A Comprehensive Review on the Role of Resting-State Functional Magnetic Resonance Imaging in Predicting Post-Stroke Motor and Sensory Outcomes
Abstract
1. Introduction
2. State-of-the-Art of Current Literature on rs-fMRI in Motor and Sensory Outcomes after Stroke
2.1. Prediction of Motor Outcomes Using rs-fMRI
2.1.1. Changes in FC Based on Traditional and Relatively New rs-fMRI Indices
2.1.2. Changes in FC and Their Association with the Application of Specific Rehabilitation Methods
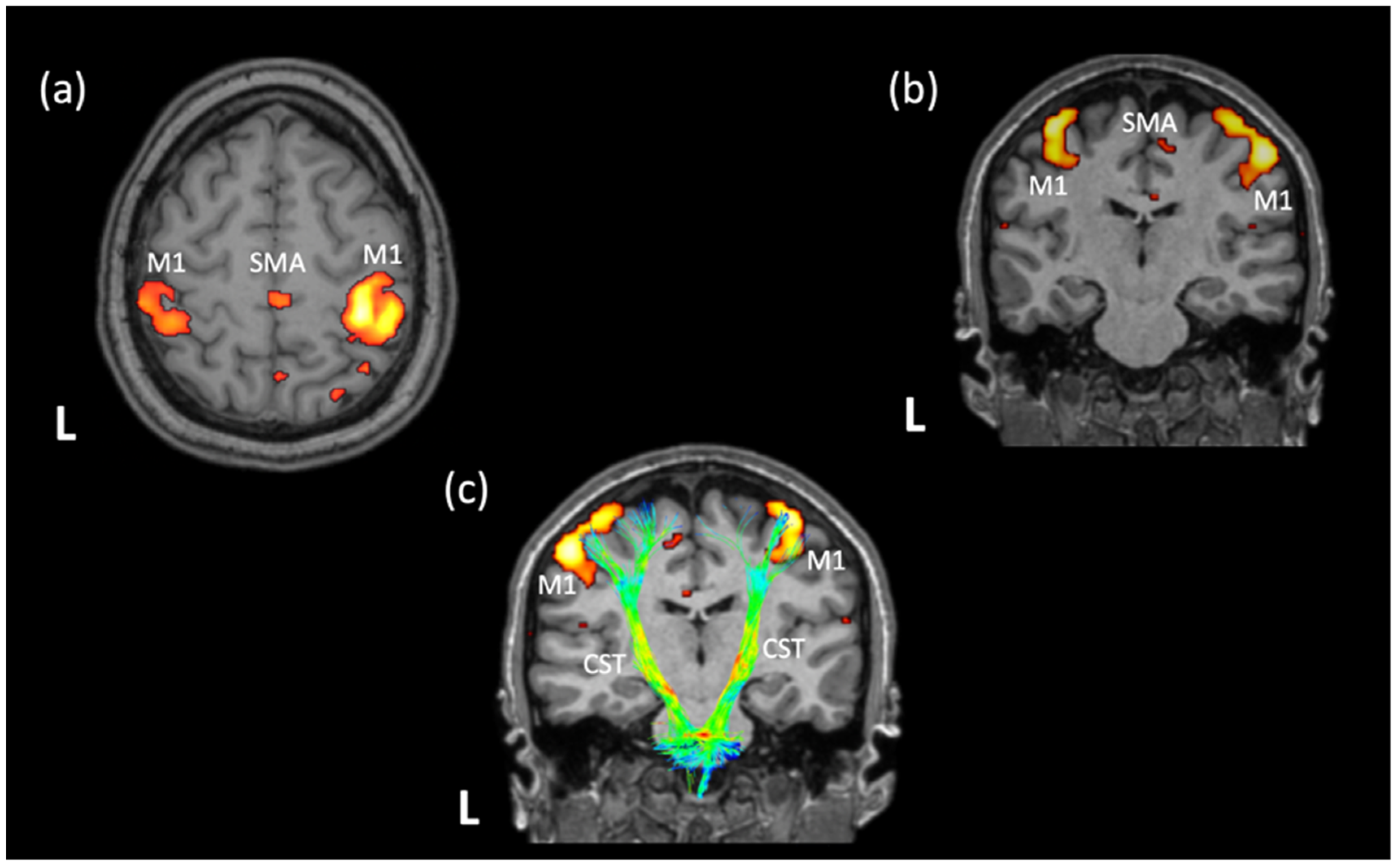
2.1.3. Changes in FC and Structural Connectivity
2.2. Prediction of Sensory Outcome Using rs-fMRI
3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sachdev, P.S.; Brodaty, H.; Valenzuela, M.J.; Lorentz, L.; Looi JC, L.; Wen, W.; Zagami, A.S. The neuropsychological profile of vascular cognitive impairment in stroke and TIA patients. Neurology 2004, 62, 912–919. [Google Scholar] [CrossRef] [PubMed]
- Loubinoux, I.; Brihmat, N.; Castel-Lacanal, E.; Marque, P. Cerebral imaging of post-stroke plasticity and tissue repair. Rev. Neurol. 2017, 173, 577–583. [Google Scholar] [CrossRef]
- Hylin, M.J.; Kerr, A.L.; Holden, R. Understanding the Mechanisms of Recovery and/or Compensation following Injury. Neural Plast. 2017, 2017, 7125057. [Google Scholar] [CrossRef]
- Hommel, M.; Detante, O.; Favre, I.; Touze, E.; Jaillard, A. How to Measure Recovery? Revisiting Concepts and Methods for Stroke Studies. Transl. Stroke Res. 2016, 7, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Sarraj, A.; Grotta, J.C. Stroke: New horizons in treatment. Lancet Neurol. 2014, 13, 2–3. [Google Scholar] [CrossRef]
- Chen, J.E.; Glover, G.H. Functional Magnetic Resonance Imaging Methods. Neuropsychol. Rev. 2015, 25, 289–313. [Google Scholar] [CrossRef] [PubMed]
- Friston, K.J.; Frith, C.D.; Liddle, P.F.; Frackowiak, R.S. Functional Connectivity: The Principal-Component Analysis of Large (PET) Data Sets. J. Cereb. Blood Flow. Metab. 1993, 13, 5–14. [Google Scholar] [CrossRef]
- Papanicolaou, A.C. The Oxford Handbook of Functional Brain Imaging in Neuropsychology and Cognitive Neurosciences; Oxford University Press: New York, NY, USA, 2017. [Google Scholar]
- Stamatakis, E.A.; Orfanidou, E.; Papanicolaou, A.C. Functional Magnetic Resonance Imaging. In The Oxford Handbook of Functional Brain Imaging in Neuropsychology and Cognitive Neurosciences; Oxford University Press: New York, NY, USA, 2017; pp. 43–60. [Google Scholar]
- Ovadia-Caro, S.; Margulies, D.S.; Villringer, A. The Value of Resting-State Functional Magnetic Resonance Imaging in Stroke. Stroke 2014, 45, 2818–2824. [Google Scholar] [CrossRef]
