Effects of Visual–Motor Illusion via Image Videos Showing Increased Exercise Intensity on the Tibial Anterior during Sit-to-Stand Movement: A Study of Healthy Participants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
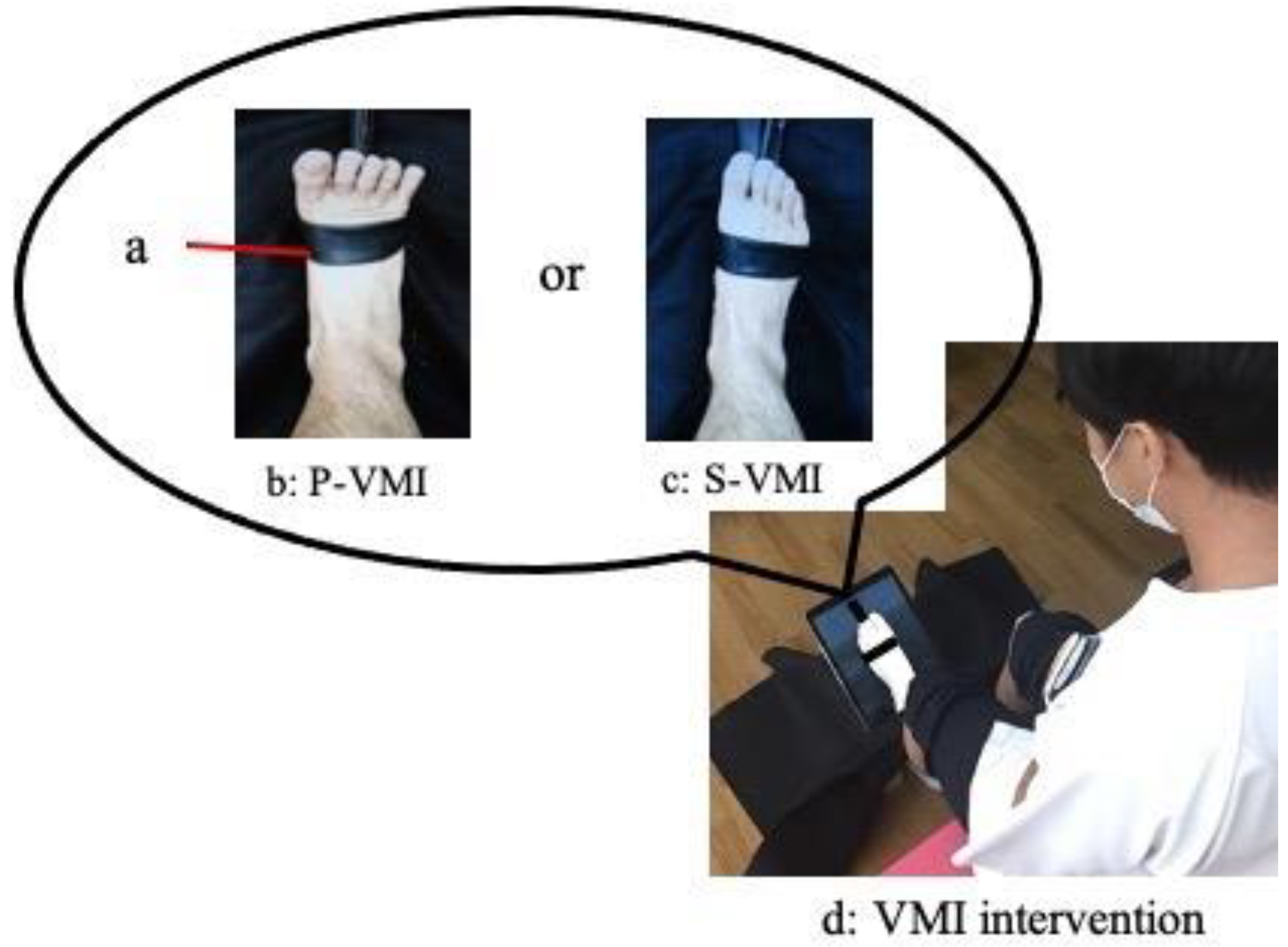
2.2. VMI Interventions
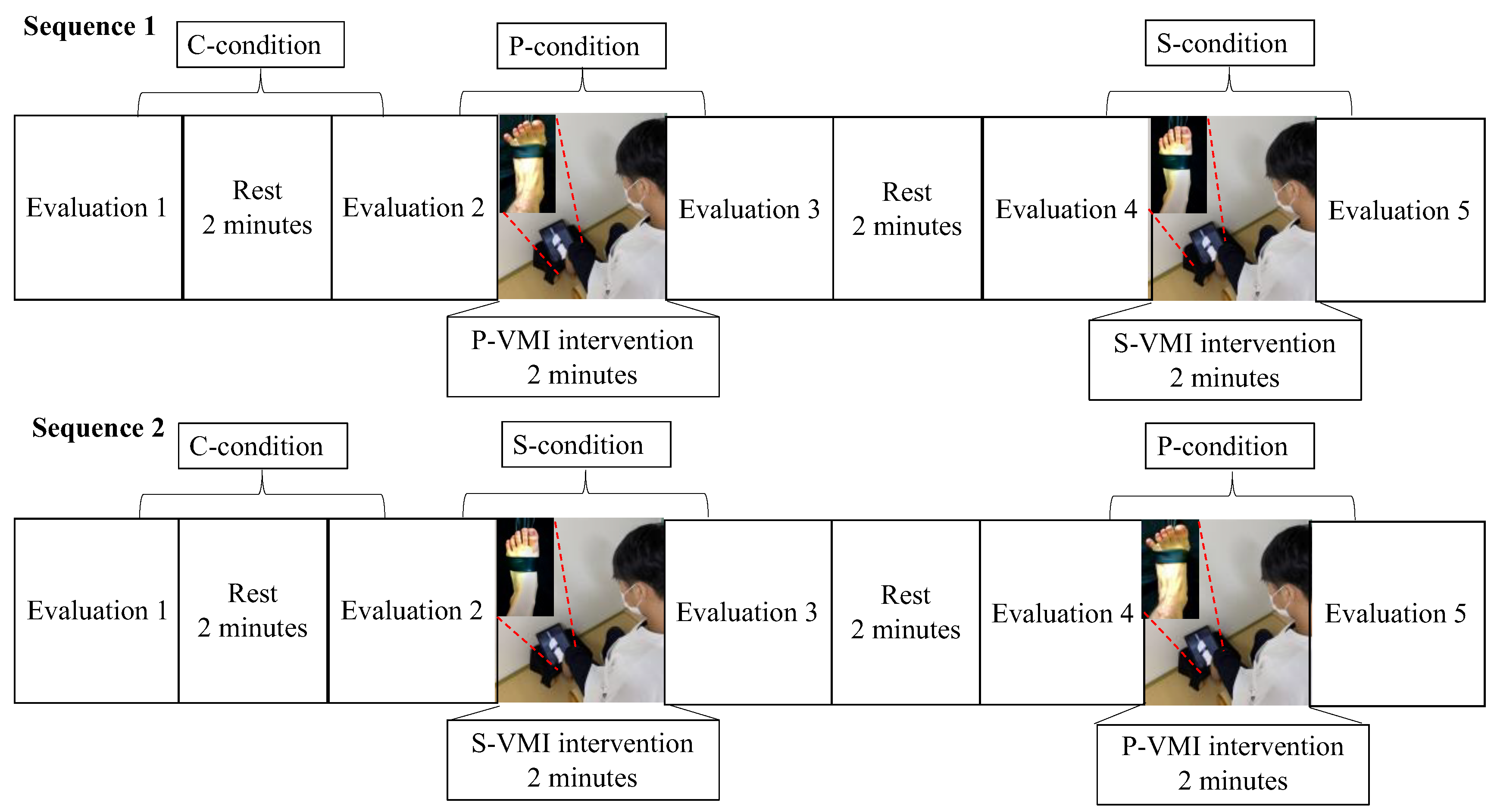
2.3. Study Protocol
2.4. Outcome Measurements
2.5. Kinematic Measurements
2.6. EMG Measurements
2.7. Subjective SOA
2.8. Test Procedure
2.9. Kinematic Data Processing
2.10. STS Duration
2.11. Movement of the Trunk and Ankle Joint during STS
2.12. Electromyographic Data Processing
2.13. Statistical Analysis
2.14. Sample Size
3. Results
3.1. STS Duration
3.2. Trunk and Ankle Joint Movements during STS
3.3. Tibialis Anterior Muscle Activity during STS and VMI Intervention
3.4. SOA
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Perin, C.; Bolis, M.; Limonta, M.; Meroni, R.; Ostasiewicz, K.; Cornaggia, C.M.; Alouche, S.R.; da Silva Matuti, G.; Cerri, C.G.; Piscitelli, D. Differences in Rehabilitation Needs after Stroke: A Similarity Analysis on the ICF Core Set for Stroke. Int. J. Environ. Res. Public Health 2020, 17, 4291. [Google Scholar] [CrossRef] [PubMed]
- Hirschfeld, H.; Thorsteinsdottir, M.; Olsson, E. Coordinated ground forces exerted by buttocks and feet are adequately programmed for weight transfer during sit-to-stand. J. Neurophysiol. 1999, 82, 3021–3029. [Google Scholar] [CrossRef] [PubMed]
- Boukadida, A.; Piotte, F.; Dehail, P.; Nadeau, S. Determinants of sit-to-stand tasks in individuals with hemiparesis post stroke: A review. Ann. Phys. Rehabil. Med. 2015, 58, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Holly-Crichlow, N.; Brichta, P.; Reeves, G.R.; Zablotny, C.M.; Nawoczenski, D.A. The effects of the lower extremity joint motions on the total body motion in sit-to-stand movement. Clin. Biomech. 2000, 15, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Prudente, C.; Rodrigues-de-Paula, F.; Faria, C.D. Lower limb muscle activation during the sit-to-stand task in subjects who have had a stroke. Am. J. Phys. Med. Rehabil. 2013, 92, 666–675. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.; Sousa, A.S.; Pinheiro, R.; Ferraz, J.; Tavares, J.M.; Santos, R.; Sousa, F. Activation timing of soleus and tibialis anterior muscles during sit-to-stand and stand-to-sit in post-stroke vs. healthy subjects. Somatosens. Mot. Res. 2013, 30, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Lomaglio, M.J.; Eng, J.J. Muscle strength and weight-bearing symmetry relate to sit-to-stand performance in individuals with stroke. Gait Posture 2005, 22, 126–131. [Google Scholar] [CrossRef]
