. MRI-Based Assessment of Risk for Stroke in Moyamoya Angiopathy (MARS-MMA): An MRI-Based Scoring System for the Severity of Moyamoya Angiopathy. Diagnostics 2024, 14, 1437. [Google Scholar] [CrossRef] [PubMed]
- Dlamini, N.; Shah-Basak, P.; Leung, J.; Kirkham, F.; Shroff, M.; Kassner, A.; Robertson, A.; Dirks, P.; Westmacott, R.; deVeber, G.; et al. Breath-Hold Blood Oxygen Level-Dependent MRI: A Tool for the Assessment of Cerebrovascular Reserve in Children with Moyamoya Disease. AJNR Am. J. Neuroradiol. 2018, 39, 1717–1723. [Google Scholar] [CrossRef] [PubMed]
- Sebok, M.; van Niftrik, C.H.B.; Winklhofer, S.; Wegener, S.; Esposito, G.; Stippich, C.; Luft, A.; Regli, L.; Fierstra, J. Mapping Cerebrovascular Reactivity Impairment in Patients With Symptomatic Unilateral Carotid Artery Disease. J. Am. Heart Assoc. 2021, 10, e020792. [Google Scholar] [CrossRef]
- Sebok, M.; van Niftrik, C.H.B.; Muscas, G.; Pangalu, A.; Seystahl, K.; Weller, M.; Regli, L.; Fierstra, J. Hypermetabolism and impaired cerebrovascular reactivity beyond the standard MRI-identified tumor border indicate diffuse glioma extended tissue infiltration. Neurooncol Adv. 2021, 3, vdab048. [Google Scholar] [CrossRef]
- Fierstra, J.; van Niftrik, C.; Warnock, G.; Wegener, S.; Piccirelli, M.; Pangalu, A.; Esposito, G.; Valavanis, A.; Buck, A.; Luft, A.; et al. Staging Hemodynamic Failure With Blood Oxygen-Level-Dependent Functional Magnetic Resonance Imaging Cerebrovascular Reactivity: A Comparison Versus Gold Standard (15O-)H2O-Positron Emission Tomography. Stroke 2018, 49, 621–629. [Google Scholar] [CrossRef]
- Sobczyk, O.; Battisti-Charbonney, A.; Poublanc, J.; Crawley, A.P.; Sam, K.; Fierstra, J.; Mandell, D.M.; Mikulis, D.J.; Duffin, J.; Fisher, J.A. Assessing cerebrovascular reactivity abnormality by comparison to a reference atlas. J. Cereb. Blood Flow. Metab. 2015, 35, 213–220. [Google Scholar] [CrossRef]