- Yin, D.; Song, F.; Xu, D.; Sun, L.; Men, W.; Zang, L.; Yan, X.; Fan, M. Altered topological properties of the cortical motor-related network in patients with subcortical stroke revealed by graph theoretical analysis. Hum. Brain Mapp. 2013, 35, 3343–3359. [Google Scholar] [CrossRef]
- Li, S.; Zhou, M.; Yu, B.; Ma, Z.; Chen, S.; Gong, Q.; He, L.; Huang, X.; Lui, S.; Wang, X.; et al. Altered default mode and affective network connectivity in stroke patients with and without dysphagia. J. Rehabil. Med. 2014, 46, 126–131. [Google Scholar] [CrossRef]
- Lam, T.K.; Binns, M.A.; Honjo, K.; Dawson, D.R.; Ross, B.; Stuss, D.T.; Black, S.E.; Chen, J.J.; Fujioka, T.; Chen, J.L. Variability in stroke motor outcome is explained by structural and functional integrity of the motor system. Sci. Rep. 2018, 8, 9480. [Google Scholar] [CrossRef]
- Chi, N.-F.; Ku, H.-L.; Chen, D.Y.-T.; Chen, D.Y.T.; Tseng, Y.C.; Chen, C.J.; Lin, Y.C.; Hsieh, Y.C.; Chan, L.; Chiou, H.Y.; et al. Cerebral Motor Functional Connectivity at the Acute Stage: An Outcome Predictor of Ischemic Stroke. Sci. Rep. 2018, 8, 16803. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Zhao, Z.; Yin, D.; Fan, M.; Small, M.; Liu, Z.; Hilgetag, C.C.; Kurths, J. Brain anomaly networks uncover heterogeneous functional reorganization patterns after stroke. NeuroImage Clin. 2018, 20, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Hong, W.; Lin, Q.; Cui, Z.; Liu, F.; Xu, R.; Tang, C. Diverse functional connectivity patterns of resting-state brain networks associated with good and poor hand outcomes following stroke. Neuroimage Clin. 2019, 24, 102065. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Dang, C.; Peng, K.; Xie, C.; Chen, H.; Xing, S.; Chen, X.; Zeng, J. Increased spontaneous neuronal activity in structurally damaged cortex is correlated with early motor recovery in patients with subcortical infarction. Eur. J. Neurol. 2015, 22, 1540–1547. [Google Scholar] [CrossRef]
- Zhao, Z.; Tang, C.; Yin, D.; Wu, J.; Gong, J.; Sun, L.; Jia, J.; Xu, D.; Fan, M. Frequency-specific alterations of regional homogeneity in subcortical stroke patients with different outcomes in hand function. Hum. Brain Mapp. 2018, 39, 4373–4384. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Hu, R.; Luo, X.; Feng, B.; Long, W.; Song, R. Reduced Complexity in Stroke with Motor Deficits: A Resting-State fMRI Study. Neuroscience 2020, 434, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Bonkhoff, A.K.; Espinoza, F.A.; Gazula, H.; Vergara, V.M.; Hensel, L.; Michely, J.; Paul, T.; Rehme, A.K.; Volz, L.J.; Fink, G.R.; et al. Acute ischaemic stroke alters the brain’s preference for distinct dynamic connectivity states. Brain 2020, 143, 1525–1540. [Google Scholar] [CrossRef]
