Dynamics of Lateral Habenula–Ventral Tegmental Area Microcircuit on Pain-Related Cognitive Dysfunctions
Abstract
1. Introduction
2. Lateral Habenula
2.1. The Role of the LHb in Pain Processing
2.2. The Role of the LHb in Mood and Cognition
3. Ventral Tegmental Area
3.1. The Role of the VTA in Pain Processing
3.2. The Role of the VTA in Mood and Cognition
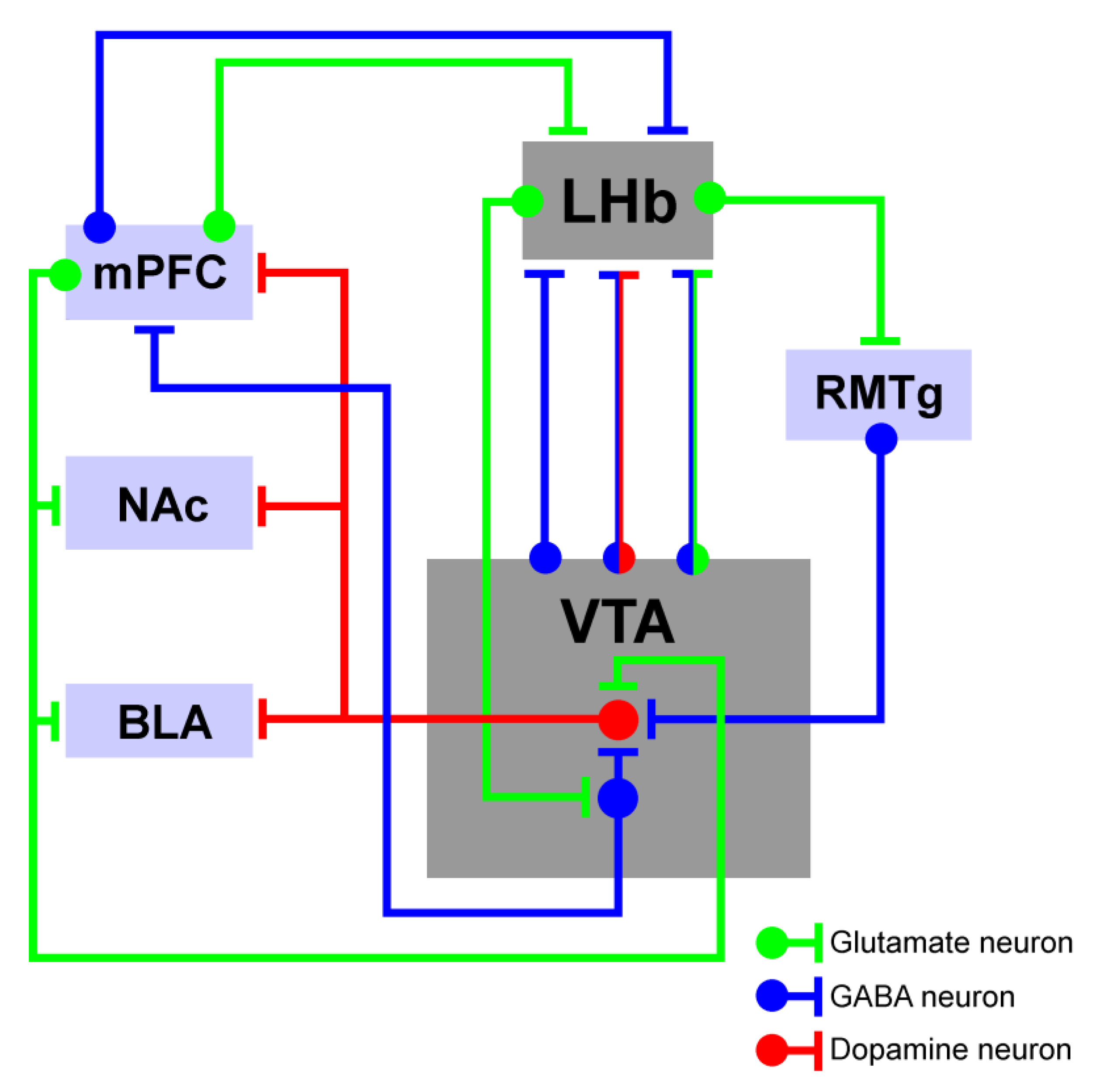
4. LHb-to-VTA Pathway Structural Connectivity
4.1. The Influence of LHb-Dependent Activity on the VTA
4.2. The Influence of VTA-Dependent Activity on the LHb
4.3. The Impact of the LHb–VTA Microcircuit Dynamics on Cognitive Activity
| Cognitive Variable | Experimental Model | Manipulation | Main Findings | Reference |
|---|---|---|---|---|
| Reward/ aversion | Primate | Electrical LHb Stimulation | LHb electrical stimulation elicits an inhibition of DA neurons; LHb input plays an important role in determining the reward-related activity of DA neurons | Matsumoto et al., 2007 [9] |
| Reward/ aversion | Rat | LHb electrical stimulation; Fasciculus retroflexus (Fr) lesion | LHb electrical stimulation elicits an inhibition of the VTA and substantia nigra (SN) DA neurons; Fr lesion attenuates LHb inhibition over DA neurons | Ji and Shepard, 2007 [19] |
| Reward/ aversion | Rat | Electrical/chemical modulation of the LHb | Inhibition of LHb increases DA release in the PFC, NAc, and dorsal striatum; LHb stimulation produces minimal opposite effects | Lecourtier et al., 2008 [151] |
| Reward/ aversion | VGLUT2-Cre mice | Activation of VTA glutamatergic neurons | VTA VGLUT2-mesohabenular neurons play a role in aversion by activating LHb glutamatergic neurons | Root et al., 2014 [152] |
| Reward/ aversion | TH-Cre mice | Activation of VTA TH-expressing neurons | This activation produces reward-related behavioral phenotypes that require GABAA signaling in the LHb | Stamatakis and Stuber, 2012 [159] |
| Reward/ aversion | VGLUT2-Cre mice | Activation of VTA glutamatergic neurons | This activation induces positive reinforcement in instrumental behavioral assays by brief stimulation and avoidance in continuous stimulation | Yoo et al., 2016 [87] |
| Reward/aversion | Rat | Modulation of LHb, RMTg, or VTA activity | Dissection of the role of this brain area in the precise coordination of DA signals that regulate future reward–risk-based responses | Stopper et al., 2014 [37] |
| Attention | Rat | Bilateral LHb lesion | This lesion promotes attention deficits through premature or impulsive responses | Lecourtier and Kelly, 2005 [70] |
| Attention | TH-Cre rat | Chemogenetic activation of VTA or SN DA neurons | Activation of VTA/SN DA neurons promotes attention deficits, without affecting impulsivity | Boekhoudt et al., 2017 [156] |
| Avoidance | VGLUT2-Cre mice | Activation of LHb neurons projecting to VTA | This activation increases aversion after LHb light stimulation; aversion for light conditioned room blocked by D1r antagonist in mPFC | Lammel et al., 2012 [38] |
| Avoidance | Mice | VTA stimulation | VTA stimulation impairs avoidance acquisition, without affecting memory retrieval or motivation | Shumake et al., 2010 [80] |
| Avoidance | Gerbils | LHb stimulation | LHb stimulation impairs acquisition of avoidance learning, without affecting consolidation or retrieval | Ilango et al., 2013 [157] |
| Avoidance | Mice | Activation of LHb glutamatergic terminals in the RMTg | This activation promotes active/passive and conditioned behavioral avoidance | Stamatakis and Stuber, 2012 [159] |
| Contextual memory | Rat | Blockade or activation of LHb DA D1r | This manipulation impairs DA D1r signaling in the LHb and affects acquisition of contextual fear memory | Chan et al., 2017a [164] |
| Contextual memory | Rat | Blockade or activation of LHb DA D1r | This manipulation promotes anxiety-like behavior and decreases depressive-like behavior; impaired aversive memory acquisition | Chan et al., 2017a [164] |
| Contextual memory | Rat | Transient inactivation of VTA | This manipulation impairs hippocampal long-term memory | Ghanbarian and Motamedi, 2013 [141] |
5. Future Directions and Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, J.; Li, Y.; Zhang, B.; Shen, X.; Zhao, H. Why depression and pain often coexist and mutually reinforce: Role of the lateral habenula. Exp. Neurol. 2016, 284, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Y.; Xuan, C.; Li, Y.; Piao, L.; Li, J.; Zhao, H. Role of the Lateral Habenula in Pain-Associated Depression. Front. Behav. Neurosci. 2017, 11, 31. [Google Scholar] [CrossRef] [PubMed]
- Borsook, D.; Linnman, C.; Faria, V.; Strassman, A.M.; Becerra, L.; Elman, I. Reward deficiency and anti-reward in pain chronification. Neurosci. Biobehav. Rev. 2016, 68, 282–297. [Google Scholar] [CrossRef] [PubMed]
- Goutagny, R.; Loureiro, M.; Jackson, J.; Chaumont, J.; Williams, S.; Isope, P.; Kelche, C.; Cassel, J.-C.; Lecourtier, L. Interactions between the lateral habenula and the hippocampus: Implication for spatial memory processes. Neuropsychopharmacology 2013, 38, 2418–2426. [Google Scholar] [CrossRef] [PubMed]
- Jacinto, L.R.; Mata, R.; Novais, A.; Marques, F.; Sousa, N. The habenula as a critical node in chronic stress-related anxiety. Exp. Neurol. 2017, 289, 46–54. [Google Scholar] [CrossRef]
- Craig, A.D. Distribution of trigeminothalamic and spinothalamic lamina I terminations in the cat. Somatosens. Mot. Res. 2003, 20, 209–222. [Google Scholar] [CrossRef]
- Kang, S.; Li, J.; Zuo, W.; Chen, P.; Gregor, D.; Fu, R.; Han, X.; Bekker, A.; Ye, J.-H. Downregulation of M-channels in lateral habenula mediates hyperalgesia during alcohol withdrawal in rats. Sci. Rep. 2019, 9, 2714. [Google Scholar] [CrossRef]
