Acute Presentation and Long-Term Rehabilitation Follow-Up of Ischemic Myelopathy Due to Clinically Suspected Fibrocartilaginous Embolism in an Adolescent Male: A Case Report and Review
Abstract
:1. Introduction
2. Case Presentation
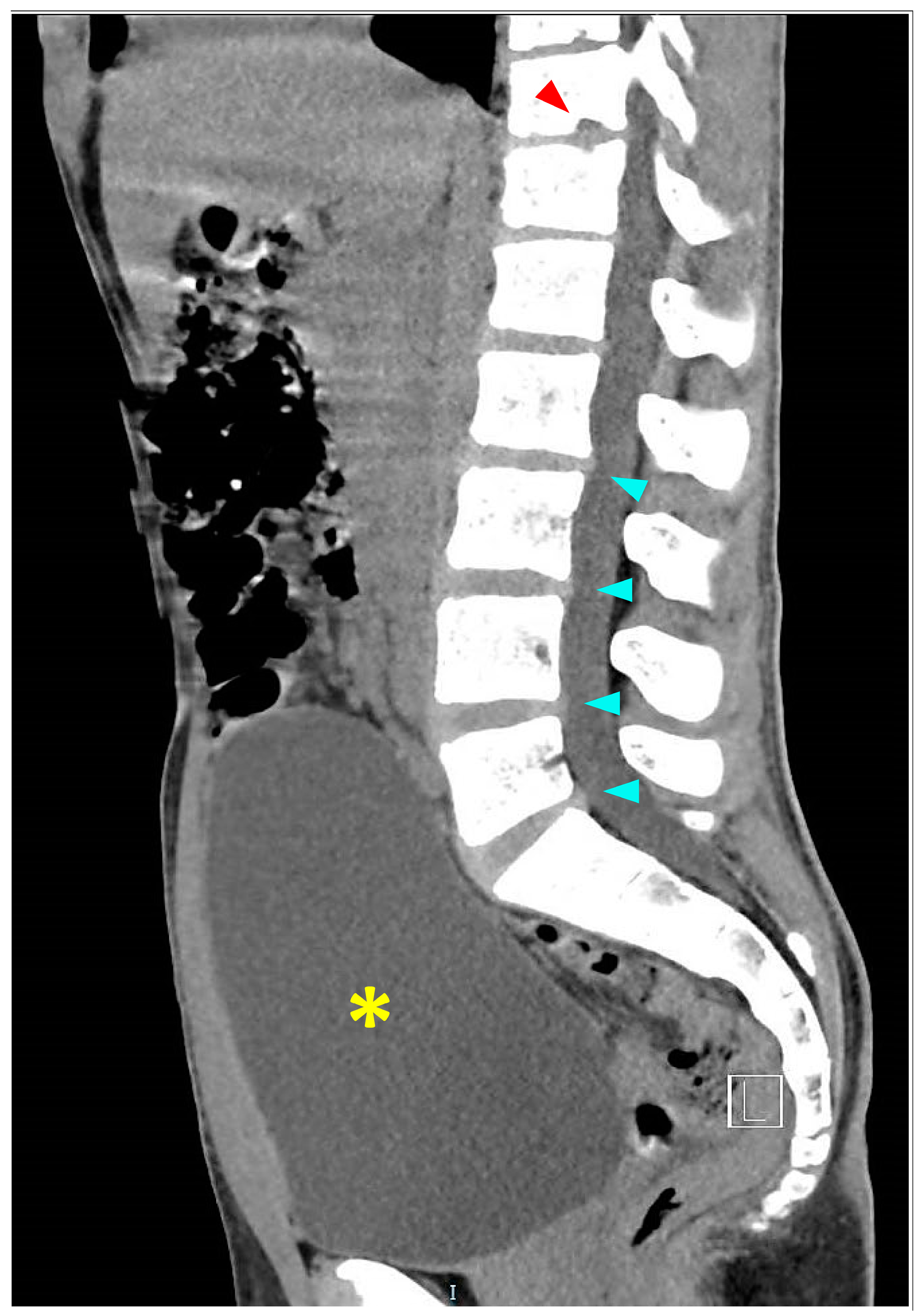
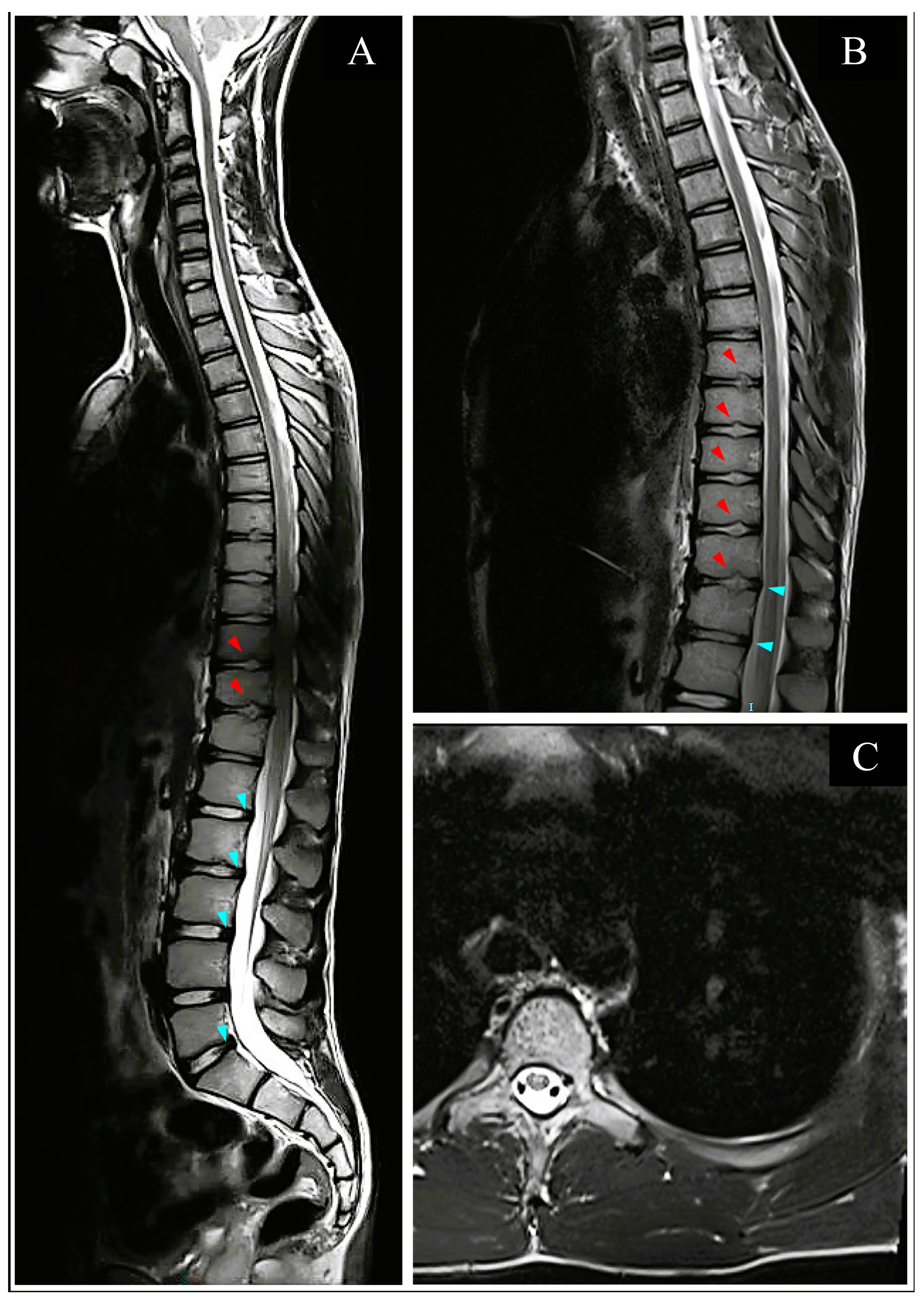
2.1. Acute Hospitalization
2.2. Inpatient Rehabilitation
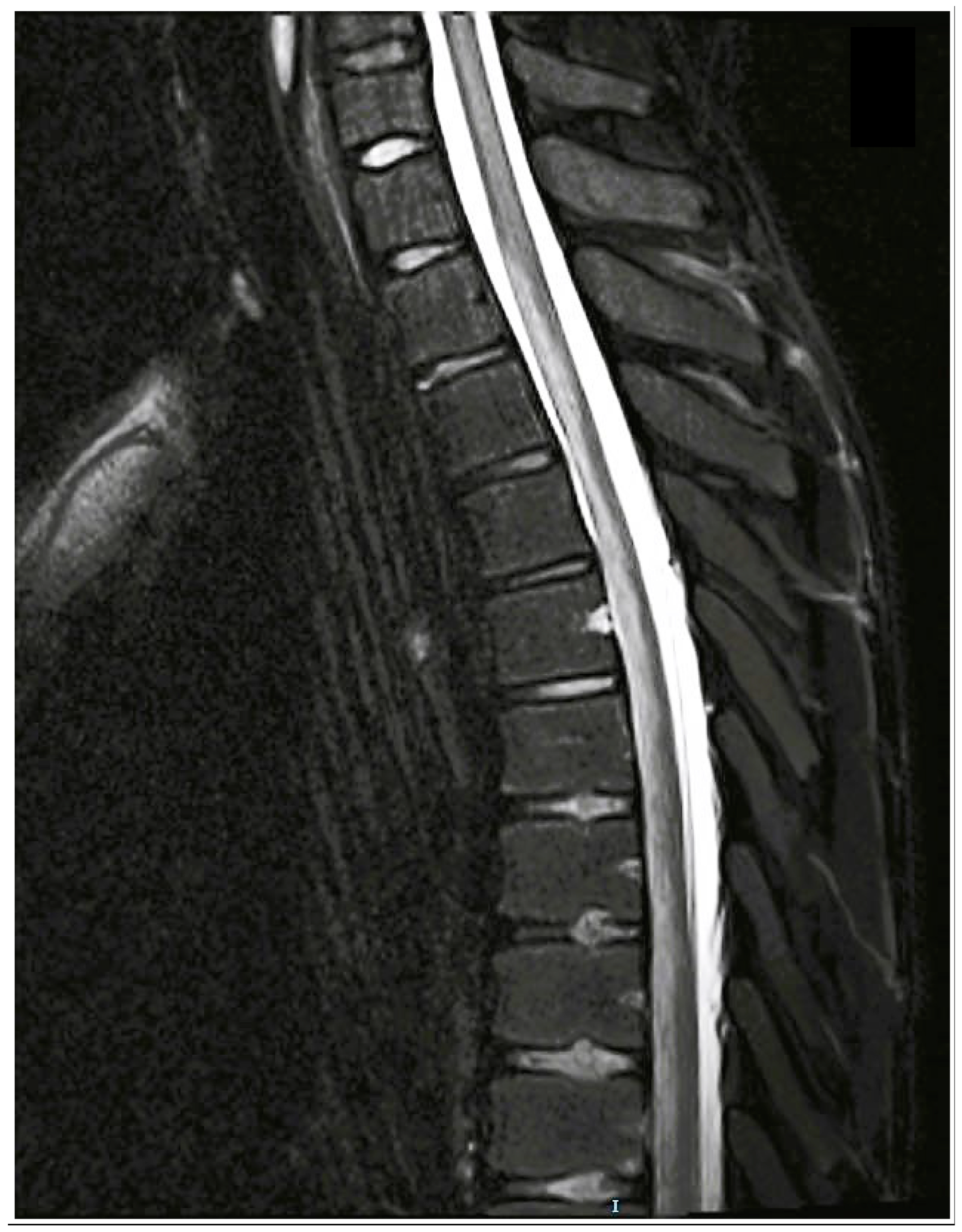
2.3. Long-Term Follow-Up
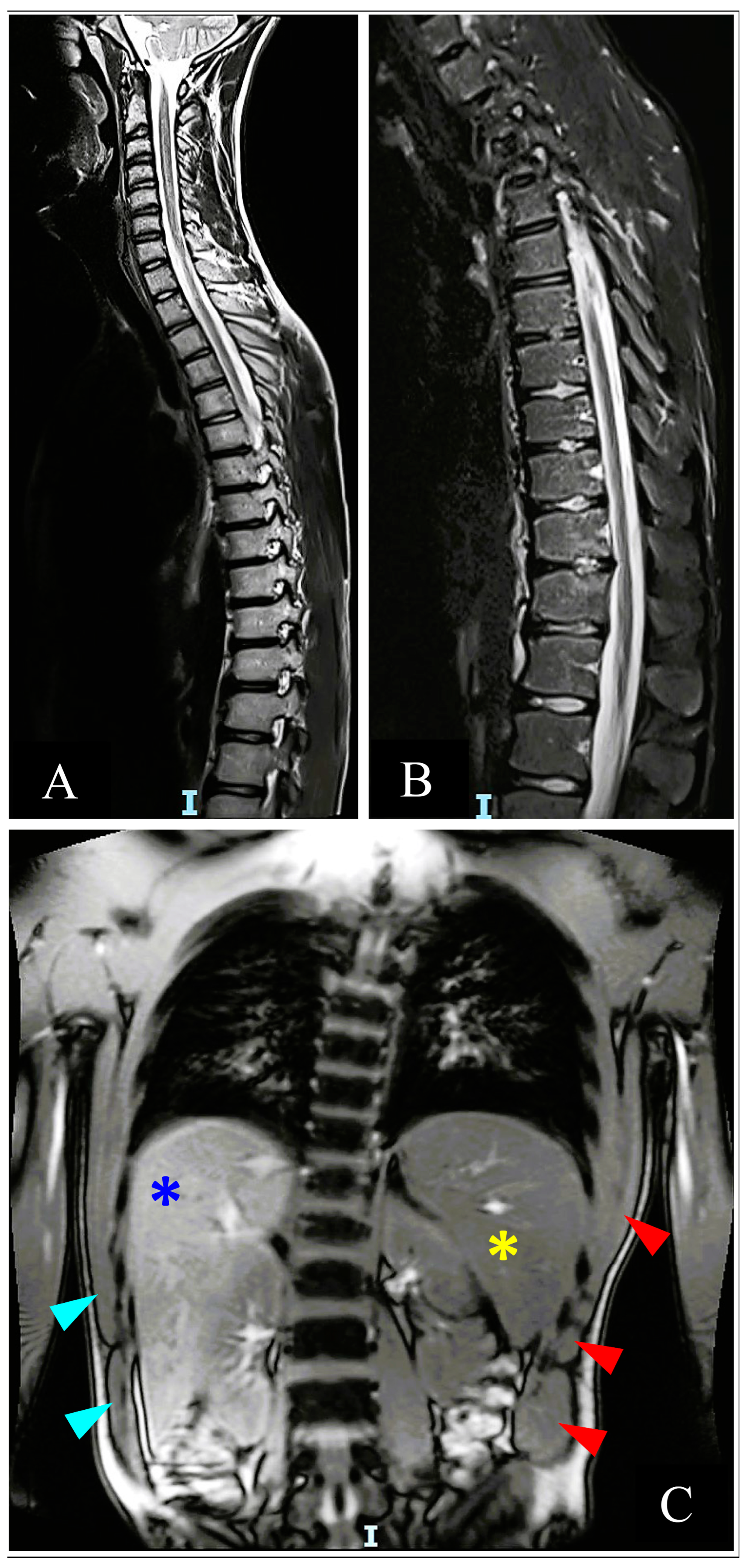
2.4. Repeat Inpatient Follow-Up
3. Discussion
3.1. Epidemiology
3.2. Pathophysiology
3.3. Diagnosis
3.3.1. Clinical Presentation
3.3.2. Imaging
3.3.3. Laboratory Tests
3.4. Treatment and Prognosis
3.4.1. Acute Phase Period
3.4.2. Long-Term Period
3.5. Limitations
3.6. Conclusions
3.7. Patient Perspective
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gupta, M.; Chhabra, H.S. Nucleus Polposus Embolism Causing Anterior Spinal Artery Occlusion: A Rare but Possible Cause of Fibrocartilaginous Embolic Myelopathy. Int. J. Spine Surg. 2020, 14, 391–396. [Google Scholar] [CrossRef]
- Ke, W.; Chen, C.; Li, S.; Wang, B.; Lu, S.; Yang, C. Clinically suspected fibrocartilaginous embolism: A case report and literature review. Int. J. Neurosci. 2022, 132, 378–383. [Google Scholar] [CrossRef]
- Al-Farsi, S.A.; Al-Abri, H.; Al-Ajmi, E.; Al-Asmi, A. Spinal Cord Infarct Due to Fibrocartilaginous Embolism in an Adolescent Boy: A Case Report and Literature Review. Cureus 2023, 15, e37319. [Google Scholar] [CrossRef]