- Sobczyk, O.; Battisti-Charbonney, A.; Fierstra, J.; Mandell, D.M.; Poublanc, J.; Crawley, A.P.; Mikulis, D.J.; Duffin, J.; Fisher, J.A. A conceptual model for CO2-induced redistribution of cerebral blood flow with experimental confirmation using BOLD MRI. Neuroimage 2014, 92, 56–68. [Google Scholar] [CrossRef]
- Pennisi, M.; Lanza, G.; Falzone, L.; Fisicaro, F.; Ferri, R.; Bella, R. SARS-CoV-2 and the Nervous System: From Clinical Features to Molecular Mechanisms. Int. J. Mol. Sci. 2020, 21, 5475. [Google Scholar] [CrossRef]
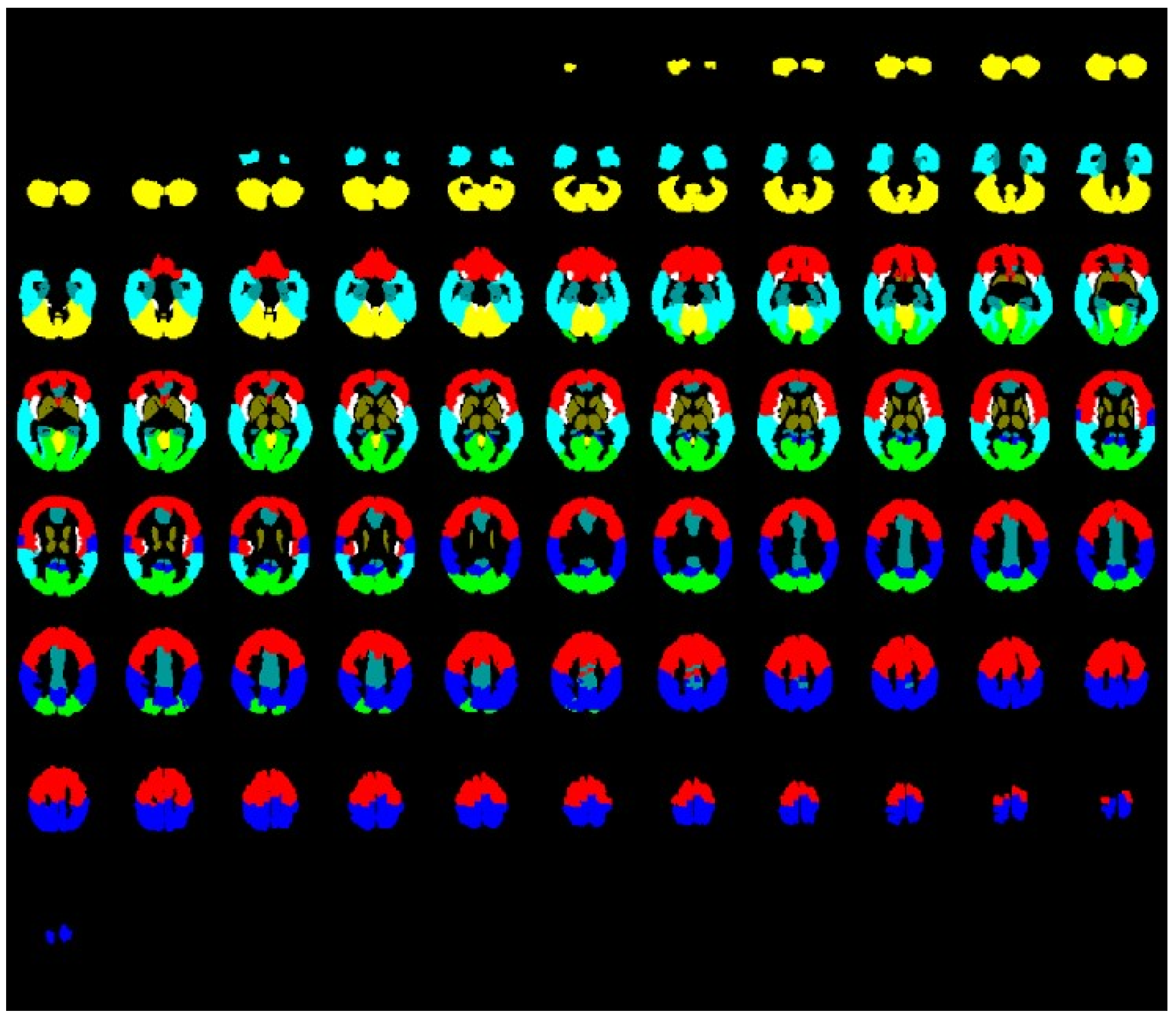
| Patients with PCSfmc 1 | Patients with PCSmn 2 | Healthy Subjects with Previous SARS-CoV-2 Infection | ||||
|---|---|---|---|---|---|---|
| Before PSM 3 | After PSM 3 | Before PSM 3 | After PSM 3 | Before PSM 3 | After PSM 3 | |
| Number of persons | 14 | 9 | 11 | 8 | 17 | 17 |
| Female/male ratio | 6.0:1 | 3.5:1 | 1.8:1 | 1.7:1 | 2.4:1 | 2.4:1 |
| Mean age [years] (SD) | 38.9 (9.9) | 38.7 (2.7) | 41.1 (7.6) | 37.8 (2.5) | 32.9 (6.3) | 33.9 (2.3) |
| Time after SARS-CoV-2 infection [months] (SD) | 22.6 (8.4) | 21.1 (2.9) | 21.4 (10.9) | 19.8 (4.0) | 19.7 (2.4) | 19.7 (2.4) |
| Fazekas score (SD) | 0.2 (0.1) | 0.2 (0.2) | 0.7 (0.2) | 0.6 (0.3) | 0.2 (0.1) | 0.2 (0.1) |
| Unadjusted | Adjusted | Unadjusted | Adjusted | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Signal Change [%] (Mean ± SD) | CI | Signal Change [%] (Mean ± SD) | CI | Time to Peak [s] (Mean ± SD) | CI | Time to Peak [s] (Mean ± SD) | CI | ||
| Global | PCSfmc 1 | 0.83 ± 0.32 | 0.63–1.03 | 0.78 ± 0.11 | 0.60–1.01 | 27.20 ± 3.19 | 25.12–29.12 | 26.41 ± 1.39 | 24.91–29.70 |
| PCSmn 2 | 0.84 ± 0.22 | 0.71–1.01 | 0.84 ± 0.10 | 0.62–1.06 | 26.10 ± 2.51 | 24.40–27.86 | 26.32 ± 1.36 | 23.10–28.70 | |
| Control group | 0.92 ± 0.25 | 0.82–1.04 | 0.87 ± 0.07 | 0.75–1.02 | 29.08 ± 3.82 | 27.41–30.97 | 29.52 ± 0.93 | 26.79–31.85 | |
| Frontal lobe | PCSfmc 1 | 0.77 ± 0.45 | 0.46–1.03 | 0.77 ± 0.13 | 0.53–0.99 | 26.97 ± 3.25 | 25.12–29.12 | 26.16 ± 1.71 | 24.46–29.61 |
| PCSmn 2 | 0.93 ± 0.17 | 0.81–1.05 | 0.91 ± 0.12 | 0.70–1.11 | 26.06 ± 2.52 | 24.40–27.86 | 25.87 ± 1.67 | 22.31–28.55 | |
