Association between Brain White Matter Lesions and Disease Activity in HAM/TSP Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Considerations
2.2. Study Design and Participants
2.3. Magnetic Resonance Imaging
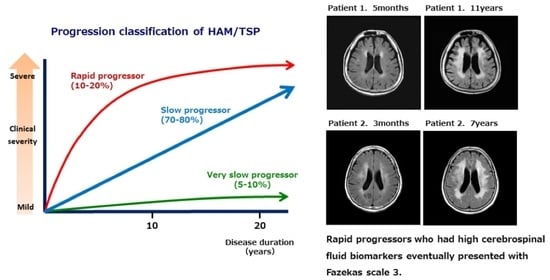
2.4. Classification Based on Disease Progression
2.5. CSF Biomarkers and PVL in PBMC
2.6. Statistical Analysis
3. Results
3.1. Fazekas Scale of Brain WM Lesions
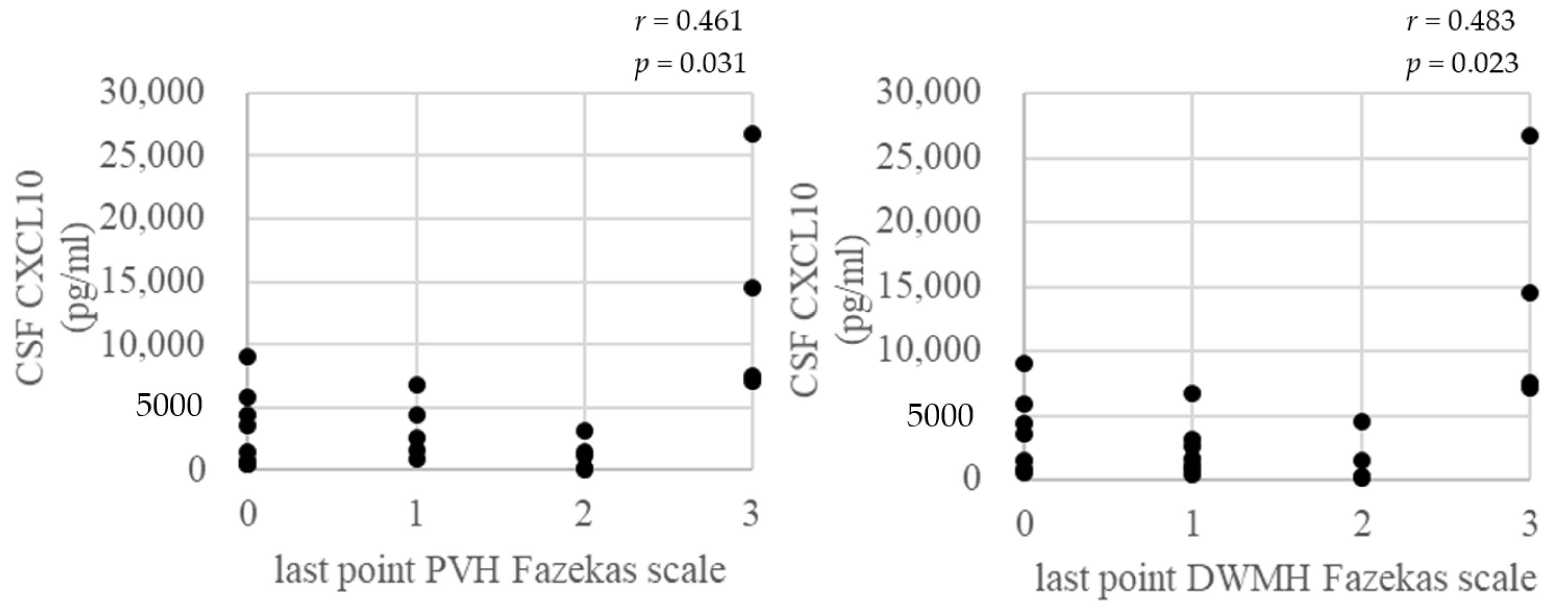
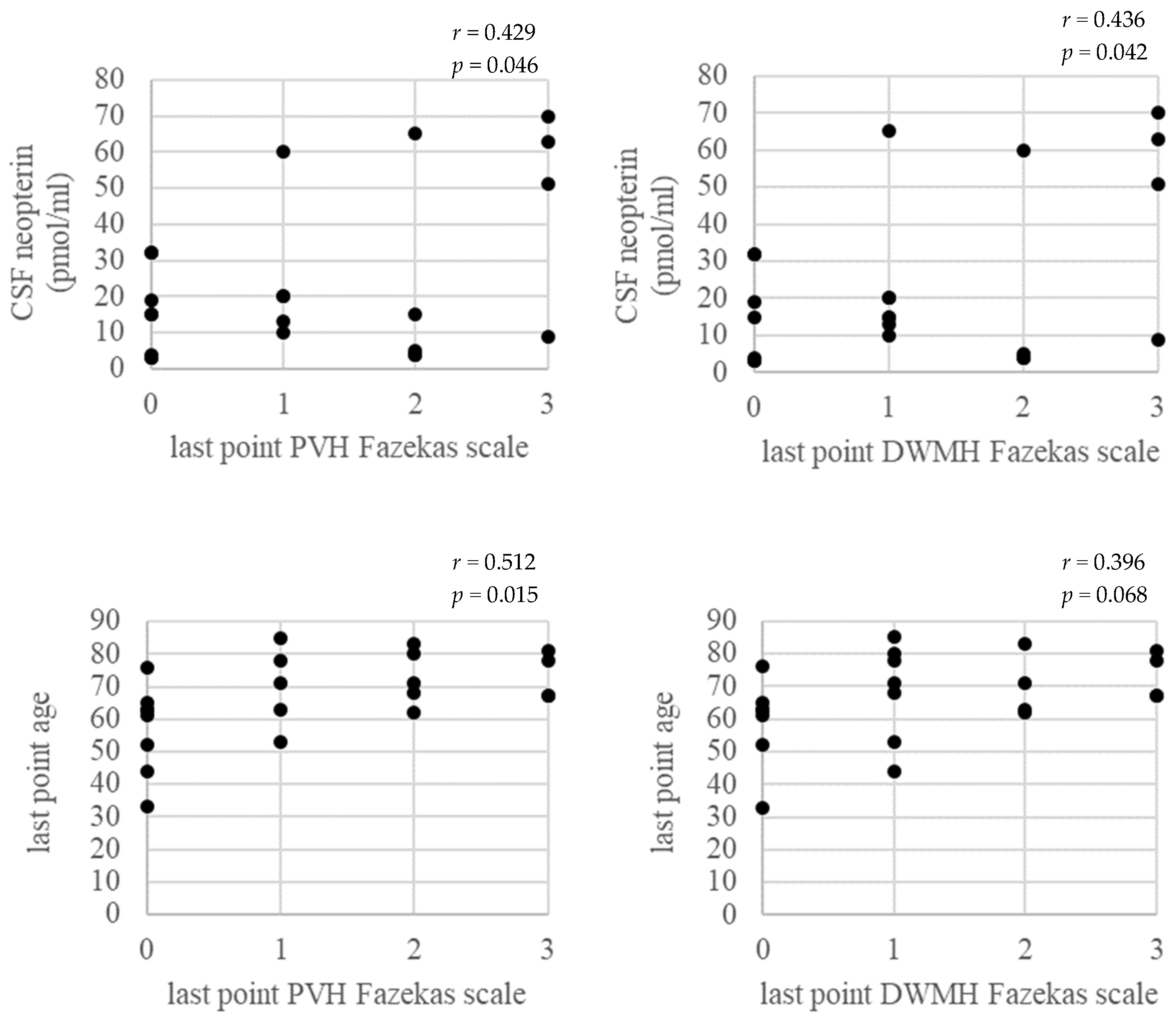
3.2. CSF Biomarkers
3.3. PVL in PBMC
3.4. Correlation between Fazekas Scale of Brain WM Lesions and Age at the Last Point, CSF Biomarkers at the Base Point, and PVL in PBMC at the Last Point
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gessain, A.; Cassar, O. Epidemiological aspects and world distribution of HTLV-1 infection. Front. Microbiol. 2012, 3, 388. [Google Scholar] [CrossRef] [PubMed]
- Satake, M.; Yamaguchi, K.; Tadokoro, K. Current prevalence of HTL-1 in Japan as determined by screening of blood donors. J. Med. Virol. 2012, 84, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Nosaka, K.; Iwanaga, M.; Imaizumi, Y.; Ishitsuka, K.; Ishizawa, K.; Ishida, Y.; Amano, M.; Ishida, T.; Uike, N.; Utunomia, A.; et al. Epidemiological and clinical features of adult T-cell leukemia-lymphoma in Japan, 2010–2011: A nationwide survey. Cancer Sci. 2017, 108, 2478–2486. [Google Scholar] [CrossRef]
- Yamano, Y.; Sato, T. Clinical pathophysiology of human T-lymphotropic virus-type 1-associated myelopathy/tropical spastic paraparesis. Front. Microbiol. 2012, 3, 389. [Google Scholar] [CrossRef] [PubMed]