- Cheng, P.T.; Chen, C.L.; Wang, C.M.; Hong, W.H. Leg muscle activation patterns of sit-to-stand movement in stroke patients. Am. J. Phys. Med. Rehabil. 2004, 83, 10–16. [Google Scholar] [CrossRef]
- Kaneko, F.; Yasojima, T.; Kizuka, T. Kinesthetic Illusory feeling induced by a finger movement movie effects on corticomotor excitability. Neuroscience 2007, 149, 976–984. [Google Scholar] [CrossRef]
- Aoyama, T.; Kaneko, F.; Hayami, T.; Shibata, E. The effects of kinesthetic illusory sensation induced by a visual stimulus on the corticomotor excitability of the leg muscles. Neurosci. Lett. 2012, 514, 106–109. [Google Scholar] [CrossRef]
- Kaneko, F.; Blanchard, C.; Lebar, N.; Nazarian, B.; Kavounoudias, A.; Romaiguère, P. Brain regions associated to a kinesthetic illusion evoked by watching a video of one’s own moving hand. PLoS ONE 2015, 10, e0131970. [Google Scholar] [CrossRef]
- Sakai, K.; Goto, K.; Watanabe, R.; Tanabe, J.; Amimoto, K.; Kumai, K.; Shibata, K.; Morikawa, K.; Ikeda, Y. Immediate effects of visual-motor illusion on resting-state functional connectivity. Brain Cogn. 2020, 146, 105632. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, F.; Inada, T.; Matsuda, N.; Shibata, E.; Koyama, S. Acute effect of visually induced kinesthetic illusion in patients with stroke: A preliminary report. Int. J. Neurorehabil. Eng. 2016, 3, 212. [Google Scholar] [CrossRef]
- Kaneko, F.; Shindo, K.; Yoneta, M.; Okawada, M.; Akaboshi, K.; Liu, M. A case series clinical trial of a novel approach using augmented reality that inspires self-body cognition in patients with stroke: Effects on motor function and resting-state brain functional connectivity. Front. Syst. Neurosci. 2019, 13, 76. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, T.; Kanazawa, A.; Kohno, Y.; Watanabe, S.; Tomita, K.; Kaneko, F. Influence of visual stimulation-induced passive reproduction of motor images in the brain on motor paralysis after stroke. Front. Hum. Neurosci. 2021, 15, 674139. [Google Scholar] [CrossRef]