| Control group | 0.97 ± 0.23 | 0.86–1.09 | 0.92 ± 0.08 | 0.77–1.14 | 27.69 ± 5.53 | 25.00–30.18 | 27.68 ± 1.17 | 23.84–31.30 | |
| Occipital lobe | PCSfmc 1 | 0.89 ± 0.38 | 0.65–1.13 | 0.80 ± 0.12 | 0.63–1.09 | 27.45 ± 3.06 | 25.58–29.70 | 27.32 ± 1.85 | 24.80–30.14 |
| PCSmn 2 | 0.87 ± 0.20 | 0.73–1.01 | 0.87 ± 0.12 | 0.63–1.07 | 27.78 ± 3.31 | 25.60–30.26 | 27.37 ± 1.81 | 22.91–30.55 | |
| Control group | 0.99 ± 0.30 | 0.86–1.14 | 0.92 ± 0.08 | 0.80–1.08 | 28.46 ± 5.75 | 25.64–30.87 | 28.66 ± 1.23 | 24.32–31.99 | |
| Parietal lobe | PCSfmc 1 | 1.01 ± 0.27 | 0.84–1.19 | 0.94 ± 0.10 | 0.82–1.21 | 27.57 ± 2.67 | 25.89–29.37 | 27.48 ± 1.70 | 25.36–30.14 |
| PCSmn 2 | 0.94 ± 0.15 | 0.83–1.04 | 0.91 ± 0.02 | 0.63–1.06 | 26.51 ± 2.44 | 24.90–28.37 | 26.50 ± 1.67 | 22.75–29.29 | |
| Control group | 1.02 ± 0.34 | 0.85–1.18 | 0.95 ± 0.07 | 0.78–1.12 | 27.71 ± 5.50 | 24.76–30.13 | 27.74± 1.14 | 22.91–31.49 | |
| Temporal lobe | PCSfmc 1 | 0.86 ± 0.36 | 0.65–1.10 | 0.77 ± 0.12 | 0.56–1.06 | 26.60 ± 3.73 | 24.63–29.36 | 25.87 ± 1.38 | 24.22–29.08 |
| PCSmn 2 | 0.94 ± 0.28 | 0.78–1.17 | 0.95 ± 0.12 | 0.71–1.24 | 26.25 ± 2.18 | 25.00–27.97 | 26.56 ± 1.36 | 24.16–28.82 | |
| Control group | 0.94 ± 0.25 | 0.82–1.06 | 0.89 ± 0.08 | 0.75–1.08 | 28.25 ± 3.67 | 26.91–30.40 | 28.72± 0.92 | 26.26–31.10 | |
| Limbic system | PCSfmc 1 | 0.73 ± 0.35 | 0.52–0.96 | 0.67 ± 0.11 | 0.46–0.92 | 27.13 ± 3.67 | 25.08–29.85 | 26.09 ± 1.22 | 24.66–29.19 |
| PCSmn 2 | 0.84 ± 0.22 | 0.72–1.03 | 0.82 ± 0.11 | 0.58–1.11 | 26.32 ± 3.52 | 24.81–28.35 | 27.16 ± 1.20 | 24.76–29.39 | |
| Control group | 0.85 ± 0.24 | 0.75–0.96 | 0.80 ± 0.07 | 0.66–0.99 | 28.16 ± 2.75 | 27.02–29.57 | 27.92± 0.82 | 26.18–29.25 | |
| Basal ganglia and thalamus | PCSfmc 1 | 0.82 ± 0.36 | 0.60–1.06 | 0.81 ± 0.13 | 0.62–1.03 | 25.40 ± 3.47 | 23.93–27.95 | 24.84 ± 1.14 | 23.76–27.04 |
| PCSmn 2 | 1.01 ± 0.34 | 0.77–1.28 | 0.94 ± 0.13 | 0.67–1.33 | 25.61 ± 2.12 | 24.24–28.35 | 26.28 ± 1.11 | 24.21–28.00 | |
