The Clinical Manifestations, Risk Factors, Etiologies, and Outcomes of Adult Patients with Infectious Meningitis and Encephalitis: Single Center Experience
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Demographics and Clinical Features
3.2. Etiology
3.3. Investigations
3.4. Management and Outcomes
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Giovane, R.A.; Lavender, P.D. Central Nervous System Infections. Prim. Care Clin. Off. Pract. 2018, 45, 505–518. [Google Scholar] [CrossRef]
- Richie, M.B.; Josephson, S.A. A Practical Approach to Meningitis and Encephalitis. Semin. Neurol. 2015, 35, 611–620. [Google Scholar] [PubMed]
- Bodilsen, J.; Dalager-Pedersen, M.; Schønheyder, H.C.; Nielsen, H. Time to antibiotic therapy and outcome in bacterial meningitis: A Danish population-based cohort study. BMC Infect. Dis. 2016, 16, 392. [Google Scholar] [CrossRef] [PubMed]
- Bodilsen, J.; Storgaard, M.; Larsen, L.; Wiese, L.; Helweg-Larsen, J.; Lebech, A.-M.; Brandt, C.; Østergaard, C.; Nielsen, H. Infectious meningitis and encephalitis in adults in Denmark: A prospective nationwide observational cohort study (DASGIB). Clin. Microbiol. Infect. 2018, 24, 1102.e1–1102.e5. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health of Saudi Arabia. National Immunization Schedule. Available online: https://www.moh.gov.sa/en/HealthAwareness/EducationalContent/HealthTips/Documents/Immunization-Schedule.pdf (accessed on 18 August 2024).
- Ministry of Health of Saudi Arabia. Immunization (Vaccines). Available online: https://www.moh.gov.sa/en/HealthAwareness/EducationalContent/vaccination/Pages/003.aspx (accessed on 18 August 2024).
- Ben Abid, F.; Abukhattab, M.; Ghazouani, H.; Khalil, O.; Gohar, A.; Al Soub, H.; Al Maslamani, M.; Al Khal, A.; Al Masalamani, E.; Al Dhahry, S.; et al. Epidemiology and clinical outcomes of viral central nervous system infections. Int. J. Infect. Dis. 2018, 73, 85–90. [Google Scholar] [CrossRef]
- Aldriweesh, M.A.; Shafaay, E.A.; Alwatban, S.M.; Alkethami, O.M.; Aljuraisi, F.N.; Bosaeed, M.; Alharbi, N.K. Viruses Causing Aseptic Meningitis: A Tertiary Medical Center Experience with a Multiplex PCR Assay. Front. Neurol. 2020, 11, 602267. [Google Scholar] [CrossRef]
- Alhazmi, A.H.; Alameer, K.M.; Abuageelah, B.M.; Gharawi, A.Y.; Hakami, E.F.; Zogel, T.A.; Almalki, A.J.; Magrashi, E.G.; Alharbi, W.A.; Manni, R.M.; et al. Epidemiology and antimicrobial resistance patterns of bacterial meningitis among hospitalized patients at a tertiary care hospital in Saudi Arabia: A six-year retrospective study. Eur. J. Clin. Microbiol. Infect. Dis. 2024. Epub ahead of print. [Google Scholar] [CrossRef]
- The Collaborative Working Group of the Pediatric Subgroup of the China Society of Infectious Diseases; Peng, X.; Zhu, Q.; Liu, J.; Zeng, M.; Qiu, Y.; Zhu, C.; Cheng, Y.; Zhou, Y.; Xu, Y.; et al. Prevalence and antimicrobial resistance patterns of bacteria isolated from cerebrospinal fluid among children with bacterial meningitis in China from 2016 to 2018: A multicenter retrospective study. Antimicrob Resist. Infect Control. 2021, 10, 24. [Google Scholar] [CrossRef]
- Runde, T.J.; Anjum, F.; Hafner, J.W. Bacterial Meningitis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK470351/ (accessed on 31 May 2024).