- Okawada, M.; Inada, T.; Matsuda, N.; Motozawa, S.; Yoneta, M.; Sasaki, S.; Shibata, E.; Kaneko, F. Effects of kinesthetic illusion induced by visual stimulation (kinvis) therapy on patients with stroke in the subacute phase: A visual analysis based on paralysis severity. Neurocase 2022, 28, 199–205. [Google Scholar] [CrossRef]
- Takahashi, R.; Koiwa, M.; Ide, W.; Okawada, M.; Akaboshi, K.; Kaneko, F. visually induced kinaesthetic illusion combined with therapeutic exercise for patients with chronic stroke: A pilot study. J. Rehabil. Med. 2022, 54, jrm00276. [Google Scholar] [CrossRef]
- Altschuler, E.L.; Wisdom, S.B.; Stone, L.; Foster, C.; Galasko, D.; Llewellyn, D.M.; Ramachandran, V.S. Rehabilitation of hemiparesis after stroke with a mirror. Lancet 1999, 353, 2035–2036. [Google Scholar] [CrossRef]
- Wakata, S.; Morioka, S. Brain activity and the perception of self-agency while viewing a video of hand grasping: A functional near-infrared spectroscopy study. NeuroReport 2015, 26, 394–398. [Google Scholar] [CrossRef]
- Ertelt, D.; Small, S.; Solodkin, A.; Dettmers, C.; McNamara, A.; Binkofski, F.; Buccino, G. Action observation has a positive impact on rehabilitation of motor deficits after stroke. NeuroImage 2007, 36, 164–173. [Google Scholar] [CrossRef]
- Rizzolatti, G.; Fadiga, L.; Gallese, V.; Fogassi, L. Premotor cortex and the recognition of motor actions. Brain Res. Cogn. Brain Res. 1996, 3, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, J.; Amimoto, K.; Sakai, K.; Osaki, S.; Yoshihiro, N. Effects of kinesthetic illusion induced by visual stimulation on the ankle joint for sit-to-stand in a hemiparesis stroke patient: ABA’ single-case design. J. Phys. Ther. Sci. 2022, 34, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Ishizaka, M.; Takeda, K.; Shimoi, T.; Maruyama, H. Relationship between intensity of toe motion and cerebral activation: A near-infrared spectroscopy study. Rigakuryoho Kagaku 2012, 27, 165–170. [Google Scholar] [CrossRef]
- Mizuguchi, N.; Umehara, I.; Nakata, H.; Kanosue, K. Modulation of corticospinal excitability dependent upon imagined force level. Exp. Brain Res. 2013, 230, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Weiss, C.; Tsakiris, M.; Haggard, P.; Schütz-Bosbach, S. Agency in the sensorimotor system and its relation to explicit action awareness. Neuropsychologia 2014, 52, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Minohara, R.; Wen, W.; Hamasaki, S.; Maeda, T.; Kato, M.; Yamakawa, H.; Yamashita, A.; Asama, H. Strength of intentional effort enhances the sense of agency. Front. Psychol. 