- Bonkhoff, A.K.; Schirmer, M.D.; Bretzner, M.; Etherton, M.; Donahue, K.; Tuozzo, C.; Nardin, M.; Giese, A.K.; Wu, O.; Calhoun, V.D.; et al. Abnormal dynamic functional connectivity is linked to recovery after acute ischemic stroke. Hum. Brain Mapp. 2021, 42, 2278–2291. [Google Scholar] [CrossRef]
- Fan, Y.; Wu, C.; Liu, H.; Lin, K.C.; Wai, Y.Y.; Chen, Y.I. Neuroplastic changes in resting-state functional connectivity after stroke rehabilitation. Front. Hum. Neurosci. 2015, 9, 546. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, H.; Wang, L.; Yang, J.; Yan, R.; Zhang, J.; Sang, L.; Li, P.; Wang, J.; Qiu, M. Relationship between functional connectivity and motor function assessment in stroke patients with hemiplegia: A resting-state functional MRI study. Neuroradiology 2016, 58, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, X.-W.; Zuo, Z.-T.; Lu, J.; Meng, C.L.; Fang, H.Y.; Xue, R.; Fan, Y.; Guan, Y.Z.; Zhang, W.H. Cerebral Functional Reorganization in Ischemic Stroke after Repetitive Transcranial Magnetic Stimulation: An fMRI Study. CNS Neurosci. Ther. 2016, 22, 952–960. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, S.; Dricot, L.; Laloux, P.; Desfontaines, P.; Evrard, F.; Peeters, A.; Jamart, J.; Vendermeeren, Y. Increased functional connectivity one week after motor learning and tDCS in stroke patients. Neuroscience 2017, 340, 424–435. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Liao, C.; Huang, W.; Wu, P. Longitudinal Brain Functional Connectivity Changes of the Cortical Motor-Related Network in Subcortical Stroke Patients with Acupuncture Treatment. Neural Plast. 2017, 2017, 5816263. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Jin, Y.; Bai, X.; Jiang, B.; He, L.; McClure, M.A.; Mu, Q. Distinction of High- and Low-Frequency Repetitive Transcranial Magnetic Stimulation on the Functional Reorganization of the Motor Network in Stroke Patients. Neural Plast. 2021, 2021, 8873221. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, F.; Lin, S.; Yu, L.; Lin, R. Effects of Virtual Reality Rehabilitation Training on Cognitive Function and Activities of Daily Living of Patients with Poststroke Cognitive Impairment: A Systematic Review and Meta-Analysis. Arch. Phys. Med. Rehabil. 2022, 103, 1422–1435. [Google Scholar] [CrossRef]
- Chen, J.L.; Schlaug, G. Resting state interhemispheric motor connectivity and white matter integrity correlate with motor impairment in chronic stroke. Front. Neurol. 2013, 4, 178. [Google Scholar] [CrossRef]