- Lehner, M.; Taracha, E.; Skorzewska, A.; Wislowska, A.; Zienowicz, M.; Maciejak, P.; Szyndler, J.; Bidzinski, A.; Plaznik, A. Sensitivity to pain and c-Fos expression in brain structures in rats. Neurosci. Lett. 2004, 370, 74–79. [Google Scholar] [CrossRef]
- Matsumoto, M.; Hikosaka, O. Lateral habenula as a source of negative reward signals in dopamine neurons. Nature 2007, 447, 1111–1115. [Google Scholar] [CrossRef]
- Baker, P.M.; Mizumori, S.J.Y. Control of behavioral flexibility by the lateral habenula. Pharmacol. Biochem. Behav. 2017, 162, 62–68. [Google Scholar] [CrossRef]
- Morales, M.; Margolis, E.B. Ventral tegmental area: Cellular heterogeneity, connectivity and behaviour. Nat. Rev. Neurosci. 2017, 18, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Oster, A.; Faure, P.; Gutkin, B.S. Mechanisms for multiple activity modes of VTA dopamine neurons. Front. Comput. Neurosci. 2015, 9, 95. [Google Scholar] [CrossRef] [PubMed]
- Martig, A.K.; Mizumori, S.J. Ventral tegmental area disruption selectively affects CA1/CA2 but not CA3 place fields during a differential reward working memory task. Hippocampus 2011, 21, 172–184. [Google Scholar] [CrossRef]
- Creed, M.C.; Ntamati, N.R.; Tan, K.R. VTA GABA neurons modulate specific learning behaviors through the control of dopamine and cholinergic systems. Front. Behav. Neurosci. 2014, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- Hentall, I.D.; Kim, J.L.; Gollapudi, L. Responses of neurons in the ventromedial midbrain to noxious mechanical stimuli. Neurosci. Lett. 1991, 133, 215–218. [Google Scholar] [CrossRef] [PubMed]
- Altier, N.; Stewart, J. Opioid receptors in the ventral tegmental area contribute to stress-induced analgesia in the formalin test for tonic pain. Brain Res. 1996, 718, 203–206. [Google Scholar] [CrossRef]
- Sotres-Bayón, F.; Torres-López, E.; López-Avila, A.; del Angel, R.; Pellicer, F. Lesion and electrical stimulation of the ventral tegmental area modify persistent nociceptive behavior in the rat. Brain Res. 2001, 898, 342–349. [Google Scholar] [CrossRef]
- Takeda, R.; Ikeda, T.; Tsuda, F.; Abe, H.; Hashiguchi, H.; Ishida, Y.; Nishimori, T. Unilateral lesions of mesostriatal dopaminergic pathway alters the withdrawal response of the rat hindpaw to mechanical stimulation. Neurosci. Res. 2005, 52, 31–36. [Google Scholar] [CrossRef]
- Ji, H.; Shepard, P.D. Lateral habenula stimulation inhibits rat midbrain dopamine neurons through a GABA(A) receptor-mediated mechanism. J. Neurosci. 2007, 27, 6923–6930. [Google Scholar] [CrossRef]
- Christoph, G.R.; Leonzio, R.J.; Wilcox, K.S. Stimulation of the lateral habenula inhibits dopamine-containing neurons in the substantia nigra and ventral tegmental area of the rat. J. Neurosci. 1986, 6, 613–619. [Google Scholar] [CrossRef]
- Niikura, K.; Narita, M.; Butelman, E.R.; Kreek, M.J.; Suzuki, T. Neuropathic and chronic pain stimuli downregulate central μ-opioid and dopaminergic transmission. Trends. Pharmacol. Sci. 2010, 31, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zhang, B.-L.; Yang, S.-J.; Rusak, B. The role of lateral habenula–dorsal raphe nucleus circuits in higher brain functions and psychiatric illness. Behav. Brain Res. 2015, 277, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Robbins, T.W. From behavior to cognition: Functions of mesostriatal, mesolimbic, and mesocortical dopamine systems. In Dopamine Handbook; Oxford University Press: Oxford, UK, 2010. [Google Scholar]
- Hauser, T.U.; Eldar, E.; Dolan, R.J. Separate mesocortical and mesolimbic pathways encode effort and reward learning signals. Proc. Natl. Acad. Sci. USA 2017, 114, E7395–E7404. [Google Scholar] [CrossRef] [PubMed]
- Shelton, L.; Becerra, L.; Borsook, D. Unmasking the mysteries of the habenula in pain and analgesia. Prog. Neurobiol. 2012, 96, 208–219. [Google Scholar] [CrossRef]
- Shelton, L.; Pendse, G.; Maleki, N.; Moulton, E.A.; Lebel, A.; Becerra, L.; Borsook, D. Mapping pain activation and connectivity of the human habenula. J. Neurophysiol. 2012, 107, 2633–2648. [Google Scholar] [CrossRef]
- Cohen, S.R.; Melzack, R. Habenular stimulation produces analgesia in the formalin test. Neurosci. Lett. 1986, 70, 165–169. [Google Scholar] [CrossRef]
- Zhang, L.; Hernandez, V.S.; Vazquez-Juarez, E.; Chay, F.K.; Barrio, R.A. Thirst Is Associated with Suppression of Habenula Output and Active Stress Coping: Is there a Role for a Non-canonical Vasopressin-Glutamate Pathway? Front. Neural. Circuits 2016, 10, 13. [Google Scholar] [CrossRef]
- Seo, J.-S.; Zhong, P.; Liu, A.; Yan, Z.; Greengard, P. Elevation of p11 in lateral habenula mediates depression-like behavior. Mol. Psychiatry 2018, 23, 1113–1119. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, H.; Hu, J.; Hu, H. Lateral habenula in the pathophysiology of depression. Curr. Opin. Neurobiol. 2018, 48, 90–96. [Google Scholar] [CrossRef]
- Matsumoto, M.; Hikosaka, O. Representation of negative motivational value in the primate lateral habenula. Nat. Neurosci. 2009, 12, 77–84. [Google Scholar] [CrossRef]
- Hong, S.; Hikosaka, O. The globus pallidus sends reward-related signals to the lateral habenula. Neuron 2008, 60, 720–729. [Google Scholar] [CrossRef]
- Flanigan, M.; Aleyasin, H.; Takahashi, A.; Golden, S.A.; Russo, S.J. An emerging role for the lateral habenula in aggressive behavior. Pharmacol. Biochem. Behav. 2017, 162, 79–86. [Google Scholar] [CrossRef]
- Mathis, V.; Cosquer, B.; Avallone, M.; Cassel, J.-C.; Lecourtier, L. Excitatory Transmission to the Lateral Habenula Is Critical for Encoding and Retrieval of Spatial Memory. Neuropsychopharmacology 2015, 40, 2843–2851. [Google Scholar] [CrossRef] [PubMed]
