Investigating the Predictive Value of Thyroid Hormone Levels for Stroke Prognosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Selection Criteria
2.3. Data Extraction
2.4. Data Analysis
3. Results
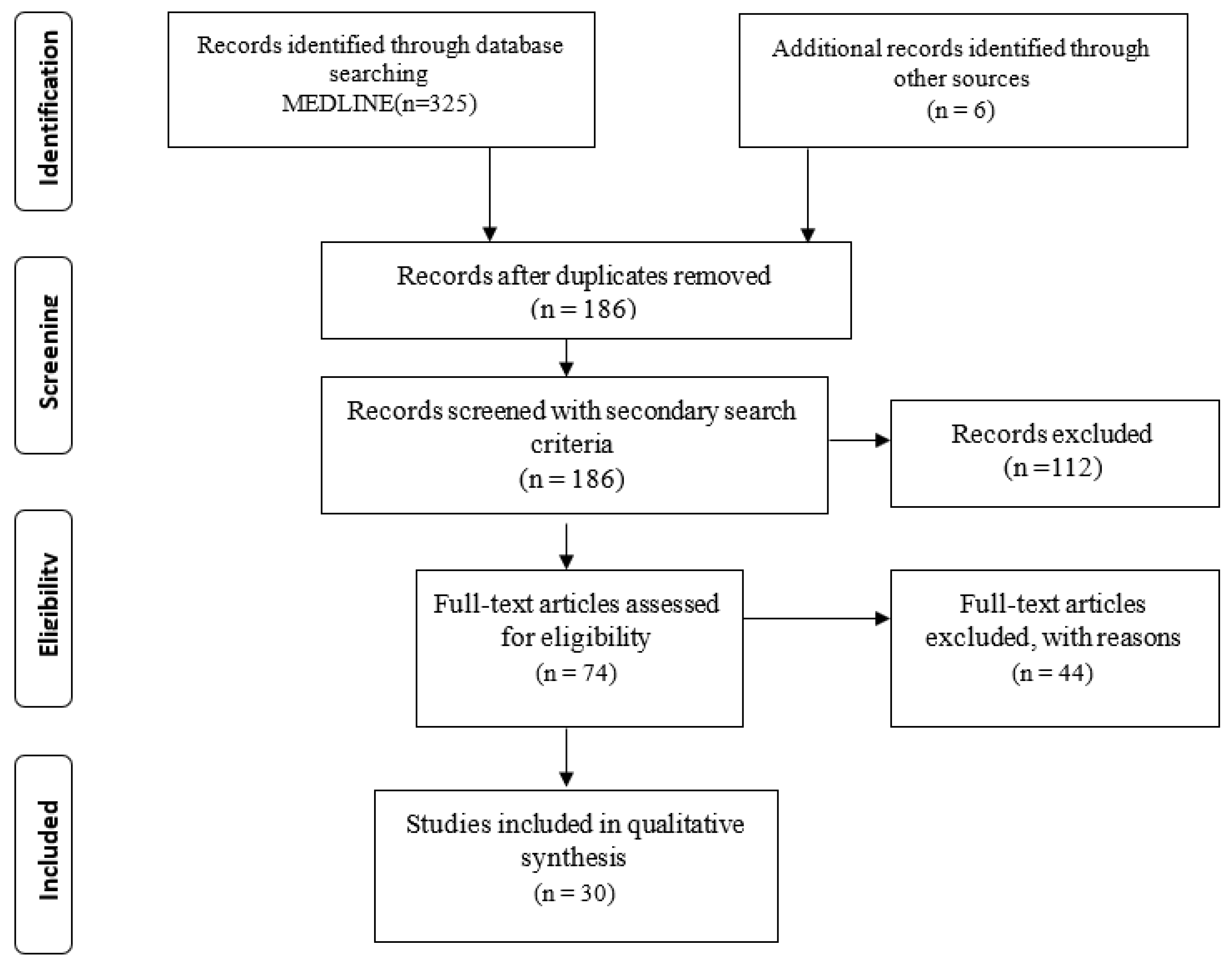
3.1. Database Searches
3.2. Study Characteristics
3.3. Study Design
3.4. Stroke Patient Groups
3.5. Demographic and Clinical Profiles
3.6. Time of Blood Sampling
3.7. Scales of Stroke Severity, Prognosis, and Functional Outcome
4. Discussion
4.1. Studies Associating Thyroid Hormone Levels with Unfavorable Stroke Prognosis
4.2. Studies Associating Thyroid Hormone Levels with Favorable Stroke Prognosis
4.3. Studies Concluding That There Is No Statistically Significant Evidence Connecting Thyroid Hormones with Stroke Outcomes
5. Study Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Feigin, V.L.; Norrving, B.; Mensah, G.A. Global Burden of Stroke. Circ. Res. 2017, 120, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, E.J.; Virani, S.S.; Callaway, C.W.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Chiuve, S.E.; Cushman, M.; Delling, F.N.; Deo, R.; et al. American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2018 Update: A Report from the American Heart Association. Circulation 2018, 137, e67–e492, Erratum in Circulation 2018, 137, e493. [Google Scholar] [CrossRef] [PubMed]
- Ingall, T. Stroke—Incidence, mortality, morbidity and risk. J. Insur. Med. 2004, 36, 143–152. [Google Scholar] [PubMed]
- Norrving, B.; Kissela, B. The global burden of stroke and need for a continuum of care. Neurology 2013, 80 (Suppl. S2), S5–S12. [Google Scholar] [CrossRef] [PubMed]
- Wafa, H.A.; Wolfe, C.D.A.; Emmett, E.; Roth, G.A.; Johnson, C.O.; Wang, Y. Burden of Stroke in Europe: Thirty-Year Projections of Incidence, Prevalence, Deaths, and Disability-Adjusted Life Years. Stroke 2020, 51, 2418–2427. [Google Scholar] [CrossRef]
- Gkantzios, A.; Tsiptsios, D.; Karatzetzou, S.; Kitmeridou, S.; Karapepera, V.; Giannakou, E.; Vlotinou, P.; Aggelousis, N.; Vadikolias, K. Stroke and Emerging Blood Biomarkers: A Clinical Prospective. Neurol. Int. 2022, 14, 784–803. [Google Scholar] [CrossRef]
- Christidi, F.; Tsiptsios, D.; Fotiadou, A.; Kitmeridou, S.; Karatzetzou, S.; Tsamakis, K.; Sousanidou, A.; Psatha, E.A.; Karavasilis, E.; Seimenis, I.; et al. Diffusion Tensor Imaging as a Prognostic Tool for Recovery in Acute and Hyperacute Stroke. Neurol. Int. 2022, 14, 841–874. [Google Scholar] [CrossRef]