2016, 7, 1165. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, J.; Amimoto, K.; Sakai, K.; Morishita, M.; Osaki, S.; Yoshihiro, N.; Kataoka, T. Effects of visual-motor illusions with different visual stimuli on the sit-to-stand of people with hemiplegia following stroke: A randomized crossover controlled trial. Hum. Mov. Sci. 2023, 87, 103021. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, J.; Amimoto, K.; Sakai, K.; Osaki, S.; Yoshihiro, N.; Kataoka, T. Effects and adaptation of visual-motor illusion using different visual stimuli on improving ankle joint paralysis of stroke survivors-a randomized crossover controlled trial. Brain Sci. 2022, 12, 1249. [Google Scholar] [CrossRef]
- Chapman, J.P.; Chapman, L.J.; Allen, J.J. The measurement of foot preference. Neuropsychologia 1987, 25, 579–584. [Google Scholar] [CrossRef]
- Fukushima, T.; Tsuji, T.; Sano, Y.; Miyata, C.; Kamisako, M.; Hohri, H.; Yoshimura, C.; Asakura, M.; Okitsu, T.; Muraoka, K.; et al. Immediate effects of active exercise with compression therapy on lower-limb lymphedema. Support. Care Cancer 2017, 25, 2603–2610. [Google Scholar] [CrossRef]
- Hermens, H.J.; Freriks, B.; Disselhorst-Klug, C.; Rau, G. Development of recommendations for SEMG sensors and sensor placement procedures. J. Electromyogr. Kinesiol. 2000, 10, 361–374. [Google Scholar] [CrossRef]
- Sidarus, N.; Vuorre, M.; Metcalfe, J.; Haggard, P. Investigating the prospective sense of agency: Effects of processing fluency, stimulus ambiguity, and response conflict. Front. Psychol. 2017, 8, 545. [Google Scholar] [CrossRef] [PubMed]
- Arborelius, U.P.; Wretenberg, P.; Lindberg, F. The effects of armrests and high seat heights on lower-limb joint load and muscular activity during sitting and rising. Ergonomics 1992, 35, 1377–1391. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, S.; Nagano, A.; Hay, D.C.; Fukashiro, S. Peak hip and knee joint moments during a sit-to-stand movement are invariant to the change of seat height within the range of low to normal seat height. Biomed. Eng. Online 2014, 13, 27. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Demura, S. Influence of the relative difference in chair seat height according to different lower thigh length on floor reaction force and lower-limb strength during sit-to-stand movement. J. Physiol. Anthropol. Appl. Hum. Sci. 2004, 23, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Kanai, A.; Kiyama, S.; Goto, H.; Tomita, H.; Tanaka, A.; Kunimi, M.; Okada, T.; Nakai, T. Use of the sit-to-stand task to evaluate motor function of older adults using telemetry. BMC Geriatr. 2016, 16, 121. [Google Scholar] [CrossRef] [PubMed]