- Coler-Reilly, A.L.; Yagishita, N.; Suzuki, H.; Sato, T.; Araya, N.; Inoue, E.; Takata, A.; Yamano, Y. Nation-wide epidemiological study of Japanese patients with rare viral myelopathy using novel registration system (HAM-net). Orphanet J. Rare Dis. 2016, 11, 69. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Yagishita, N.; Tamaki, K.; Inoue, E.; Hasegawa, D.; Nagasaka, M.; Suzuki, H.; Araya, N.; Coler-Reilly, A.; Hasegawa, Y.; et al. Proposal of classification criteria for HTLV-1-associated myelopathy/tropical spastic paraparesis disease activity. Front. Microbiol. 2018, 25, 1651. [Google Scholar] [CrossRef] [PubMed]
- Olindo, S.; Lézin, A.; Cabre, P.; Merle, H.; Saint-Vil, M.; Edimonana Kaptue, M.; Signate, A.; Césaire, R.; Smadja, D. HTLV-1 proviral load in peripheral blood mononuclear cells quantified in 100 HAM/TSP patients: A marker of disease progression. J. Neurol. Sci. 2005, 237, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, E.; Nozuma, S.; Tashiro, Y.; Kubota, R.; Izumo, S.; Takashima, H. HTLV-1 associated myelopathy/tropical spastic paraparesis (HAM/TSP): A comparative study to identify factors that influence disease progression. J. Neurol. Sci. 2016, 371, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Bagnato, F.; Butman, J.A.; Mora, C.A.; Gupta, S.; Yamano, Y.; Tasciyan, T.A.; Solomon, J.M.; Santos, W.J.; Stone, R.D.; McFarland, H.F.; et al. Conventional magnetic resonance imaging features in patients with tropical spastic paraparesis. J. Neurovirol. 2005, 11, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Howard, A.K.; Li, D.K.; Oger, J. MRI contributes to the differentiation between MS and HTLV-I associated myelopathy in British Columbian coastal natives. Can. J. Neurol. Sci. 2003, 30, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Gascón, M.R.P.; Casseb, J.; Smid, J.; Vidal, J.E.; Fonseca, L.A.M.; Paiva, A.; Haziot, M.J.; Penalva de Oliveira, A.C. Cognitive impairment is frequent among symptomatic carriers of human T-cell lymphotropic virus type 1 (HTLV-1), regardless of their clinical status. J. Neurol. Sci. 2017, 377, 185–189. [Google Scholar] [CrossRef]