- Naiman, J.L.; Donohue, W.L.; Richard, J.S. Fatal nucleus pulposus embolism of spinal cord after trauma. Neurology 1961, 11, 83. [Google Scholar] [CrossRef]
- Fedaravičius, A.; Feinstein, Y.; Lazar, I.; Gidon, M.; Shelef, I.; Avraham, E.; Tamašauskas, A.; Melamed, I. Successful management of spinal cord ischemia in a pediatric patient with fibrocartilaginous embolism: Illustrative case. J. Neurosurg. Case Lessons 2021, 2, CASE21380. [Google Scholar] [CrossRef] [PubMed]
- McLean, J.M.; Palagallo, G.L.; Henderson, J.P.; Kimm, J.A. Myelopathy associated with fibrocartilaginous emboli (FE): Review and two suspected cases. Surg. Neurol. 1995, 44, 228–235. [Google Scholar] [CrossRef]
- AbdelRazek, M.A.; Mowla, A.; Farooq, S.; Silvestri, N.; Sawyer, R.; Wolfe, G. Fibrocartilaginous embolism: A comprehensive review of an under-studied cause of spinal cord infarction and proposed diagnostic criteria. J. Spinal Cord Med. 2016, 39, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Moore, B.J.; Batterson, A.M.; Luetmer, M.T.; Reeves, R.K. Fibrocartilaginous embolic myelopathy: Demographics, clinical presentation, and functional outcomes. Spinal Cord 2018, 56, 1144–1150. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Nagase, H.; Nishiyama, M.; Tokumoto, S.; Toyoshima, D.; Akasaka, Y.; Maruyama, A.; Iijima, K. Fibrocartilaginous Embolism of the Spinal Cord in Children: A Case Report and Review of Literature. Pediatr. Neurol. 2019, 99, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Nakstad, I.; Randjelovic, I.; Bergan, H.; Evensen, K. Fibrocartilaginøs emboli som årsak til arteria spinalis anterior-syndrom? Tidsskr. Nor. Legeforening 2020, 140. [Google Scholar] [CrossRef] [PubMed]