- Kalinosky, B.T.; Berrios Barillas, R.; Schmit, B.D. Structurofunctional resting-state networks correlate with motor function in chronic stroke. Neuroimage Clin. 2017, 16, 610–623. [Google Scholar] [CrossRef]
- Lin, L.Y.; Ramsey, L.; Metcalf, N.V.; Rengachary, J.; Shulman, G.L.; Shimony, J.S.; Corbetta, M. Stronger prediction of motor recovery and outcome post-stroke by cortico-spinal tract integrity than functional connectivity. PLoS ONE 2018, 13, e0202504. [Google Scholar] [CrossRef]
- Lee, J.H.; Kyeong, S.; Kang, H.; Kim, D.H. Structural and functional connectivity correlates with motor impairment in chronic supratentorial stroke: A multimodal magnetic resonance imaging study. NeuroReport 2019, 30, 526–531. [Google Scholar] [CrossRef]
- Lu, Q.; Huang, G.; Chen, L.; Li, W.; Liang, Z. Structural and functional reorganization following unilateral internal capsule infarction contribute to neurological function recovery. Neuroradiology 2019, 61, 1181–1190. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Huang, G.; Quan, X.; Qin, Q.; Li, H.; Xu, C.; Liang, Z. Dynamic Structural and Functional Reorganizations Following Motor Stroke. Med. Sci. Monit. 2021, 27, e929092. [Google Scholar] [CrossRef] [PubMed]
- Bannister, L.C.; Crewther, S.G.; Gavrilescu, M.; Carey, L.M. Improvement in Touch Sensation after Stroke is Associated with Resting Functional Connectivity Changes. Front. Neurol. 2015, 6, 165. [Google Scholar] [CrossRef] [PubMed]
- Goodin, P.; Lamp, G.; Vidyasagar, R.; McArdle, D.; Seitz, R.J.; Carey, L.M. Altered functional connectivity differs in stroke survivors with impaired touch sensation following left and right hemisphere lesions. NeuroImage Clin. 2018, 18, 342–355. [Google Scholar] [CrossRef] [PubMed]
- Fox, M.D.; Raichle, M.E. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat. Rev. Neurosci. 2007, 8, 700–711. [Google Scholar] [CrossRef] [PubMed]
- Fox, M.D.; Snyder, A.Z.; Vincent, J.L.; Raichle, M.E. Intrinsic fluctuations within cortical systems account for intertrial variability in human behavior. Neuron 2007, 56, 171–184. [Google Scholar] [CrossRef] [PubMed]
- Fox, M.D.; Snyder, A.Z.; Zacks, J.M.; Raichle, M.E. Coherent spontaneous activity accounts for trial-to-trial variability in human evoked brain responses. Nat. Neurosci. 2006, 9, 23–25. [Google Scholar] [CrossRef] [PubMed]
- Park, K.Y.; Lee, J.J.; Dierker, D.; Marple, L.M.; Hacker, C.D.; Roland, J.L.; Marcus, D.S.; Milchenko, M.; Miller-Thomas, M.M.; Benzinger, T.L.; et al. Mapping language function with task-based vs. resting-state functional MRI. PLoS ONE 2020, 15, e0236423. [Google Scholar] [CrossRef]