- Osame, M. Review of WHO kagoshima meeting and diagnostic guidelines for HAM/TSP. In Human Retrovirology; Blattner, W.A., Ed.; Raven Press: New York, NY, USA, 1990; pp. 191–197. [Google Scholar]
- Fazekas, F.; Chawluk, J.B.; Alavi, A.; Hurtig, H.I.; Zimmerman, R.A. MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJR Am. J. Roentgenol. 1987, 149, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Morgan, D.J.; Caskey, M.F.; Abbehusen, C.; Oliveira-Filho, J.; Araujo, C.; Porto, A.F.; Santos, S.B.; Orge, G.O.; Joia, M.J.; Muniz, A.L.; et al. Brain magnetic resonance imaging white matter lesions are frequent in HTLV-I carriers and do not discriminate from HAM/TSP. AIDS Res. Hum. Retroviruses 2007, 23, 1499–1504. [Google Scholar] [CrossRef] [PubMed]
- Kalil, R.S.; Vasconcellos, I.; Rosadas, C.; Cony, A.; Lima, D.P.; Gonçalves, C.C.; Batista, E.; Grassi, M.F.; Galvão-Castro, B.P.; Taylor, G.; et al. Association between high proviral load, cognitive impairment, and white matter brain lesions in HTLV-1-infected individuals. J. Neurovirol. 2021, 27, 810–819. [Google Scholar] [CrossRef] [PubMed]
- Puccioni-Sohler, M.; Gasparetto, E.; Cabral-Castro, M.J.; Slatter, C.; Vidal, C.M.; Cortes, R.D.; Rosen, B.R.; Mainero, C. HAM/TSP: Association between white matter lesions on magnetic resonance imaging, clinical and cerebrospinal fluid findings. Arq. Neuropsiquiatr. 2012, 70, 246–251. [Google Scholar] [CrossRef]
- Kira, J.; Fujihara, K.; Itoyama, Y.; Goto, I.; Hasuo, K. Leukoencephalopathy in HTLV-I-associated myelopathy/tropical spastic paraparesis: MRI analysis and a two year follow-up study after corticosteroid therapy. J. Neurol. Sci. 1991, 106, 41–49. [Google Scholar] [CrossRef]