- Ahluwalia, R.; Hayes, L.; Chandra, T.; Maugans, T.A. Pediatric fibrocartilaginous embolism inducing paralysis. Child’s Nerv. Syst. 2020, 36, 441–446. [Google Scholar] [CrossRef]
- Riley, D.S.; Barber, M.S.; Kienle, G.S.; Aronson, J.K.; von Schoen-Angerer, T.; Tugwell, P.; Kiene, H.; Helfand, M.; Altman, D.G.; Sox, H.; et al. CARE guidelines for case reports: Explanation and elaboration document. J. Clin. Epidemiol. 2017, 89, 218–235. [Google Scholar] [CrossRef]
- Weidauer, S.; Nichtweiß, M.; Hattingen, E.; Berkefeld, J. Spinal cord ischemia: Aetiology, clinical syndromes and imaging features. Neuroradiology 2015, 57, 241–257. [Google Scholar] [CrossRef] [PubMed]
- Sandson, T.A.; Friedman, J.H. Spinal cord infarction. Report of 8 cases and review of the literature. Medicine 1989, 68, 282–292. [Google Scholar] [PubMed]
- Nedeltchev, K.; Loher, T.J.; Stepper, F.; Arnold, M.; Schroth, G.; Mattle, H.P.; Sturzenegger, M.; Kanellopoulos, G.K.; Kato, H.; Hsu, C.Y.; et al. Long-Term Outcome of Acute Spinal Cord Ischemia Syndrome. Stroke 2004, 35, 560–565. [Google Scholar] [CrossRef]
- Mateen, F.J.; Monrad, P.A.; Hunderfund, A.N.L.; Robertson, C.E.; Sorenson, E.J. Clinically suspected fibrocartilaginous embolism: Clinical characteristics, treatments, and outcomes. Eur. J. Neurol. 2011, 18, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Bansal, S.; Brown, W.; Dayal, A.; Carpenter, J.L. Posterior Spinal Cord Infarction Due to Fibrocartilaginous Embolization in a 16-Year-Old Athlete. Pediatrics 2014, 134, e289–e292. [Google Scholar] [CrossRef]