- Mathis, V.; Barbelivien, A.; Majchrzak, M.; Mathis, C.; Cassel, J.-C.; Lecourtier, L. The Lateral Habenula as a Relay of Cortical Information to Process Working Memory. Cereb. Cortex 2017, 27, 5485–5495. [Google Scholar] [CrossRef] [PubMed]
- Hikosaka, O. The habenula: From stress evasion to value-based decision-making. Nat. Rev. Neurosci. 2010, 11, 503–513. [Google Scholar] [CrossRef]
- Stopper, C.M.; Tse, M.T.L.; Montes, D.R.; Wiedman, C.R.; Floresco, S.B. Overriding phasic dopamine signals redirects action selection during risk/reward decision making. Neuron 2014, 84, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Lammel, S.; Lim, B.K.; Ran, C.; Huang, K.W.; Betley, M.J.; Tye, K.M.; Deisseroth, K.; Malenka, R.C. Input-specific control of reward and aversion in the ventral tegmental area. Nature 2012, 491, 212–217. [Google Scholar] [CrossRef]
- Cohen, J.Y.; Haesler, S.; Vong, L.; Lowell, B.B.; Uchida, N. Neuron-type-specific signals for reward and punishment in the ventral tegmental area. Nature 2012, 482, 85–88. [Google Scholar] [CrossRef]
- Bano-Otalora, B.; Piggins, H.D. Contributions of the lateral habenula to circadian timekeeping. Pharmacol. Biochem. Behav. 2017, 162, 46–54. [Google Scholar] [CrossRef]
- Vincenz, D.; Wernecke, K.E.A.; Fendt, M.; Goldschmidt, J. Habenula and interpeduncular nucleus differentially modulate predator odor-induced innate fear behavior in rats. Behav. Brain Res. 2017, 332, 164–171. [Google Scholar] [CrossRef]
- Stamatakis, A.M.; Van Swieten, M.; Basiri, M.L.; Blair, G.A.; Kantak, P.; Stuber, G.D. Lateral Hypothalamic Area Glutamatergic Neurons and Their Projections to the Lateral Habenula Regulate Feeding and Reward. J. Neurosci. 2016, 36, 302–311. [Google Scholar] [CrossRef]
- Fore, S.; Yaksi, E. Habenula: A Role in Brain State Transitions during Coping Behavior. Curr. Biol. 2019, 29, R692–R694. [Google Scholar] [CrossRef] [PubMed]
- Pobbe, R.L.; Zangrossi, H., Jr. Involvement of the lateral habenula in the regulation of generalized anxiety- and panic-related defensive responses in rats. Life Sci. 2008, 82, 1256–1261. [Google Scholar] [CrossRef] [PubMed]
- Goto, M.; Canteras, N.S.; Burns, G.; Swanson, L.W. Projections from the subfornical region of the lateral hypothalamic area. J. Comp. Neurol. 2005, 493, 412–438. [Google Scholar] [CrossRef]
- Ferraro, G.; Montalbano, M.E.; Sardo, P.; La Grutta, V. Lateral habenular influence on dorsal raphe neurons. Brain Res. Bull. 1996, 41, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Mahieux, G.; Benabid, A.L. Naloxone-reversible analgesia induced by electrical stimulation of the habenula in the rat. Brain Res. 1987, 406, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.M.; Hoffman, D.; Benabid, A.L. Simultaneous recording of spontaneous activities and nociceptive responses from neurons in the pars compacta of substantia nigra and in the lateral habenula. Eur. J. Neurosci. 1996, 8, 1474–1478. [Google Scholar] [CrossRef]
- Shen, X.; Ruan, X.; Zhao, H. Stimulation of midbrain dopaminergic structures modifies firing rates of rat lateral habenula neurons. PLoS ONE 2012, 7, e34323. [Google Scholar] [CrossRef]
- Erpelding, N.; Sava, S.; Simons, L.E.; Lebel, A.; Serrano, P.; Becerra, L.; Borsook, D. Habenula functional resting-state connectivity in pediatric CRPS. J. Neurophysiol. 2014, 111, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wang, S. Different effect of L-glutamate microinjection into medial or lateral habenular nucleu on pain threshold. Sheng Li Xue Bao 1995, 47, 292–296. [Google Scholar]
- Smith, W.J.; Stewart, J.; Pfaus, J.G. Tail pinch induces fos immunoreactivity within several regions of the male rat brain: Effects of age. Physiol. Behav. 1997, 61, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Dai, D.; Li, W.; Chen, A.; Gao, X.-F.; Xiong, L. Lateral Habenula and Its Potential Roles in Pain and Related Behaviors. ACS Chem. Neurosci. 2022, 13, 1108–1118. [Google Scholar] [CrossRef] [PubMed]
- Purvis, E.M.; Klein, A.K.; Ettenberg, A. Lateral habenular norepinephrine contributes to states of arousal and anxiety in male rats. Behav. Brain Res. 2018, 347, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Fomberstein, K.; Qadri, S.; Ramani, R. Functional MRI and pain. Curr. Opin. Anaesthesiol. 2013, 26, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Boulos, L.J.; Darcq, E.; Kieffer, B.L. Translating the Habenula-From Rodents to Humans. Biol. Psychiatry 2017, 81, 296–305. [Google Scholar] [CrossRef]
- Sacher, J.; Neumann, J.; Funfstuck, T.; Soliman, A.; Villringer, A.; Schroeter, M.L. Mapping the depressed brain: A meta-analysis of structural and functional alterations in major depressive disorder. J. Affect. Disord. 2012, 140, 142–148. [Google Scholar] [CrossRef]
- Ranft, K.; Dobrowolny, H.; Krell, D.; Bielau, H.; Bogerts, B.; Bernstein, H.-G. Evidence for structural abnormalities of the human habenular complex in affective disorders but not in schizophrenia. Psychol. Med. 2010, 40, 557–567. [Google Scholar] [CrossRef]
- Savitz, J.B.; Nugent, A.C.; Bogers, W.; Roiser, J.P.; Bain, E.E.; Neumeister, A.; Zarate, C.A., Jr.; Manji, H.K.; Cannon, D.M.; Marrett, S.; et al. Habenula volume in bipolar disorder and major depressive disorder: A high-resolution magnetic resonance imaging study. Biol. Psychiatry 2011, 69, 336–343. [Google Scholar] [CrossRef]
- Johnston, B.A.; Steele, J.D.; Tolomeo, S.; Christmas, D.; Matthews, K. Structural MRI-Based Predictions in Patients with Treatment-Refractory Depression (TRD). PLoS ONE 2015, 10, e0132958. [Google Scholar] [CrossRef]