- Ungureanu, A.; van der Meer, J.; Bicvic, A.; Abbuehl, L.; Chiffi, G.; Jaques, L.; Suter-Riniker, F.; Leib, S.L.; Bassetti, C.L.A.; Dietmann, A. Meningitis, meningoencephalitis and encephalitis in Bern: An observational study of 258 patients. BMC Neurol. 2021, 21, 474. [Google Scholar] [CrossRef]
- Mailles, A.; Stahl, J.-P. Steering Committee and Investigators Group. Infectious encephalitis in france in 2007: A national prospective study. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2009, 49, 1838–1847. [Google Scholar] [CrossRef]
- Granerod, J.; Ambrose, H.E.; Davies, N.W.; Clewley, J.P.; Walsh, A.L.; Morgan, D.; Cunningham, R.; Zuckerman, M.; Mutton, K.J.; Solomon, T.; et al. Causes of encephalitis and differences in their clinical presentations in England: A multicentre, population-based prospective study. Lancet Infect. Dis. 2010, 10, 835–844. [Google Scholar] [CrossRef] [PubMed]
- Carter, E.; McGill, F. The management of acute meningitis: An update. Clin. Med. Lond. Engl. 2022, 22, 396–400. [Google Scholar] [CrossRef] [PubMed]
- Rogers, T.; Sok, K.; Erickson, T.; Aguilera, E.; Wootton, S.H.; O Murray, K.; Hasbun, R. Impact of Antibiotic Therapy in the Microbiological Yield of Healthcare-Associated Ventriculitis and Meningitis. Open Forum Infect. Dis. 2019, 6, ofz050. [Google Scholar] [CrossRef] [PubMed]
- McGill, F.; Griffiths, M.J.; Bonnett, L.J.; Geretti, A.M.; Michael, B.D.; Beeching, N.J.; McKee, D.; Scarlett, P.; Hart, I.J.; Mutton, K.J.; et al. Incidence, aetiology, and sequelae of viral meningitis in UK adults: A multicentre prospective observational cohort study. Lancet Infect. Dis. 2018, 18, 992–1003. [Google Scholar] [CrossRef]
- Shukla, B.; Aguilera, E.A.; Salazar, L.; Wootton, S.H.; Kaewpoowat, Q.; Hasbun, R. Aseptic meningitis in adults and children: Diagnostic and management challenges. J. Clin. Virol. Off. Publ. Pan Am. Soc. Clin. Virol. 2017, 94, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.R.; Sample, H.A.; Zorn, K.C.; Arevalo, S.; Yu, G.; Neuhaus, J.; Federman, S.; Stryke, D.; Briggs, B.; Langelier, C.; et al. Clinical Metagenomic Sequencing for Diagnosis of Meningitis and Encephalitis. N. Engl. J. Med. 2019, 380, 2327–2340. [Google Scholar] [CrossRef]