- Tosi, L.; Rigoli, G.; Beltramello, A. Fibrocartilaginous embolism of the spinal cord: A clinical and pathogenetic reconsideration. J. Neurol. Neurosurg. Psychiatry 1996, 60, 55–60. [Google Scholar] [CrossRef]
- New, P.W. A Narrative Review of Pediatric Nontraumatic Spinal Cord Dysfunction. Top. Spinal Cord Inj. Rehabil. 2019, 25, 112–120. [Google Scholar] [CrossRef]
- Wang, J.-Z.; Yang, M.; Meng, M.; Li, Z.-H. Clinical characteristics and treatment of spinal cord injury in children and adolescents. Chin. J. Traumatol. 2023, 26, 8–13. [Google Scholar] [CrossRef]
- Alexander, R.T.; Cummings, T.J. Pathologic Quiz Case: Acute-Onset Paraplegia in a 60-Year-Old Woman. Arch. Pathol. Lab. Med. 2003, 127, 1047–1048. [Google Scholar] [CrossRef] [PubMed]
- Ciceri, E.F.; Opancina, V.; Pellegrino, C.; Scarabelli, A.; Botturi, A.G.; Bersano, A.; D’arrigo, S.; Erbetta, A.; Chiapparini, L. Fibrocartilaginous embolism: A rare cause leading to spinal cord infarction? Neurol. Sci. 2023, 44, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Eid, R.; Raj, A.; Farber, D.; Puri, V.; Bertolone, S. Spinal Cord Infarction in Hemoglobin SC Disease as an Amusement Park Accident. Pediatrics 2016, 138, e20154020. [Google Scholar] [CrossRef]