- Mirrione, M.M.; Schulz, D.; Lapidus, K.A.B.; Zhang, S.; Goodman, W.; Henn, F.A. Increased metabolic activity in the septum and habenula during stress is linked to subsequent expression of learned helplessness behavior. Front. Hum. Neurosci. 2014, 8, 29. [Google Scholar] [CrossRef]
- Huang, Y.; Sun, B.; Debarros, J.; Zhang, C.; Zhan, S.; Li, D.; Zhang, C.; Wang, T.; Huang, P.; Lai, Y.; et al. Increased theta/alpha synchrony in the habenula-prefrontal network with negative emotional stimuli in human patients. Elife 2021, 10, e65444. [Google Scholar] [CrossRef] [PubMed]
- Mao, C.P.; Wu, Y.; Yang, H.J.; Qin, J.; Song, Q.C.; Zhang, B.; Zhou, X.Q.; Zhang, L.; Sun, H.H. Altered habenular connectivity in chronic low back pain: An fMRI and machine learning study. Hum. Brain Mapp. 2023, 44, 4407–4421. [Google Scholar] [CrossRef] [PubMed]
- Geisler, S.; Trimble, M. The lateral habenula: No longer neglected. CNS Spectr. 2008, 13, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Manssuer, L.; Ding, Q.; Zhang, Y.; Gong, H.; Liu, W.; Yang, R.; Zhang, C.; Zhao, Y.; Pan, Y.; Zhan, S.; et al. Risk and aversion coding in human habenula high gamma activity. Brain 2023, 146, 2642–2653. [Google Scholar] [CrossRef] [PubMed]
- Thornton, E.W.; Murray, M.; Connors-Eckenrode, T.; Haun, F. Dissociation of behavioral changes in rats resulting from lesions of the habenula versus fasciculus retroflexus and their possible anatomical substrates. Behav. Neurosci. 1994, 108, 1150–1162. [Google Scholar] [CrossRef]
- Thornton, E.W.; Evans, J.C. The role of habenular nuclei in the selection of behavioral strategies. Physiol. Psychol. 1982, 10, 361–367. [Google Scholar] [CrossRef]
- Thornton, E.W.; Bradbury, G.E.; Davies, C. Increased immobility in an automated forced swimming test following lesion of the habenula in rats: Absence of evidence for a contribution from motor impairment. Behav. Neurosci. 1990, 104, 37–43. [Google Scholar] [CrossRef]
- Lecourtier, L.; Neijt, H.C.; Kelly, P.H. Habenula lesions cause impaired cognitive performance in rats: Implications for schizophrenia. Eur. J. Neurosci. 2004, 19, 2551–2560. [Google Scholar] [CrossRef]
- Lecourtier, L.; Kelly, P.H. Bilateral lesions of the habenula induce attentional disturbances in rats. Neuropsychopharmacology 2005, 30, 484–496. [Google Scholar] [CrossRef]
- Tomaiuolo, M.; Gonzalez, C.; Medina, J.H.; Piriz, J. Lateral Habenula determines long-term storage of aversive memories. Front. Behav. Neurosci. 2014, 8, 170. [Google Scholar] [CrossRef]
- Du, C.X.; Liu, J.; Guo, Y.; Zhang, L.; Zhang, Q.J. Lesions of the lateral habenula improve working memory performance in hemiparkinsonian rats. Neurosci. Lett. 2018, 662, 162–166. [Google Scholar] [CrossRef]
- Kim, U.; Lee, T. Topography of descending projections from anterior insular and medial prefrontal regions to the lateral habenula of the epithalamus in the rat. Eur. J. Neurosci. 2012, 35, 1253–1269. [Google Scholar] [CrossRef]
- Metz, A.E.; Yau, H.-J.; Centeno, M.V.; Apkarian, A.V.; Martina, M. Morphological and functional reorganization of rat medial prefrontal cortex in neuropathic pain. Proc. Natl. Acad. Sci. USA 2009, 106, 2423–2428. [Google Scholar] [CrossRef] [PubMed]
- Cardoso-Cruz, H.; Laranjeira, I.; Monteiro, C.; Galhardo, V. Altered prefrontal-striatal theta-band oscillatory dynamics underlie working memory deficits in neuropathic pain rats. Eur. J. Pain 2022, 26, 1546–1568. [Google Scholar] [CrossRef] [PubMed]
- Cardoso-Cruz, H.; Paiva, P.; Monteiro, C.; Galhardo, V. Selective optogenetic inhibition of medial prefrontal glutamatergic neurons reverses working memory deficits induced by neuropathic pain. Pain 2019, 160, 805–823. [Google Scholar] [CrossRef] [PubMed]
- Stopper, C.M.; Floresco, S.B. What’s better for me? Fundamental role for lateral habenula in promoting subjective decision biases. Nat. Neurosci. 2014, 17, 33–35. [Google Scholar] [CrossRef] [PubMed]
- van der Meulen, J.A.J.; Joosten, R.N.J.M.A.; de Bruin, J.P.C.; Feenstra, M.G.P. Dopamine and noradrenaline efflux in the medial prefrontal cortex during serial reversals and extinction of instrumental goal-directed behavior. Cereb. Cortex 2007, 17, 1444–1453. [Google Scholar] [CrossRef][Green Version]
- Baker, P.M.; Oh, S.E.; Kidder, K.S.; Mizumori, S.J.Y. Ongoing behavioral state information signaled in the lateral habenula guides choice flexibility in freely moving rats. Front. Behav. Neurosci. 2015, 9, 295. [Google Scholar] [CrossRef] [PubMed]
- Shumake, J.; Ilango, A.; Scheich, H.; Wetzel, W.; Ohl, F.W. Differential neuromodulation of acquisition and retrieval of avoidance learning by the lateral habenula and ventral tegmental area. J. Neurosci. 2010, 30, 5876–5883. [Google Scholar] [CrossRef]
- Poller, W.C.; Madai, V.I.; Bernard, R.; Laube, G.; Veh, R.W. A glutamatergic projection from the lateral hypothalamus targets VTA-projecting neurons in the lateral habenula of the rat. Brain Res. 2013, 1507, 45–60. [Google Scholar] [CrossRef]
- Huang, S.; Borgland, S.L.; Zamponi, G.W. Dopaminergic modulation of pain signals in the medial prefrontal cortex: Challenges and perspectives. Neurosci. Lett. 2019, 702, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Jarcho, J.M.; Mayer, E.A.; Jiang, Z.K.; Feier, N.A.; London, E.D. Pain, affective symptoms, and cognitive deficits in patients with cerebral dopamine dysfunction. Pain 2012, 153, 744–754. [Google Scholar] [CrossRef] [PubMed]
- Nair-Roberts, R.G.; Chatelain-Badie, S.D.; Benson, E.; White-Cooper, H.; Bolam, J.P.; Ungless, M.A. Stereological estimates of dopaminergic, GABAergic and glutamatergic neurons in the ventral tegmental area, substantia nigra and retrorubral field in the rat. Neuroscience 2008, 152, 1024–1031. [Google Scholar] [CrossRef] [PubMed]