- Stinear, C.M. Prediction of motor recovery after stroke: Advances in biomarkers. Lancet Neurol. 2017, 16, 826–836. [Google Scholar] [CrossRef]
- Andone, S.; Bajko, Z.; Motataianu, A.; Mosora, O.; Balasa, R. The Role of Biomarkers in Atherothrombotic Stroke-A Systematic Review. Int. J. Mol. Sci. 2021, 22, 9032. [Google Scholar] [CrossRef]
- Gkantzios, A.; Tsiptsios, D.; Karapepera, V.; Karatzetzou, S.; Kiamelidis, S.; Vlotinou, P.; Giannakou, E.; Karampina, E.; Paschalidou, K.; Kourkoutsakis, N.; et al. Monocyte to HDL and Neutrophil to HDL Ratios as Potential Ischemic Stroke Prognostic Biomarkers. Neurol. Int. 2023, 15, 301–317. [Google Scholar] [CrossRef]
- Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: Preferred definitions and conceptual framework. Clin. Pharmacol. Ther. 2001, 69, 89–95. [Google Scholar] [CrossRef]
- Karatzetzou, S.; Tsiptsios, D.; Sousanidou, A.; Fotiadou, S.; Christidi, F.; Kokkotis, C.; Gkantzios, A.; Stefas, E.; Vlotinou, P.; Kaltsatou, A.; et al. Copeptin Implementation on Stroke Prognosis. Neurol. Int. 2023, 15, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Soldozy, S.; Yağmurlu, K.; Norat, P.; Elsarrag, M.; Costello, J.; Farzad, F.; Sokolowski, J.D.; Sharifi, K.A.; Elarjani, T.; Burks, J.; et al. Biomarkers Predictive of Long-Term Outcome After Ischemic Stroke: A Meta-Analysis. World Neurosurg. 2022, 163, e1–e42. [Google Scholar] [CrossRef]
- Di Liegro, I. Thyroid hormones and the central nervous system of mammals (Review). Mol. Med. Rep. 2008, 1, 279–295. [Google Scholar] [CrossRef] [PubMed]
- Cramer, S.C. Repairing the human brain after stroke: I. Mechanisms of spontaneous recovery. Ann. Neurol. 2008, 63, 272–287. [Google Scholar] [CrossRef]
- Lamba, N.; Liu, C.; Zaidi, H.; Broekman, M.L.D.; Simjian, T.; Shi, C.; Doucette, J.; Ren, S.; Smith, T.R.; Mekary, R.A.; et al. A prognostic role for Low tri-iodothyronine syndrome in acute stroke patients: A systematic review and meta-analysis. Clin. Neurol. Neurosurg. 2018, 169, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Talhada, D.; Santos, C.R.A.; Gonçalves, I.; Ruscher, K. Thyroid Hormones in the Brain and Their Impact in Recovery Mechanisms after Stroke. Front. Neurol. 2019, 10, 1103. [Google Scholar] [CrossRef]