- Han, J.J.; Massagli, T.L.; Jaffe, K.M. Fibrocartilaginous embolism—An uncommon cause of spinal cord infarction: A case report and review of the literature1. Arch. Phys. Med. Rehabil. 2004, 85, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Davis, G.A.; Klug, G.L. Acute-onset nontraumatic paraplegia in childhood: Fibrocartilaginous embolism or acute myelitis? Child’s Nerv. Syst. 2000, 16, 551–554. [Google Scholar] [CrossRef] [PubMed]
- Liskova, Z.; Lehotska, V.; Liska, M.; Mikula, P. Fibrocartilaginous Embolization—A Rare Cause of Spinal Cord Infarction: Case Report. J. Radiol. Case Rep. 2018, 12, 1–9. [Google Scholar] [CrossRef]
- Shah, S.; Bryant, P. Fibrocartilaginous emboli in the pediatric population: The role of rehabilitation in facilitating functional recovery. J. Pediatr. Rehabil. Med. 2018, 11, 53–56. [Google Scholar] [CrossRef]
- Verhey, L.H.; Banwell, B.L. Chapter 104—Inflammatory, vascular, and infectious myelopathies in children. In Handbook of Clinical Neurology; Dulac, O., Lassonde, M., Sarnat, H.B., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 112, pp. 999–1017. [Google Scholar] [CrossRef]
- Manara, R.; Calderone, M.; Severino, M.S.; Citton, V.; Toldo, I.; Laverda, A.M.; Sartori, S. Spinal Cord Infarction Due to Fibrocartilaginous Embolization: The Role of Diffusion Weighted Imaging and Short-Tau Inversion Recovery Sequences. J. Child Neurol. 2010, 25, 1024–1028. [Google Scholar] [CrossRef]
- Elsayh, K.I.; Zahran, A.M.; El-Abaseri, T.B.; Mohamed, A.O.; El-Metwally, T.H. Hypoxia Biomarkers, Oxidative Stress, and Circulating Microparticles in Pediatric Patients with Thalassemia in Upper Egypt. Clin. Appl. Thromb./Hemost. 2014, 20, 536–545. [Google Scholar] [CrossRef]