- Tritsch, N.X.; Ding, J.B.; Sabatini, B.L. Dopaminergic neurons inhibit striatal output through non-canonical release of GABA. Nature 2012, 490, 262–266. [Google Scholar] [CrossRef]
- Mingote, S.; Chuhma, N.; Kusnoor, S.V.; Field, B.; Deutch, A.Y.; Rayport, S. Functional Connectome Analysis of Dopamine Neuron Glutamatergic Connections in Forebrain Regions. J. Neurosci. 2015, 35, 16259–16271. [Google Scholar] [CrossRef]
- Yoo, J.H.; Zell, V.; Gutierrez-Reed, N.; Wu, J.; Ressler, R.; Shenasa, M.A.; Johnson, A.B.; Fife, K.H.; Faget, L.; Hnasko, T.S. Ventral tegmental area glutamate neurons co-release GABA and promote positive reinforcement. Nat. Commun. 2016, 7, 13697. [Google Scholar] [CrossRef]
- Margolis, E.B.; Lock, H.; Hjelmstad, G.O.; Fields, H.L. The ventral tegmental area revisited: Is there an electrophysiological marker for dopaminergic neurons? J. Physiol. 2006, 577, 907–924. [Google Scholar] [CrossRef]
- Lammel, S.; Hetzel, A.; Hackel, O.; Jones, I.; Liss, B.; Roeper, J. Unique properties of mesoprefrontal neurons within a dual mesocorticolimbic dopamine system. Neuron 2008, 57, 760–773. [Google Scholar] [CrossRef]
- Lammel, S.; Ion, D.I.; Roeper, J.; Malenka, R.C. Projection-specific modulation of dopamine neuron synapses by aversive and rewarding stimuli. Neuron 2011, 70, 855–862. [Google Scholar] [CrossRef]
- Ford, C.P.; Mark, G.P.; Williams, J.T. Properties and opioid inhibition of mesolimbic dopamine neurons vary according to target location. J. Neurosci. 2006, 26, 2788–2797. [Google Scholar] [CrossRef]
- Brischoux, F.; Chakraborty, S.; Brierley, D.I.; Ungless, M.A. Phasic excitation of dopamine neurons in ventral VTA by noxious stimuli. Proc. Natl. Acad. Sci. USA 2009, 106, 4894–4899. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Takada, M. Distinct representations of cognitive and motivational signals in midbrain dopamine neurons. Neuron 2013, 79, 1011–1024. [Google Scholar] [CrossRef] [PubMed]
- Bromberg-Martin, E.S.; Matsumoto, M.; Hikosaka, O. Dopamine in motivational control: Rewarding, aversive, and alerting. Neuron 2010, 68, 815–834. [Google Scholar] [CrossRef] [PubMed]
- Keiflin, R.; Pribut, H.J.; Shah, N.B.; Janak, P.H. Ventral Tegmental Dopamine Neurons Participate in Reward Identity Predictions. Curr. Biol. 2019, 29, 93–103.e3. [Google Scholar] [CrossRef]
- Nestler, E.J.; Carlezon, W.A., Jr. The mesolimbic dopamine reward circuit in depression. Biol. Psychiatry 2006, 59, 1151–1159. [Google Scholar] [CrossRef]
- Volkow, N.D.; Morales, M. The Brain on Drugs: From Reward to Addiction. Cell 2015, 162, 712–725. [Google Scholar] [CrossRef]
- Kohler, S.; Wagner, G.; Bar, K.J. Activation of brainstem and midbrain nuclei during cognitive control in medicated patients with schizophrenia. Hum. Brain Mapp. 2019, 40, 202–213. [Google Scholar] [CrossRef]
- Duvarci, S.; Simpson, E.H.; Schneider, G.; Kandel, E.R.; Roeper, J.; Sigurdsson, T. Impaired recruitment of dopamine neurons during working memory in mice with striatal D2 receptor overexpression. Nat. Commun. 2018, 9, 2822. [Google Scholar] [CrossRef]
- Nicola, S.M.; Taha, S.A.; Kim, S.W.; Fields, H.L. Nucleus accumbens dopamine release is necessary and sufficient to promote the behavioral response to reward-predictive cues. Neuroscience 2005, 135, 1025–1033. [Google Scholar] [CrossRef]
- Mantz, J.; Thierry, A.M.; Glowinski, J. Effect of noxious tail pinch on the discharge rate of mesocortical and mesolimbic dopamine neurons: Selective activation of the mesocortical system. Brain Res. 1989, 476, 377–381. [Google Scholar] [CrossRef]
- Guarraci, F.A.; Kapp, B.S. An electrophysiological characterization of ventral tegmental area dopaminergic neurons during differential pavlovian fear conditioning in the awake rabbit. Behav. Brain Res. 1999, 99, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Ungless, M.A.; Magill, P.J.; Bolam, J.P. Uniform inhibition of dopamine neurons in the ventral tegmental area by aversive stimuli. Science 2004, 303, 2040–2042. [Google Scholar] [CrossRef] [PubMed]
- Elman, I.; Borsook, D. Common Brain Mechanisms of Chronic Pain and Addiction. Neuron 2016, 89, 11–36. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Centeno, M.V.; Berger, S.; Wu, Y.; Na, X.; Liu, X.; Kondapalli, J.; Apkarian, A.V.; Martina, M.; Surmeier, D.J. The indirect pathway of the nucleus accumbens shell amplifies neuropathic pain. Nat. Neurosci. 2016, 19, 220–222. [Google Scholar] [CrossRef]
- Narita, M.; Ozaki, S.; Narita, M.; Ise, Y.; Yajima, Y.; Suzuki, T. Change in the expression of c-fos in the rat brain following sciatic nerve ligation. Neurosci. Lett. 2003, 352, 231–233. [Google Scholar] [CrossRef]
- Sagheddu, C.; Aroni, S.; De Felice, M.; Lecca, S.; Luchicchi, A.; Melis, M.; Muntoni, A.L.; Romano, R.; Palazzo, E.; Guida, F.; et al. Enhanced serotonin and mesolimbic dopamine transmissions in a rat model of neuropathic pain. Neuropharmacology 2015, 97, 383–393. [Google Scholar] [CrossRef]
- Wood, P.B. Role of central dopamine in pain and analgesia. Expert. Rev. Neurother. 2008, 8, 781–797. [Google Scholar] [CrossRef]
- Jarcho, J.M.; Feier, N.A.; Labus, J.S.; Naliboff, B.; Smith, S.R.; Hong, J.-Y.; Colloca, L.; Tillisch, K.; Mandelkern, M.A.; Mayer, E.A.; et al. Placebo analgesia: Self-report measures and preliminary evidence of cortical dopamine release associated with placebo response. Neuroimage Clin. 2016, 10, 107–114. [Google Scholar] [CrossRef]
- Ford, G.K.; Moriarty, O.; McGuire, B.E.; Finn, D.P. Investigating the effects of distracting stimuli on nociceptive behaviour and associated alterations in brain monoamines in rats. Eur. J. Pain 2008, 12, 970–979. [Google Scholar] [CrossRef]