| Control group | 0.93 ± 0.30 | 0.79–1.09 | 0.89 ± 0.09 | 0.75–1.08 | 26.03 ± 2.46 | 25.03–27.21 | 26.16± 0.76 | 25.03–27.13 | |
| Cerebellum | PCSfmc 1 | 0.87 ± 0.27 | 0.59–0.97 | 0.82 ± 0.11 | 0.61–1.05 | 28.93 ± 4.00 | 26.67–31.63 | 28.76 ± 1.49 | 27.08–31.64 |
| PCSmn 2 | 0.90 ± 0.26 | 0.80–1.02 | 0.74 ± 0.11 | 0.53–1.01 | 28.16 ± 3.49 | 25.72–30.51 | 28.07 ± 1.45 | 24.81–30.71 | |
| Control group | 0.85 ± 0.27 | 0.77–0.94 | 0.85 ± 0.08 | 0.72–1.02 | 30.53 ± 3.74 | 28.82–32.27 | 31.31± 0.99 | 28.99–33.34 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zerweck, L.; Klose, U.; Mengel, A.; Hoheisel, T.; Eikemeier, M.; Richter, V.; Joos, N.S.; Ernemann, U.; Bender, B.; Hauser, T.-K. Cerebrovascular Reactivity Assessed by Breath-Hold Functional MRI in Patients with Neurological Post-COVID-19 Syndrome—A Pilot Study. Neurol. Int. 2024, 16, 992-1004. https://doi.org/10.3390/neurolint16050075
Zerweck L, Klose U, Mengel A, Hoheisel T, Eikemeier M, Richter V, Joos NS, Ernemann U, Bender B, Hauser T-K. Cerebrovascular Reactivity Assessed by Breath-Hold Functional MRI in Patients with Neurological Post-COVID-19 Syndrome—A Pilot Study. Neurology International. 2024; 16(5):992-1004. https://doi.org/10.3390/neurolint16050075
Chicago/Turabian StyleZerweck, Leonie, Uwe Klose, Annerose Mengel, Tobias Hoheisel, Melinda Eikemeier, Vivien Richter, Natalie Sophie Joos, Ulrike Ernemann, Benjamin Bender, and Till-Karsten Hauser. 2024. "Cerebrovascular Reactivity Assessed by Breath-Hold Functional MRI in Patients with Neurological Post-COVID-19 Syndrome—A Pilot Study" Neurology International 16, no. 5: 992-1004. https://doi.org/10.3390/neurolint16050075
APA StyleZerweck, L., Klose, U., Mengel, A., Hoheisel, T., Eikemeier, M., Richter, V., Joos, N. S., Ernemann, U., Bender, B., & Hauser, T.-K. (2024). Cerebrovascular Reactivity Assessed by Breath-Hold Functional MRI in Patients with Neurological Post-COVID-19 Syndrome—A Pilot Study. Neurology International, 16(5), 992-1004. https://doi.org/10.3390/neurolint16050075