- Zhang, H.-C.; Ai, J.-W.; Cui, P.; Zhu, Y.-M.; Hong-Long, W.; Li, Y.-J.; Zhang, W.-H. Incremental value of metagenomic next generation sequencing for the diagnosis of suspected focal infection in adults. J. Infect. 2019, 79, 419–425. [Google Scholar] [CrossRef]
- Liang, M.; Fan, Y.; Zhang, D.; Yang, L.; Wang, X.; Wang, S.; Xu, J.; Zhang, J. Metagenomic next-generation sequencing for accurate diagnosis and management of lower respiratory tract infections. Int. J. Infect. Dis. 2022, 122, 921–929. [Google Scholar] [CrossRef]
- van de Beek, D.; de Gans, J.; Spanjaard, L.; Weisfelt, M.; Reitsma, J.B.; Vermeulen, M. Clinical features and prognostic factors in adults with bacterial meningitis. N. Engl. J. Med. 2004, 351, 1849–1859. [Google Scholar] [CrossRef]
- Wood, G.K.; Babar, R.; Ellul, M.A.; Thomas, R.H.; Tooren, H.V.D.; Easton, A.; Tharmaratnam, K.; Burnside, G.; Alam, A.M.; Castell, H.; et al. Acute seizure risk in patients with encephalitis: Development and validation of clinical prediction models from two independent prospective multicentre cohorts. BMJ Neurol. Open 2022, 4, e000323. [Google Scholar] [CrossRef]
- Akaishi, T.; Tarasawa, K.; Fushimi, K.; Yaegashi, N.; Aoki, M.; Fujimori, K. Demographic profiles and risk factors for mortality in acute meningitis: A nationwide population-based observational study. Acute Med. Surg. 2024, 11, e920. [Google Scholar] [CrossRef]
- Mailles, A.; Argemi, X.; Biron, C.; Fillatre, P.; De Broucker, T.; Buzelé, R.; Gagneux-Brunon, A.; Gueit, I.; Henry, C.; Patrat-Delon, S.; et al. Changing profile of encephalitis: Results of a 4-year study in France. Infect. Dis. Now. 2022, 52, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ai, J.; Xie, Z.; Liu, G.; Chen, Z.; Yang, Y.; Li, Y.; Chen, J.; Zheng, G.; Shen, K. Etiology and prognosis of acute viral encephalitis and meningitis in Chinese children: A multicentre prospective study. BMC Infect. Dis. 2017, 17, 494. [Google Scholar] [CrossRef] [PubMed]
- Jiménez Caballero, P.E.; Muñoz Escudero, F.; Murcia Carretero, S.; Pérezb, A.V. Análisis descriptivo de las meningitis víricas en nuestro hospital. Características diferenciales entre niños y adultos. Neurología 2011, 26, 468–473. [Google Scholar] [CrossRef]