- Nestler, E.J. Is there a common molecular pathway for addiction? Nat. Neurosci. 2005, 8, 1445–1449. [Google Scholar] [CrossRef]
- Huang, S.; Borgland, S.L.; Zamponi, G.W. Peripheral nerve injury-induced alterations in VTA neuron firing properties. Mol. Brain 2019, 12, 89. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Narita, M.; Hamada, Y.; Yamashita, A.; Tamura, H.; Ikegami, D.; Kondo, T.; Shinzato, T.; Shimizu, T.; Fukuchi, Y.; et al. Activation of ventral tegmental area dopaminergic neurons reverses pathological allodynia resulting from nerve injury or bone cancer. Mol. Pain 2018, 14, 1744806918756406. [Google Scholar] [CrossRef] [PubMed]
- Li, A.-L.; Sibi, J.E.; Yang, X.; Chiao, J.-C.; Peng, Y.B. Stimulation of the ventral tegmental area increased nociceptive thresholds and decreased spinal dorsal horn neuronal activity in rat. Exp. Brain Res. 2016, 234, 1505–1514. [Google Scholar] [CrossRef]
- Navratilova, E.; Xie, J.Y.; Okun, A.; Qu, C.; Eyde, N.; Ci, S.; Ossipov, M.H.; King, T.; Fields, H.L.; Porreca, F. Pain relief produces negative reinforcement through activation of mesolimbic reward-valuation circuitry. Proc. Natl. Acad. Sci. USA 2012, 109, 20709–20713. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.W.; North, R.A. Opioids excite dopamine neurons by hyperpolarization of local interneurons. J. Neurosci. 1992, 12, 483–488. [Google Scholar] [CrossRef]
- Leone, P.; Pocock, D.; Wise, R.A. Morphine-dopamine interaction: Ventral tegmental morphine increases nucleus accumbens dopamine release. Pharmacol. Biochem. Behav. 1991, 39, 469–472. [Google Scholar] [CrossRef]
- Clark, K.L.; Noudoost, B. The role of prefrontal catecholamines in attention and working memory. Front. Neural. Circuits 2014, 8, 33. [Google Scholar] [CrossRef]
- Cardoso-Cruz, H.; Dourado, M.; Monteiro, C.; Galhardo, V. Blockade of dopamine D2 receptors disrupts intrahippocampal connectivity and enhances pain-related working memory deficits in neuropathic pain rats. Eur. J. Pain 2018, 22, 1002–1015. [Google Scholar] [CrossRef]
- Klanker, M.; Feenstra, M.; Denys, D. Dopaminergic control of cognitive flexibility in humans and animals. Front. Neurosci. 2013, 7, 201. [Google Scholar] [CrossRef]
- Takahashi, Y.K.; Roesch, M.R.; Stalnaker, T.A.; Haney, R.Z.; Calu, D.J.; Taylor, A.R.; Burke, K.A.; Schoenbaum, G. The orbitofrontal cortex and ventral tegmental area are necessary for learning from unexpected outcomes. Neuron 2009, 62, 269–280. [Google Scholar] [CrossRef]
- Fields, H.L.; Hjelmstad, G.O.; Margolis, E.B.; Nicola, S.M. Ventral tegmental area neurons in learned appetitive behavior and positive reinforcement. Annu. Rev. Neurosci. 2007, 30, 289–316. [Google Scholar] [CrossRef] [PubMed]
- Arsenault, J.T.; Rima, S.; Stemmann, H.; Vanduffel, W. Role of the primate ventral tegmental area in reinforcement and motivation. Curr. Biol. 2014, 24, 1347–1353. [Google Scholar] [CrossRef] [PubMed]
- Nasser, H.M.; Calu, D.J.; Schoenbaum, G.; Sharpe, M.J. The Dopamine Prediction Error: Contributions to Associative Models of Reward Learning. Front. Psychol. 2017, 8, 244. [Google Scholar] [CrossRef] [PubMed]
- Pais-Vieira, M.; Mendes-Pinto, M.M.; Lima, D.; Galhardo, V. Cognitive impairment of prefrontal-dependent decision-making in rats after the onset of chronic pain. Neuroscience 2009, 161, 671–679. [Google Scholar] [CrossRef]
- Salamone, J.D.; Correa, M. The mysterious motivational functions of mesolimbic dopamine. Neuron 2012, 76, 470–485. [Google Scholar] [CrossRef]
- Wimmer, G.E.; Daw, N.D.; Shohamy, D. Generalization of value in reinforcement learning by humans. Eur. J. Neurosci. 2012, 35, 1092–1104. [Google Scholar] [CrossRef]
- Braver, T.S.; Barch, D.M. A theory of cognitive control, aging cognition, and neuromodulation. Neurosci. Biobehav. Rev. 2002, 26, 809–817. [Google Scholar] [CrossRef]
- Roitman, M.F.; Stuber, G.D.; Phillips, P.E.M.; Wightman, R.M.; Carelli, R.M. Dopamine operates as a subsecond modulator of food seeking. J. Neurosci. 2004, 24, 1265–1271. [Google Scholar] [CrossRef]
- Nishino, H.; Ono, T.; Muramoto, K.; Fukuda, M.; Sasaki, K. Neuronal activity in the ventral tegmental area (VTA) during motivated bar press feeding in the monkey. Brain. Res. 1987, 413, 302–313. [Google Scholar] [CrossRef]
- MacInnes, J.J.; Dickerson, K.C.; Chen, N.-K.; Adcock, R.A. Cognitive Neurostimulation: Learning to Volitionally Sustain Ventral Tegmental Area Activation. Neuron 2016, 89, 1331–1342. [Google Scholar] [CrossRef]
- Schultz, W. Dopamine reward prediction-error signalling: A two-component response. Nat. Rev. Neurosci. 2016, 17, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Guitart-Masip, M.; Duzel, E.; Dolan, R.; Dayan, P. Action versus valence in decision making. Trends Cogn. Sci. 2014, 18, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Nieoullon, A. Dopamine and the regulation of cognition and attention. Prog. Neurobiol. 2002, 67, 53–83. [Google Scholar] [CrossRef] [PubMed]
- Ott, T.; Nieder, A. Dopamine and Cognitive Control in Prefrontal Cortex. Trends Cogn. Sci. 2019, 23, 213–234. [Google Scholar] [CrossRef]
- Puig, M.V.; Rose, J.; Schmidt, R.; Freund, N. Dopamine modulation of learning and memory in the prefrontal cortex: Insights from studies in primates, rodents, and birds. Front. Neural Circuits 2014, 8, 93. [Google Scholar] [CrossRef] [PubMed]
- Carr, D.B.; Sesack, S.R. Projections from the rat prefrontal cortex to the ventral tegmental area: Target specificity in the synaptic associations with mesoaccumbens and mesocortical neurons. J. Neurosci. 2000, 20, 3864–3873. [Google Scholar] [CrossRef] [PubMed]