- Lemée, J.-M.; Berro, D.H.; Bernard, F.; Chinier, E.; Leiber, L.M.; Menei, P.; Minassian, A.T. Resting-state functional magnetic resonance imaging versus task-based activity for language mapping and correlation with perioperative cortical mapping. Brain Behav. 2019, 9, e01362. [Google Scholar] [CrossRef]
- Christidi, F.; Tsiptsios, D.; Sousanidou, A.; Karamanidis, S.; Kitmeridou, S.; Karatzetzou, S.; Aitsidou, S.; Tsamakis, K.; Psatha, E.A.; Karavasilis, E.; et al. The Clinical Utility of Leukoaraiosis as a Prognostic Indicator in Ischemic Stroke Patients. Neurol. Int. 2022, 14, 76. [Google Scholar] [CrossRef]
- Gkantzios, A.; Karapepera, V.; Tsiptsios, D.; Liaptsi, E.; Christidi, F.; Gkartzonika, E.; Karatzetzou, S.; Kokkotis, C.; Kyrtsopoulos, M.; Tsiakiri, A.; et al. Investigating the Predictive Value of Thyroid Hormone Levels for Stroke Prognosis. Neurol. Int. 2023, 15, 926–953. [Google Scholar] [CrossRef] [PubMed]
- Karatzetzou, S.; Tsiptsios, D.; Sousanidou, A.; Fotiadou, S.; Christidi, F.; Kokkotis, C.; Gkantzios, A.; Stefas, E.; Vlotinou, P.; Kaltsatou, A.; et al. Elucidating the Role of Baseline Leukoaraiosis on Forecasting Clinical Outcome of Acute Ischemic Stroke Patients Undergoing Reperfusion Therapy. Neurol. Int. 2022, 14, 923–942. [Google Scholar] [CrossRef] [PubMed]
- Karatzetzou, S.; Tsiptsios, D.; Sousanidou, A.; Fotiadou, S.; Christidi, F.; Kokkotis, C.; Gkantzios, A.; Stefas, E.; Sousanidou, A.; Kaltsatou, A.; et al. Copeptin Implementation on Stroke Prognosis. Neurol. Int. 2023, 15, 83–99. [Google Scholar] [CrossRef]
- Liaptsi, E.; Merkouris, E.; Polatidou, E.; Tsiptsios, D.; Gkantzios, A.; Kokkotis, C.; Petridis, F.; Christidi, F.; Karatzetzou, S.; Karaoglanis, C.; et al. Targeting Neutrophil Extracellular Traps for Stroke Prognosis: A Promising Path. Neurol. Int. 2023, 15, 1212–1226. [Google Scholar] [CrossRef] [PubMed]
- Orgianelis, I.; Merkouris, E.; Kitmeridou, S.; Tsiptsio, D.; Karatzetzou, S.; Sousanidou, A.; Gkantzios, A.; Christidi, F.; Polatidou, E.; Beliani, A.; et al. Exploring the Utility of Autonomic Nervous System Evaluation for Stroke Prognosis. Neurol. Int. 2023, 15, 661–696. [Google Scholar] [CrossRef]
- Tziaka, E.; Christidi, F.; Tsiptsios, D.; Sousanidou, A.; Karatzetzou, S.; Tsiakiri, A.; Doskas, T.K.; Tsamakis, K.; Retzepis, N.; Konstantinidis, C.; et al. Leukoaraiosis as a Predictor of Depression and Cognitive Impairment among Stroke Survivors: A Systematic Review. Neurol. Int. 2023, 15, 238–272. [Google Scholar] [CrossRef] [PubMed]
- Khatri, P.; Conaway, M.R.; Johnston, K.C. Ninety-day outcome rates of a prospective cohort of consecutive patients with mild ischemic stroke. Stroke 2012, 43, 560–562. [Google Scholar] [CrossRef]