- Tan, L.V.; Thai, L.H.; Phu, N.H.; Nghia, H.D.T.; Van Chuong, L.; Sinh, D.X.; Phong, N.D.; Mai, N.T.H.; Man, D.N.H.; Hien, V.M. Viral Aetiology of Central Nervous System Infections in Adults Admitted to a Tertiary Referral Hospital in Southern Vietnam over 12 Years. PLoS Negl. Trop. Dis. 2014, 8, e3127. [Google Scholar] [CrossRef] [PubMed]
- Jarrin, I.; Sellier, P.; Lopes, A.; Morgand, M.; Makovec, T.; Delcey, V.; Champion, K.; Simoneau, G.; Green, A.; Mouly, S.; et al. Etiologies and Management of Aseptic Meningitis in Patients Admitted to an Internal Medicine Department. Medicine 2016, 95, e2372. [Google Scholar] [CrossRef]
- The General Authority for Statistics (GASTAT) Is the Official Source of Statistical Data in the Kingdom of Saudi Arabia. Available online: https://portal.saudicensus.sa/portal/public/1/15/101461?type=TABLE (accessed on 18 August 2024).
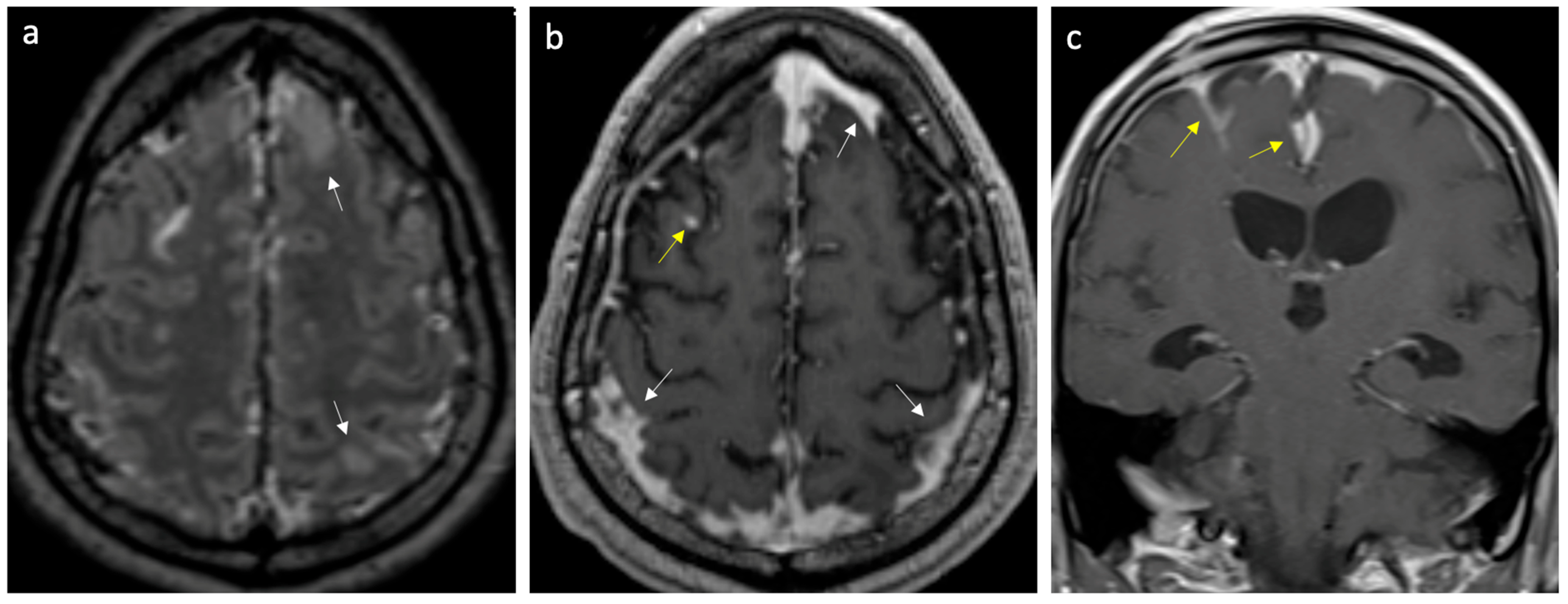
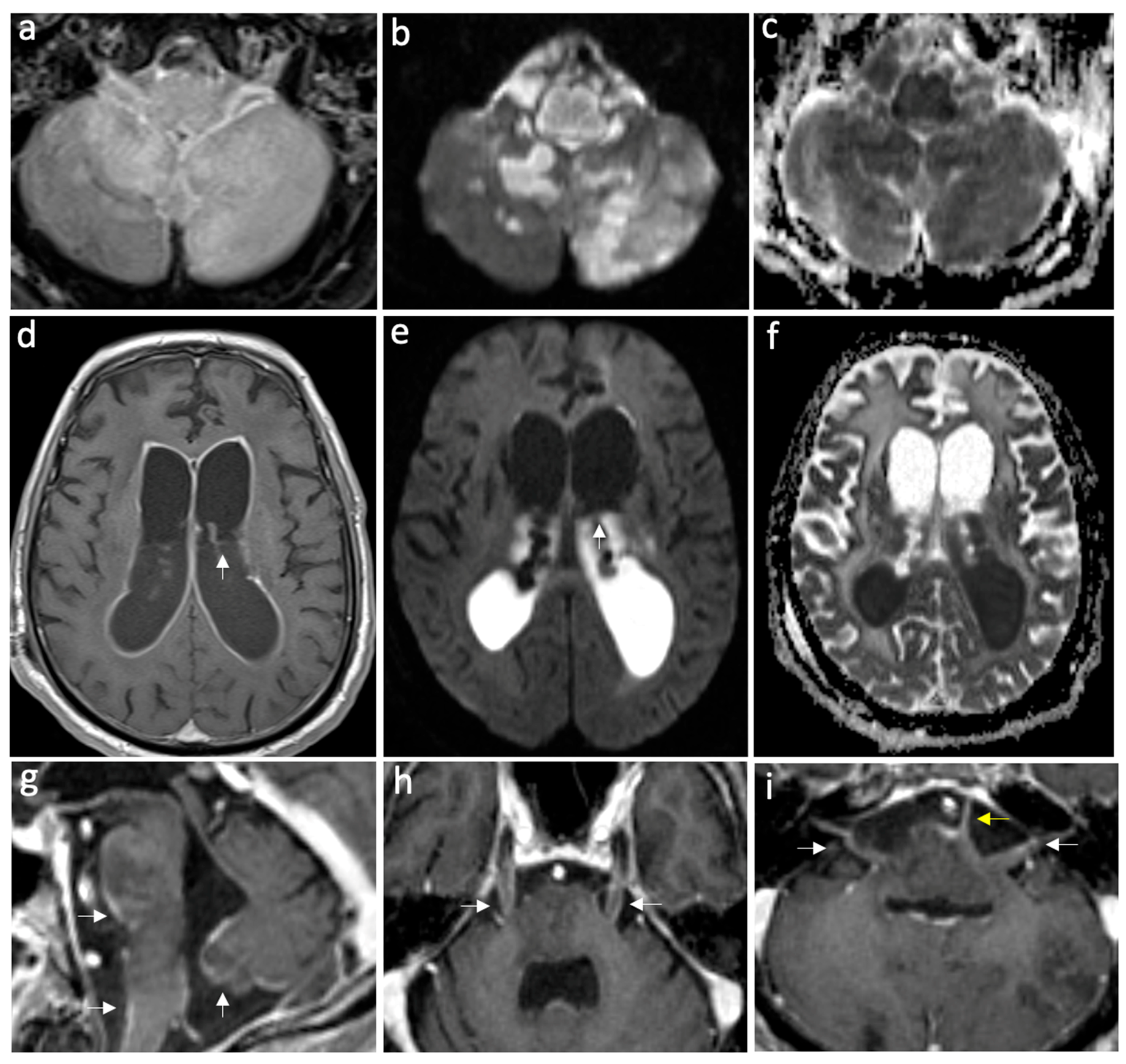
| Variables | Encephalitis n (%) n = 14 | Meningitis n (%) n = 36 |
|---|---|---|
| Male gender | 11 (78.6%) | 23 (63.9%) |
| Median age (IQR) | 59 (33.3) | 47 (33) |
| Comorbidities and risk factors | ||
| Diabetes mellitus | 5 (35.7%) | 14 (38.9%) |
| Use of Immunosuppressive drugs | 2 (14.3%) | 6 (16.7%) |
| Autoimmune disease | 2 (14.3%) | 2 (5.6%) |
| Recent neurosurgery | 0 | 7 (19.4%) |
| Ventriculoperitoneal shunt | 0 | 4 (11.1%) |
| Human immunodeficiency virus | 0 | 1 (2.8%) |
| Signs and symptoms | ||
| Seizure | 13 (92.9%) | 8 (22.2%) |
| Altered mental status | 12 (85.7%) | 25 (69.4%) |
| Fever | 6 (42.9%) | 21 (58.8%) |
| Headache | 3 (21.4%) | 23 (63.9%) |
| Nausea/Vomiting | 3 (21.4%) | 19 (52.8%) |
| Photophobia | 0 | 15 (41.7%) |
| Neck stiffness | 0 | 9 (25%) |
| Outcomes | ||
| Median Length of Stay (days) | 23.5 (17) | 14 (15) |
| Intensive care unit (ICU) admissions | 10 (71.4%) | 9 (25%) |
| In-hospital mortality | 5 (35.7%) | 2 (5.6%) |
| Variables | Encephalitis n (%) n = 14 | Meningitis n (%) n = 36 |
|---|---|---|
| Herpes simplex virus 1 | 5 (35.7%) | 0 |
| Varicella zoster virus | 2 (14.3%) | 1 (2.7%) |
| Neisseria meningitidis | 0 | 2 (5.6%) |
| Klebsiella pneumoniae | 0 | 2 (5.6%) |
| Acinetobacter baumannii | 0 | 1 (2.8%) |