- Sesack, S.R.; Grace, A.A. Cortico-Basal Ganglia reward network: Microcircuitry. Neuropsychopharmacology 2010, 35, 27–47. [Google Scholar] [CrossRef]
- Fujisawa, S.; Buzsaki, G. A 4 Hz oscillation adaptively synchronizes prefrontal, VTA, and hippocampal activities. Neuron 2011, 72, 153–165. [Google Scholar] [CrossRef]
- Lisman, J.E.; Grace, A.A. The hippocampal-VTA loop: Controlling the entry of information into long-term memory. Neuron 2005, 46, 703–713. [Google Scholar] [CrossRef]
- Ghanbarian, E.; Motamedi, F. Ventral tegmental area inactivation suppresses the expression of CA1 long term potentiation in anesthetized rat. PLoS ONE 2013, 8, e58844. [Google Scholar] [CrossRef]
- Omelchenko, N.; Bell, R.; Sesack, S.R. Lateral habenula projections to dopamine and GABA neurons in the rat ventral tegmental area. Eur. J. Neurosci. 2009, 30, 1239–1250. [Google Scholar] [CrossRef] [PubMed]
- Brinschwitz, K.; Dittgen, A.; Madai, V.I.; Lommel, R.; Geisler, S.; Veh, R.W. Glutamatergic axons from the lateral habenula mainly terminate on GABAergic neurons of the ventral midbrain. Neuroscience 2010, 168, 463–476. [Google Scholar] [CrossRef]
- Root, D.H.; Mejias-Aponte, C.A.; Zhang, S.; Wang, H.-L.; Hoffman, A.F.; Lupica, C.R.; Morales, M. Single rodent mesohabenular axons release glutamate and GABA. Nat. Neurosci. 2014, 17, 1543–1551. [Google Scholar] [CrossRef] [PubMed]
- Stamatakis, A.M.; Jennings, J.H.; Ung, R.L.; Blair, G.A.; Weinberg, R.J.; Neve, R.L.; Boyce, F.; Mattis, J.; Ramakrishnan, C.; Deisseroth, K.; et al. A unique population of ventral tegmental area neurons inhibits the lateral habenula to promote reward. Neuron 2013, 80, 1039–1053. [Google Scholar] [CrossRef]
- Jhou, T.C.; Fields, H.L.; Baxter, M.G.; Saper, C.B.; Holland, P.C. The rostromedial tegmental nucleus (RMTg), a GABAergic afferent to midbrain dopamine neurons, encodes aversive stimuli and inhibits motor responses. Neuron 2009, 61, 786–800. [Google Scholar] [CrossRef] [PubMed]
- Benekareddy, M.; Stachniak, T.J.; Bruns, A.; Knoflach, F.; von Kienlin, M.; Kunnecke, B.; Ghosh, A. Identification of a Corticohabenular Circuit Regulating Socially Directed Behavior. Biological. Psychiatry 2018, 83, 607–617. [Google Scholar] [CrossRef]
- Li, Y.-Q.; Takada, M.; Mizuno, N. Demonstration of habenular neurons which receive afferent fibers from the nucleus accumbens and send their axons to the midbrain periaqueductal gray. Neurosci. Lett. 1993, 158, 55–58. [Google Scholar] [CrossRef]
- Brown, P.L.; Palacorolla, H.; Brady, D.; Riegger, K.; Elmer, G.I.; Shepard, P.D. Habenula-Induced Inhibition of Midbrain Dopamine Neurons Is Diminished by Lesions of the Rostromedial Tegmental Nucleus. J. Neurosci. 2017, 37, 217–225. [Google Scholar] [CrossRef]
- Brown, P.L.; Shepard, P.D. Functional evidence for a direct excitatory projection from the lateral habenula to the ventral tegmental area in the rat. J. Neurophysiol. 2016, 116, 1161–1174. [Google Scholar] [CrossRef]
- Lecourtier, L.; Defrancesco, A.; Moghaddam, B. Differential tonic influence of lateral habenula on prefrontal cortex and nucleus accumbens dopamine release. Eur. J. Neurosci. 2008, 27, 1755–1762. [Google Scholar] [CrossRef]
- Root, D.H.; Mejias-Aponte, C.A.; Qi, J.; Morales, M. Role of glutamatergic projections from ventral tegmental area to lateral habenula in aversive conditioning. J. Neurosci. 2014, 34, 13906–13910. [Google Scholar] [CrossRef] [PubMed]
- Bechara, A.; Martin, E.M. Impaired decision making related to working memory deficits in individuals with substance addictions. Neuropsychology 2004, 18, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Gruber, A.J.; Calhoon, G.G.; Shusterman, I.; Schoenbaum, G.; Roesch, M.R.; O’Donnell, P. More is less: A disinhibited prefrontal cortex impairs cognitive flexibility. J. Neurosci. 2010, 30, 17102–17110. [Google Scholar] [CrossRef] [PubMed]
- Floresco, S.B. Prefrontal dopamine and behavioral flexibility: Shifting from an “inverted-U” toward a family of functions. Front. Neurosci. 2013, 7, 62. [Google Scholar] [CrossRef] [PubMed]
- Boekhoudt, L.; Voets, E.S.; Flores-Dourojeanni, J.P.; Luijendijk, M.C.; Vanderschuren, L.J.; Adan, R.A. Chemogenetic Activation of Midbrain Dopamine Neurons Affects Attention, but not Impulsivity, in the Five-Choice Serial Reaction Time Task in Rats. Neuropsychopharmacology 2017, 42, 1315–1325. [Google Scholar] [CrossRef]
- Ilango, A.; Shumake, J.; Wetzel, W.; Scheich, H.; Ohl, F.W. Electrical stimulation of lateral habenula during learning: Frequency-dependent effects on acquisition but not retrieval of a two-way active avoidance response. PLoS ONE 2013, 8, e65684. [Google Scholar] [CrossRef]
- Wilson, J.R.; Mitchell, J.C.; Van Hoesen, G.W. Epithalamic and ventral tegmental contributions to avoidance behavior in rats. J. Comp. Physiol. Psychol. 1972, 78, 442–449. [Google Scholar] [CrossRef]
- Stamatakis, A.M.; Stuber, G.D. Activation of lateral habenula inputs to the ventral midbrain promotes behavioral avoidance. Nat. Neurosci. 2012, 15, 1105–1107. [Google Scholar] [CrossRef]
- Wang, J.; Bast, T.; Wang, Y.-C.; Zhang, W.-N. Hippocampus and two-way active avoidance conditioning: Contrasting effects of cytotoxic lesion and temporary inactivation. Hippocampus 2015, 25, 1517–1531. [Google Scholar] [CrossRef]
- Coco, M.L.; Weiss, J.M. Neural substrates of coping behavior in the rat: Possible importance of mesocorticolimbic dopamine system. Behav. Neurosci. 2005, 119, 429–445. [Google Scholar] [CrossRef]