- Ota, S.; Goto, H.; Fujita, R.; Haruta, M.; Noda, Y.; Tamakoshi, K. Application of pole walking to day service centers for use by community-dwelling frail elderly people. Int. J. Gerontol. 2014, 8, 6–11. [Google Scholar] [CrossRef]
- Mazzà, C.; Stanhope, S.J.; Taviani, A.; Cappozzo, A. Biomechanic modeling of sit-to-stand to upright posture for mobility assessment of persons with chronic stroke. Arch. Phys. Med. Rehabil. 2006, 87, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Dehail, P.; Bestaven, E.; Muller, F.; Mallet, A.; Robert, B.; Bourdel-Marchasson, I.; Petit, J. Kinematic and electromyographic analysis of rising from a chair during a “sit-to-walk” task in elderly subjects: Role of strength. Clin. Biomech. 2007, 22, 1096–1103. [Google Scholar] [CrossRef]
- Morishita, M.; Mori, S.; Yamagami, S.; Mizutani, M. Effect of carbonated beverages on pharyngeal swallowing in young individuals and elderly inpatients. Dysphagia 2014, 29, 213–222. [Google Scholar] [CrossRef]
- Soma, M.; Murata, S.; Kai, Y.; Nakae, H.; Satou, Y. Activity of the crural muscle during the foot-gripping action. Rigakuryoho Kagaku 2013, 28, 491–494. [Google Scholar] [CrossRef]
- Cheon, S.; Lee, J.H.; Jun, H.P.; An, Y.W.; Chang, E. Acute effects of open kinetic chain exercise versus those of closed kinetic chain exercise on quadriceps muscle thickness in healthy adults. Int. J. Environ. Res. Public Health 2020, 17, 4669. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Routledge: London, UK, 2013. [Google Scholar]
- Kawasaki, T.; Aramaki, H.; Tozawa, R. An Effective Model for Observational Learning to Improve Novel Motor Performance. J. Phys. Ther. Sci. 2015, 27, 3829–3832. [Google Scholar] [CrossRef]
- Park, H.R.; Kim, J.M.; Lee, M.K.; Oh, D.W. Clinical feasibility of action observation training for walking function of patients with post-stroke hemiparesis: A randomized controlled trial. Clin. Rehabil. 2014, 28, 794–803. [Google Scholar] [CrossRef]

| C-Condition | P-Condition | S-Condition | ||||
|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | |
| STS duration (s) | ||||||
| Period 1 c | 0.69 (0.10) | 0.70 (0.08) | 0.69 (0.09) | 0.65 (0.07) a,† | 0.69 (0.08) | 0.67 (0.09) |
| Period 2 d | 0.66 (0.36) | 0.66 (0.38) | 0.65 (0.37) | 0.63 (0.32) | 0.65 (0.36) | 0.64 (0.35) |
| Period 3 e | 1.35 (0.42) | 1.36 (0.43) | 1.34 (0.42) | 1.28 (0.36) | 1.34 (0.41) | 1.32 (0.41) |
| Trunk and ankle joint movement | ||||||
| Trunk forward inclination angle (°) | 44.2 (7.9) | 43.5 (7.5) | 42.9 (6.4) | 44.2 (6.9) | 44.4 (7.8) | 44.2 (7.7) |
| Trunk forward inclination angular velocity (°/s) | 93.6 (18.4) | 93.3 (21.1) | 92.1 (18.0) | 104.8 (22.2) a,b,§ | 97.4 (19.2) | 99.6 (18.2) |
| Ankle dorsiflexion angle (°) | 28.0 (6.9) | 27.9 (6.6) | 28.2 (7.0) | 28.4 (6.9) | 27.5 (6.7) | 27.7 (5.8) |
| Ankle dorsiflexion angular velocity (°/s) | 72.5 (23.2) | 70.2 (20.6) | 73.6 (21.1) | 86.1 (27.8) a,b,§ | 75.9 (25.7) | 78.4 (23.3) |
| Tibialis anterior muscle activity | ||||||
| IEMG of the tibialis anterior muscle (%) | 49.0 (12.3) | 46.6 (14.2) | 47.3 (14.4) | 53.4 (14.0) a,b,§ | 46.4 (15.1) | 49.4 (14.3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanabe, J.; Amimoto, K.; Sakai, K.; Morishita, M. Effects of Visual–Motor Illusion via Image Videos Showing Increased Exercise Intensity on the Tibial Anterior during Sit-to-Stand Movement: A Study of Healthy Participants. Neurol. Int. 2023, 15, 1290-1302. https://doi.org/10.3390/neurolint15040081
Tanabe J, Amimoto K, Sakai K, Morishita M. Effects of Visual–Motor Illusion via Image Videos Showing Increased Exercise Intensity on the Tibial Anterior during Sit-to-Stand Movement: A Study of Healthy Participants. Neurology International. 2023; 15(4):1290-1302. https://doi.org/10.3390/neurolint15040081
Chicago/Turabian StyleTanabe, Junpei, Kazu Amimoto, Katsuya Sakai, and Motoyoshi Morishita. 2023. "Effects of Visual–Motor Illusion via Image Videos Showing Increased Exercise Intensity on the Tibial Anterior during Sit-to-Stand Movement: A Study of Healthy Participants" Neurology International 15, no. 4: 1290-1302. https://doi.org/10.3390/neurolint15040081
APA StyleTanabe, J., Amimoto, K., Sakai, K., & Morishita, M. (2023). Effects of Visual–Motor Illusion via Image Videos Showing Increased Exercise Intensity on the Tibial Anterior during Sit-to-Stand Movement: A Study of Healthy Participants. Neurology International, 15(4), 1290-1302. https://doi.org/10.3390/neurolint15040081