- Ogata, A.; Nagashima, K.; Tashiro, K.; Miyakawa, A.; Mikuni, C. MRI-pathological correlate of brain lesions in a necropsy case of HTLV-I associated myelopathy. J. Neurol. Neurosurg. Psychiatry 1993, 56, 194–196. [Google Scholar] [CrossRef]
- Tamaki, K.; Sato, T.; Tsugawa, J.; Fujioka, S.; Yagishita, N.; Araya, N.; Yamauchi, J.; Coler-Reilly, A.L.G.; Nagasaka, M.; Hasegawa, Y.; et al. Cerebrospinal Fluid CXCL10 as a Candidate Surrogate Marker for HTLV-1-Associated Myelopathy/Tropical Spastic Paraparesis. Front. Microbiol. 2019, 10, 2110. [Google Scholar] [CrossRef] [PubMed]
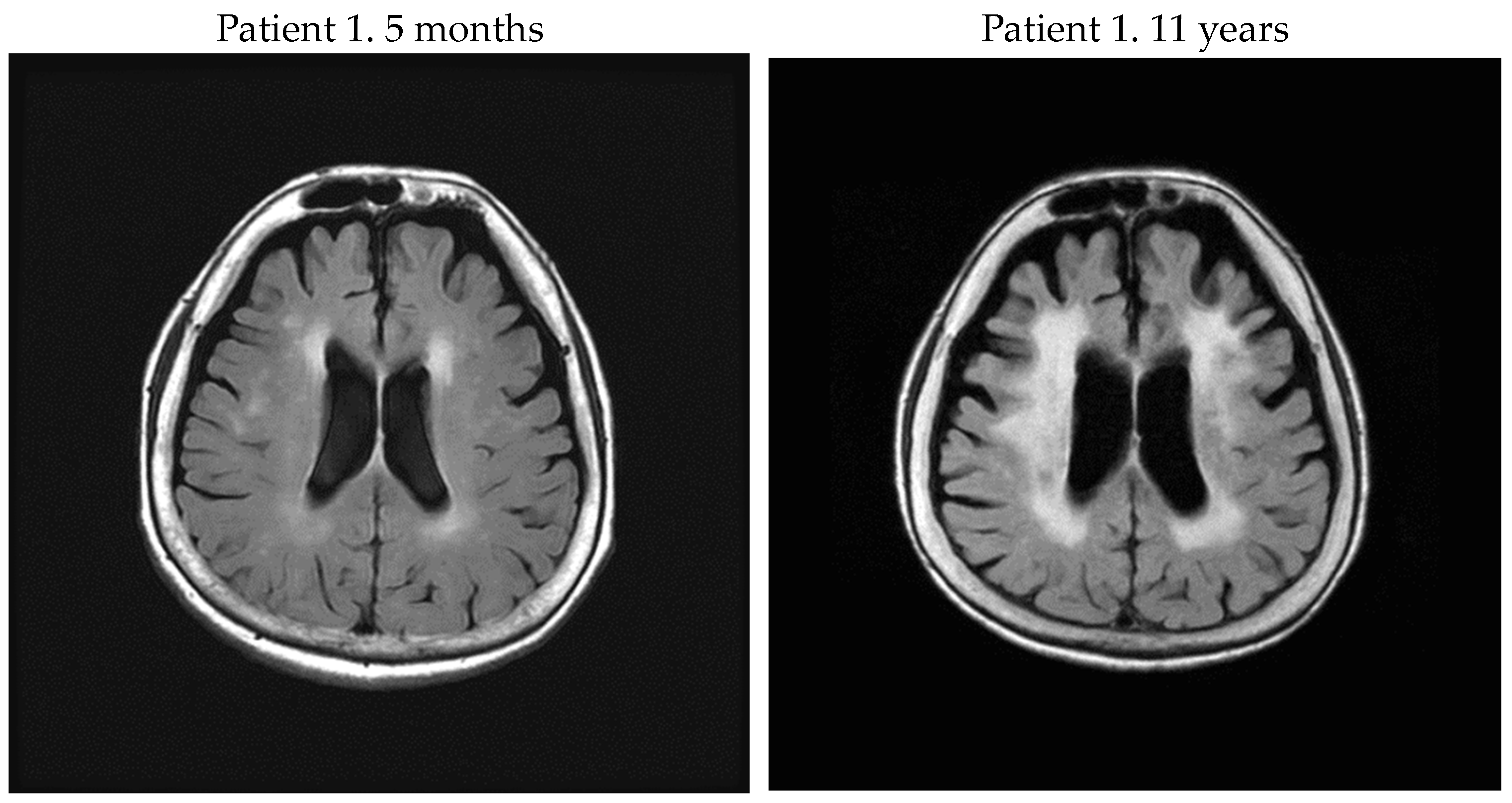

| Patient No. | Sex | Onset Age | Age at Exam | DD | DPP | Brain MRI PVH Fazekas Scale | Brain MRI DWMH Fazekas Scale | PVL in PBMC (Copy/100 Cells) | Anti HTLV-1 Ab Titer in CSF (PA Method) | CSF CXCL10 (pg/mL) | CSF Neopterin (pmol/mL) | Steroid Therapy | OMDS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 71 | 71 | 5 m | rapid | 1 | 1 | — | 2048 | 26,772.2 | 63 | no | 9 |
| 71 | 6 m | — | — | 4.30 | 256 | 1056.2 | 17 | yes | 7 | ||||
| 81 | 11 y | 3 | 3 | 14.98 | — | — | — | yes | 10 | ||||
| 2 | F | 61 | 61 | 3 m | rapid | 2 | 2 | — | 2048 | 14,492.8 | 70 | no | 7 |
| 67 | 7 y | 3 | 3 | 3.89 | 1024 | 5083.7 | 43 | yes | 6 | ||||
| 3 | F | 70 | 72 | 2 y | rapid | 1 | 1 | 0.70 | 128 | 1137.2 | 65 | no | 5 |
| 80 | 10 y | 2 | 1 | 1.69 | 64 | 577.0 | 8 | yes | 6 | ||||
| 4 | F | 65 | 78 | 13 y | rapid | 3 | 3 | 18.69 | 2048 | 7065.4 | 51 | yes | 5 |
| 79 | 14 y | — | — | 17.55 | — | — | — | yes | 5 | ||||
| 5 | F | 81 | 81 | 3 m | rapid | 1 | 1 | — | 1024 | 3173.6 | 20 | no | 9 |
| 81 | 4 m | — | — | — | 512 | 485.4 | 9 | yes | 5 | ||||
| 85 | 4 y | 1 | 1 | — | — | — | — | yes | 5 | ||||
| 6 | F | 58 | 59 | 1 y | slow | 1 | 1 | 13.29 | 128 | 6775.1 | 60 | no | 3 |
| 60 | 2 y | 1 | 1 | 7.88 | 64 | 4072.4 | 41 | yes | 4 | ||||
| 63 | 5 y | 1 | 2 | 8.41 | — | — | — | yes | 4 | ||||