- Stark, H.; Bischof, A.; Wagner, T.; Scheich, H. Activation of the dopaminergic system of medial prefrontal cortex of gerbils during formation of relevant associations for the avoidance strategy in the shuttle-box. Prog. Neuropsychopharmacol. Biol. Psychiatry 2001, 25, 409–426. [Google Scholar] [CrossRef] [PubMed]
- Stark, H.; Rothe, T.; Wagner, T.; Scheich, H. Learning a new behavioral strategy in the shuttle-box increases prefrontal dopamine. Neuroscience 2004, 126, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.; Guan, X.; Ni, Y.; Luo, L.; Yang, L.; Zhang, P.; Zhang, J.; Chen, Y. Dopamine D1-like receptor in lateral habenula nucleus affects contextual fear memory and long-term potentiation in hippocampal CA1 in rats. Behav. Brain Res. 2017, 321, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.; Ni, Y.; Zhang, P.; Zhang, J.; Chen, Y. D1-like dopamine receptor dysfunction in the lateral habenula nucleus increased anxiety-like behavior in rat. Neuroscience 2017, 340, 542–550. [Google Scholar] [CrossRef] [PubMed]
- Gothard, K.M.; Skaggs, W.E.; McNaughton, B.L. Dynamics of mismatch correction in the hippocampal ensemble code for space: Interaction between path integration and environmental cues. J. Neurosci. 1996, 16, 8027–8040. [Google Scholar] [CrossRef] [PubMed]
- Moser, M.B.; Moser, E.I. Functional differentiation in the hippocampus. Hippocampus 1998, 8, 608–619. [Google Scholar] [CrossRef]
- Kahn, I.; Shohamy, D. Intrinsic connectivity between the hippocampus, nucleus accumbens, and ventral tegmental area in humans. Hippocampus 2013, 23, 187–192. [Google Scholar] [CrossRef]
- Kalen, P.; Lindvall, O.; Bjorklund, A. Electrical stimulation of the lateral habenula increases hippocampal noradrenaline release as monitored by in vivo microdialysis. Exp. Brain Res. 1989, 76, 239–245. [Google Scholar] [CrossRef]
- Pintus, R.; Riggi, M.; Cannarozzo, C.; Valeri, A.; de Leo, G.; Romano, M.; Gulino, R.; Leanza, G. Essential role of hippocampal noradrenaline in the regulation of spatial working memory and TDP-43 tissue pathology. J. Comp. Neurol. 2018, 526, 1131–1147. [Google Scholar] [CrossRef]
- Jackisch, R.; Moll, S.; Feuerstein, T.J.; Hertting, G. Dopaminergic modulation of hippocampal noradrenaline release. Evidence for alpha 2-antagonistic effects of some dopamine receptor agonists and antagonists. Naunyn. Schmiedebergs Arch. Pharmacol. 1985, 330, 105–113. [Google Scholar] [CrossRef]
- Jay, T.M. Dopamine: A potential substrate for synaptic plasticity and memory mechanisms. Prog. Neurobiol. 2003, 69, 375–390. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Kato, M.; Takano, H.; Arakawa, R.; Okumura, M.; Otsuka, T.; Kodaka, F.; Hayashi, M.; Okubo, Y.; Ito, H.; et al. Differential contributions of prefrontal and hippocampal dopamine D1 and D2 receptors in human cognitive functions. J. Neurosci. 2008, 28, 12032–12038. [Google Scholar] [CrossRef] [PubMed]
- Cardoso-Cruz, H.; Dourado, M.; Monteiro, C.; Matos, M.R.; Galhardo, V. Activation of dopaminergic D2/D3 receptors modulates dorsoventral connectivity in the hippocampus and reverses the impairment of working memory after nerve injury. J. Neurosci. 2014, 34, 5861–5873. [Google Scholar] [CrossRef] [PubMed]
- Coffeen, U.; Ortega-Legaspi, J.M.; de Gortari, P.; Simón-Arceo, K.; Jaimes, O.; Amaya, M.I.; Pellicer, F. Inflammatory nociception diminishes dopamine release and increases dopamine D2 receptor mRNA in the rat’s insular cortex. Mol. Pain 2010, 6, 75. [Google Scholar] [CrossRef]
- Cobacho, N.; de la Calle, J.L.; Paíno, C.L. Dopaminergic modulation of neuropathic pain: Analgesia in rats by a D2-type receptor agonist. Brain Res. Bull. 2014, 106, 62–71. [Google Scholar] [CrossRef]
- Ledermann, K.; Jenewein, J.; Sprott, H.; Hasler, G.; Schnyder, U.; Warnock, G.; Johayem, A.; Kollias, S.; Buck, A.; Martin-Soelch, C. Relation of dopamine receptor 2 binding to pain perception in female fibromyalgia patients with and without depression—A [11C] raclopride PET-study. Eur Neuropsychopharmacol 2016, 26, 320–330. [Google Scholar] [CrossRef]
- Alemi, M.; Pereira, A.R.; Cerqueira-Nunes, M.; Monteiro, C.; Galhardo, V.; Cardoso-Cruz, H. Role of Glutamatergic Projections from Lateral Habenula to Ventral Tegmental Area in Inflammatory Pain-Related Spatial Working Memory Deficits. Biomedicines 2023, 11, 820. [Google Scholar] [CrossRef]
- Guo, F.; Du, Y.; Qu, F.-H.; Lin, S.-D.; Chen, Z.; Zhang, S.-H. Dissecting the Neural Circuitry for Pain Modulation and Chronic Pain: Insights from Optogenetics. Neurosci. Bull. 2022, 38, 440–452. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, A.R.; Alemi, M.; Cerqueira-Nunes, M.; Monteiro, C.; Galhardo, V.; Cardoso-Cruz, H. Dynamics of Lateral Habenula–Ventral Tegmental Area Microcircuit on Pain-Related Cognitive Dysfunctions. Neurol. Int. 2023, 15, 1303-1319. https://doi.org/10.3390/neurolint15040082
Pereira AR, Alemi M, Cerqueira-Nunes M, Monteiro C, Galhardo V, Cardoso-Cruz H. Dynamics of Lateral Habenula–Ventral Tegmental Area Microcircuit on Pain-Related Cognitive Dysfunctions. Neurology International. 2023; 15(4):1303-1319. https://doi.org/10.3390/neurolint15040082
Chicago/Turabian StylePereira, Ana Raquel, Mobina Alemi, Mariana Cerqueira-Nunes, Clara Monteiro, Vasco Galhardo, and Helder Cardoso-Cruz. 2023. "Dynamics of Lateral Habenula–Ventral Tegmental Area Microcircuit on Pain-Related Cognitive Dysfunctions" Neurology International 15, no. 4: 1303-1319. https://doi.org/10.3390/neurolint15040082
APA StylePereira, A. R., Alemi, M., Cerqueira-Nunes, M., Monteiro, C., Galhardo, V., & Cardoso-Cruz, H. (2023). Dynamics of Lateral Habenula–Ventral Tegmental Area Microcircuit on Pain-Related Cognitive Dysfunctions. Neurology International, 15(4), 1303-1319. https://doi.org/10.3390/neurolint15040082