- Chang, W.H. Personalized Approaches to Stroke: One Step Forward for Functional Recovery of Stroke Patients. J. Pers. Med. 2022, 12, 822. [Google Scholar] [CrossRef]
- Tsiakiri, A.; Vlotinou, P.; Paschalidou, A.; Konstantinidis, C.; Christidi, F.; Tsiptsios, D.; Detsaridou, G.; Petridou, A.; Gkantzios, A.; Karatzetzou, S.; et al. A Scoping Review on Coping Strategies and Quality of Life of Stroke Caregivers: Often Underestimated Variables in Stroke Recovery Process? BioMed 2023, 3, 349–368. [Google Scholar] [CrossRef]
- Cramer, S.C. Repairing the human brain after stroke: I. Mechanisms of spontaneous recovery. Ann. Neurol. 2008, 63, 272–287. [Google Scholar] [CrossRef]
- Christidi, F.; Tsiptsios, D.; Fotiadou, A.; Kitmeridou, S.; Karatzetzou, S.; Tsamakis, K.; Sousanidou, A.; Psatha, E.A.; Karavasilis, E.; Seimenis, I.; et al. Diffusion Tensor Imaging as a Prognostic Tool for Recovery in Acute and Hyperacute Stroke. Neurol. Int. 2022, 14, 841–874. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Christidi, F.; Orgianelis, I.; Merkouris, E.; Koutsokostas, C.; Tsiptsios, D.; Karavasilis, E.; Psatha, E.A.; Tsiakiri, A.; Serdari, A.; Aggelousis, N.; et al. A Comprehensive Review on the Role of Resting-State Functional Magnetic Resonance Imaging in Predicting Post-Stroke Motor and Sensory Outcomes. Neurol. Int. 2024, 16, 189-201. https://doi.org/10.3390/neurolint16010012
Christidi F, Orgianelis I, Merkouris E, Koutsokostas C, Tsiptsios D, Karavasilis E, Psatha EA, Tsiakiri A, Serdari A, Aggelousis N, et al. A Comprehensive Review on the Role of Resting-State Functional Magnetic Resonance Imaging in Predicting Post-Stroke Motor and Sensory Outcomes. Neurology International. 2024; 16(1):189-201. https://doi.org/10.3390/neurolint16010012
Chicago/Turabian StyleChristidi, Foteini, Ilias Orgianelis, Ermis Merkouris, Christos Koutsokostas, Dimitrios Tsiptsios, Efstratios Karavasilis, Evlampia A. Psatha, Anna Tsiakiri, Aspasia Serdari, Nikolaos Aggelousis, and et al. 2024. "A Comprehensive Review on the Role of Resting-State Functional Magnetic Resonance Imaging in Predicting Post-Stroke Motor and Sensory Outcomes" Neurology International 16, no. 1: 189-201. https://doi.org/10.3390/neurolint16010012
APA StyleChristidi, F., Orgianelis, I., Merkouris, E., Koutsokostas, C., Tsiptsios, D., Karavasilis, E., Psatha, E. A., Tsiakiri, A., Serdari, A., Aggelousis, N., & Vadikolias, K. (2024). A Comprehensive Review on the Role of Resting-State Functional Magnetic Resonance Imaging in Predicting Post-Stroke Motor and Sensory Outcomes. Neurology International, 16(1), 189-201. https://doi.org/10.3390/neurolint16010012