| 7 | F | 33 | 48 | 15 y | slow | — | — | 7.97 | 8 | 4463.2 | 20 | no | 4 |
| 53 | 20 y | 1 | 1 | 12.58 | 8 | 3192.8 | 16 | yes | 5 | ||||
| 8 | F | 59 | 74 | 15 y | slow | 1 | 1 | 8.13 | 64 | 1605.7 | 13 | no | 5 |
| 78 | 19 y | 1 | 1 | 9.10 | — | — | — | yes | 6 | ||||
| 9 | F | 48 | 61 | 13 y | slow | — | — | 3.09 | 128 | 888.2 | 4 | no | 5 |
| 62 | 14 y | 2 | 2 | 1.54 | — | — | — | no | 5 | ||||
| 10 | F | 49 | 69 | 20 y | slow | 0 | 0 | 1.46 | 16 | 82.1 | 3 | no | 5 |
| 76 | 27 y | 0 | 0 | 1.76 | — | — | — | no | 6 | ||||
| 11 | M | 40 | 66 | 26 y | slow | — | — | 1.64 | 64 | 784.6 | 5 | no | 5 |
| 71 | 31 y | 2 | 2 | 5.79 | — | — | — | no | 6 | ||||
| 12 | F | 68 | 79 | 11 y | slow | — | — | 0.22 | 8 | 220.7 | 4 | no | 9 |
| 83 | 15 y | 2 | 2 | 0.42 | — | — | — | no | 9 | ||||
| 13 | F | 27 | 60 | 33 y | slow | 3 | 3 | — | 128 | 1499.1 | 9 | no | 13 |
| 67 | 40 y | 3 | 3 | 4.96 | 32 | 467.8 | 6 | yes | 13 | ||||
| 14 | F | 40 | 44 | 4 y | slow | 0 | 0 | 2.77 | 64 | 7499.5 | 32 | no | 4 |
| 52 | 12 y | 0 | 0 | 1.79 | — | — | — | no | 5 | ||||
| 15 | F | 55 | 57 | 2 y | slow | 0 | 0 | 2.02 | 128 | 4372.0 | 32 | no | 3 |
| 63 | 8 y | 0 | 0 | 3.06 | — | — | — | yes | 4 | ||||
| 16 | F | 10 | 28 | 18 y | slow | 0 | 0 | 6.37 | 2048 | 9107.8 | 49 | no | 9 |
| 28 | 18 y | — | — | — | 2048 | 3381.1 | 19 | yes | 6 | ||||
| 33 | 23 y | 0 | 0 | 4.88 | — | — | — | yes | 6 | ||||
| 17 | F | 34 | 44 | 10 y | slow | 0 | 1 | 8.87 | 256 | 3576.9 | 15 | yes | 6 |
| 18 | M | 51 | 56 | 5 y | very slow | 0 | 0 | 2.07 | 128 | 458.2 | 4 | no | 3 |
| 63 | 12 y | 0 | 0 | 0.81 | — | — | — | no | 3 | ||||
| 19 | M | 54 | 56 | 2 y | slow | 0 | 0 | 8.39 | 32 | 5841.1 | 15 | yes | 4 |
| 61 | 7 y | 0 | 0 | 4.04 | — | — | — | yes | 4 | ||||
| 20 | M | 61 | 65 | 4 y | slow | 0 | 0 | 14.59 | 32 | 519.3 | 3 | no | 3 |