- Olgar, S.; Kara, A.; Hicyilmaz, H.; Balta, N.; Canatan, D. Evaluation of angiogenesis with vascular endothelial growth factor in patients with thalassemia major. Pediatr. Int. 2010, 52, 247–251. [Google Scholar] [CrossRef]
- Shitrit, D.; Tamary, H.; Koren, A.; Levin, C.; Bargil-Shitrit, A.; Sulkes, J.; Kramer, M.R. Correlation of vascular endothelial growth factor with the severity of thalassemia intermedia. Blood Coagul. Fibrinolysis 2008, 19, 611–614. [Google Scholar] [CrossRef]
- Kerzhner, O.; Berla, E.; Har-Even, M.; Ratmansky, M.; Goor-Aryeh, I. Consistency of inconsistency in long-COVID-19 pain symptoms persistency: A systematic review and meta-analysis. Pain Pract. 2023. [Google Scholar] [CrossRef]
- Khoueiry, M.; Moussa, H.; Sawaya, R. Spinal cord infarction in a young adult: What is the culprit? J. Spinal Cord Med. 2021, 44, 1015–1018. [Google Scholar] [CrossRef]
- Rodrigues, M.; Beça, G.; Almeida, A.; Natário, I.; Vilabril, F.; Pereira, M.; Barreto, J.; Dias, L.; Gandarez, F. Spinal cord infarction in children: Can gymnastics be a cause? J. Pediatr. Rehabil. Med. 2021, 14, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Reisner, A.; Gary, M.F.; Chern, J.J.; Grattan-Smith, J.D. Spinal cord infarction following minor trauma in children: Fibrocartilaginous embolism as a putative cause. J. Neurosurg. Pediatr. 2013, 11, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Draganich, C.; Wenzel, L.R. Fibrocartilagenous embolism case series: Is it a zebra? Spinal Cord Ser. Cases 2021, 7, 28. [Google Scholar] [CrossRef] [PubMed]