| Bacillus megaterium | 0 | 1 (2.8%) |
| Brucella spp. | 0 | 1 (2.8%) |
| Enterovirus | 0 | 1 (2.8%) |
| Mycobacterium tuberculosis | 0 | 1 (2.8%) |
| Salmonella enterica | 1 (2.8%) | 1 (2.8%) |
| Streptococcus gordonii | 0 | 1 (2.8%) |
| Streptococcus intermedius | 0 | 1 (2.8%) |
| Streptococcus pneumoniae | 0 | 1 (2.8%) |
| Unknown | 6 (42.9%) | 22 (61.1%) |
| Variables | Encephalitis n (%) n = 14 | Meningitis n (%) n = 36 |
|---|---|---|
| Median CSF WBC * (IQR) | 14 (100) | 235 (449) |
| Median CSF RBC ** (IQR) | 4 (10) | 20 (124.5) |
| Median CSF glucose *** (IQR) | 4.95 (2.5) | 3.5 (2.1) |
| Median CSF protein **** (IQR) | 0.66 (0.4) | 1.13 (1.5) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makkawi, S.; Alqurashi, S.; Hubayni, W.; Almahdawi, S.; Bahkali, S.; Alharbi, A.; Khojah, O.; Halawani, A.; Malli, I. The Clinical Manifestations, Risk Factors, Etiologies, and Outcomes of Adult Patients with Infectious Meningitis and Encephalitis: Single Center Experience. Neurol. Int. 2024, 16, 966-975. https://doi.org/10.3390/neurolint16050073
Makkawi S, Alqurashi S, Hubayni W, Almahdawi S, Bahkali S, Alharbi A, Khojah O, Halawani A, Malli I. The Clinical Manifestations, Risk Factors, Etiologies, and Outcomes of Adult Patients with Infectious Meningitis and Encephalitis: Single Center Experience. Neurology International. 2024; 16(5):966-975. https://doi.org/10.3390/neurolint16050073
Chicago/Turabian StyleMakkawi, Seraj, Shatha Alqurashi, Wejdan Hubayni, Saleha Almahdawi, Sadeem Bahkali, Abeer Alharbi, Osama Khojah, Aisha Halawani, and Israa Malli. 2024. "The Clinical Manifestations, Risk Factors, Etiologies, and Outcomes of Adult Patients with Infectious Meningitis and Encephalitis: Single Center Experience" Neurology International 16, no. 5: 966-975. https://doi.org/10.3390/neurolint16050073
APA StyleMakkawi, S., Alqurashi, S., Hubayni, W., Almahdawi, S., Bahkali, S., Alharbi, A., Khojah, O., Halawani, A., & Malli, I. (2024). The Clinical Manifestations, Risk Factors, Etiologies, and Outcomes of Adult Patients with Infectious Meningitis and Encephalitis: Single Center Experience. Neurology International, 16(5), 966-975. https://doi.org/10.3390/neurolint16050073