| 21 | F | 63 | 66 | 3 y | slow | — | — | 3.84 | 64 | 1464.9 | 10 | no | 4 |
| 71 | 8 y | 1 | 1 | 2.98 | — | — | — | no | 6 | ||||
| 22 | F | 67 | 68 | 1 y | slow | 2 | 1 | 1.31 | 64 | 2651.7 | 15 | no | 3 |
| Grade | Motor Disability |
|---|---|
| 0 | No walking or running abnormalities |
| 1 | Normal gait but runs slowly |
| 2 | Abnormal gait (stumbling, stiffness) |
| 3 | Unable to run |
| 4 | Needs handrail to climb stairs |
| 5 | Needs a cane (unilateral support) to walk |
| 6 | Needs bilateral support to walk |
| 7 | Can walk 5–10 m with bilateral support |
| 8 | Can walk 1–5 m with bilateral support |
| 9 | Cannot walk, but able to crawl |
| 10 | Cannot crawl, but able to move using arms |
| 11 | Cannot move around, but able to turn over in bed |
| 12 | Cannot turn over in bed |
| 13 | Cannot even move toes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamaki, K.; Ouma, S.; Takahashi, N.; Fujioka, S.; Tsuboi, Y. Association between Brain White Matter Lesions and Disease Activity in HAM/TSP Patients. Neurol. Int. 2024, 16, 202-209. https://doi.org/10.3390/neurolint16010013
Tamaki K, Ouma S, Takahashi N, Fujioka S, Tsuboi Y. Association between Brain White Matter Lesions and Disease Activity in HAM/TSP Patients. Neurology International. 2024; 16(1):202-209. https://doi.org/10.3390/neurolint16010013
Chicago/Turabian StyleTamaki, Keiko, Shinji Ouma, Nobutaka Takahashi, Shinsuke Fujioka, and Yoshio Tsuboi. 2024. "Association between Brain White Matter Lesions and Disease Activity in HAM/TSP Patients" Neurology International 16, no. 1: 202-209. https://doi.org/10.3390/neurolint16010013
APA StyleTamaki, K., Ouma, S., Takahashi, N., Fujioka, S., & Tsuboi, Y. (2024). Association between Brain White Matter Lesions and Disease Activity in HAM/TSP Patients. Neurology International, 16(1), 202-209. https://doi.org/10.3390/neurolint16010013