- Roberts, T.T.; Leonard, G.R.; Cepela, D.J. Classifications In Brief: American Spinal Injury Association (ASIA) Impairment Scale. Clin. Orthop. Relat. Res. 2017, 475, 1499–1504. [Google Scholar] [CrossRef]
- Nas, K.; Yazmalar, L.; Şah, V.; Aydin, A.; Öneş, K. Rehabilitation of spinal cord injuries. World J. Orthop. 2015, 6, 8–16. [Google Scholar] [CrossRef]
- Van Middendorp, J.J.; Goss, B.; Urquhart, S.; Atresh, S.; Williams, R.P.; Schuetz, M. Diagnosis and Prognosis of Traumatic Spinal Cord Injury. Glob. Spine J. 2011, 1, 1–7. [Google Scholar] [CrossRef]

| Diagnostic Studies Assessments | Time after Infarct | |||
|---|---|---|---|---|
| 16 Days | 2.5 Months | 4 Months | 16 Months | |
| ASIA exam | ||||
| UERMS | 25 | 25 | 25 | 25 |
| UELMS | 25 | 25 | 25 | 25 |
| UEMS Total | 50 | 50 | 50 | 50 |
| LERMS | 0 | 7 | 7 | 18 |
| LELMS | 18 | 25 | 25 | 25 |
| LEMS Total | 18 | 32 | 32 | 43 |
| LTR | 39 | 56 | 56 | 56 |
| LTL | 39 | 56 | 56 | 56 |
| LT Total | 78 | 112 | 112 | 112 |
| PPR | 24 | 28 | 33 | 41 |
| PPL | 25 | 30 | 43 | 45 |
| PP Total | 49 | 58 | 76 | 86 |
| VAC | Yes | Yes | Yes | Yes |
| DAP | Yes | Yes | Yes | Yes |
| NLI | T4 | T4 | T4 | T4 |
| AIS | C | D | D | D |
| SCI classification | T4 AIS C | T4 AIS D | T4 AIS D | T4 AIS D |
| SCIM | 45 | N/A | 66 | 74 |
| UDS | ||||
| Compliance |
| N/A |
|
|
| Voluntary bladder contraction | No | N/A | Yes | Yes |
| Voluntary voiding | No | N/A | Able to produce inconsistent stream with interrupted voiding. | Able to produce inconsistent stream with interrupted voiding. |
| VUR | No | N/A | No | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berla, E.; Kerzhner, O.; Caspi, T.; Shaklai, S.; Michaeli, D. Acute Presentation and Long-Term Rehabilitation Follow-Up of Ischemic Myelopathy Due to Clinically Suspected Fibrocartilaginous Embolism in an Adolescent Male: A Case Report and Review. Neurol. Int. 2023, 15, 1273-1289. https://doi.org/10.3390/neurolint15040080
Berla E, Kerzhner O, Caspi T, Shaklai S, Michaeli D. Acute Presentation and Long-Term Rehabilitation Follow-Up of Ischemic Myelopathy Due to Clinically Suspected Fibrocartilaginous Embolism in an Adolescent Male: A Case Report and Review. Neurology International. 2023; 15(4):1273-1289. https://doi.org/10.3390/neurolint15040080
Chicago/Turabian StyleBerla, Einat, Oleg Kerzhner, Tomm Caspi, Sharon Shaklai, and Dianne Michaeli. 2023. "Acute Presentation and Long-Term Rehabilitation Follow-Up of Ischemic Myelopathy Due to Clinically Suspected Fibrocartilaginous Embolism in an Adolescent Male: A Case Report and Review" Neurology International 15, no. 4: 1273-1289. https://doi.org/10.3390/neurolint15040080
APA StyleBerla, E., Kerzhner, O., Caspi, T., Shaklai, S., & Michaeli, D. (2023). Acute Presentation and Long-Term Rehabilitation Follow-Up of Ischemic Myelopathy Due to Clinically Suspected Fibrocartilaginous Embolism in an Adolescent Male: A Case Report and Review. Neurology International, 15(4), 1273-1289. https://doi.org/10.3390/neurolint15040080