- Rodriguez-Peña, A.; Ibarrola, N.; Iñiguez, M.A.; Muñoz, A.; Bernal, J. Neonatal hypothyroidism affects the timely expression of myelin-associated glycoprotein in the rat brain. J. Clin. Investig. 1993, 91, 812–818. [Google Scholar] [CrossRef]
- Schwarz, S.; Schwab, S.; Klinga, K.; Maser-Gluth, C.; Bettendorf, M. Neuroendocrine changes in patients with acute space occupying ischaemic stroke. J. Neurol. Neurosurg. Psychiatry 2003, 74, 725–727. [Google Scholar] [CrossRef]
- Morreale de Escobar, G.; Obregon, M.J.; Escobar del Rey, F. Role of thyroid hormone during early brain development. Eur. J. Endocrinol. 2004, 151 (Suppl. S3), U25–U37. [Google Scholar] [CrossRef]
- Kolb, B.; Cote, S.; Ribeiro-da-Silva, A.; Cuello, A.C. Nerve growth factor treatment prevents dendritic atrophy and promotes recovery of function after cortical injury. Neuroscience 1997, 76, 1139–1151. [Google Scholar] [CrossRef] [PubMed]
- Bunevicius, A.; Smith, T.; Laws, E.R. Low Tri-Iodothyronine Syndrome in Neurosurgical Patients: A Systematic Review of Literature. World Neurosurg. 2016, 95, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Hiroi, Y.; Kim, H.H.; Ying, H.; Furuya, F.; Huang, Z.; Simoncini, T.; Noma, K.; Ueki, K.; Nguyen, N.H.; Scanlan, T.S.; et al. Rapid nongenomic actions of thyroid hormone. Proc. Natl. Acad. Sci. USA 2006, 103, 14104–14109. [Google Scholar] [CrossRef] [PubMed]
- Crupi, R.; Paterniti, I.; Campolo, M.; Di Paola, R.; Cuzzocrea, S.; Esposito, E. Exogenous T3 administration provides neuroprotection in a murine model of traumatic brain injury. Pharmacol. Res. 2013, 70, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Shulga, A.; Blaesse, A.; Kysenius, K.; Huttunen, H.J.; Tanhuanpää, K.; Saarma, M.; Rivera, C. Thyroxin regulates BDNF expression to promote survival of injured neurons. Mol. Cell. Neurosci. 2009, 42, 408–418. [Google Scholar] [CrossRef]
- Losi, G.; Garzon, G.; Puia, G. Nongenomic regulation of glutamatergic neurotransmission in hippocampus by thyroid hormones. Neuroscience 2008, 151, 155–163. [Google Scholar] [CrossRef]
- Badaut, J.; Lasbennes, F.; Magistretti, P.J.; Regli, L. Aquaporins in brain: Distribution, physiology, and pathophysiology. J. Cereb. Blood Flow Metab. 2002, 22, 367–378. [Google Scholar] [CrossRef]
- Roeder, L.M.; Hopkins, I.B.; Kaiser, J.R.; Hanukoglu, L.; Tildon, J.T. Thyroid hormone action on glucose transporter activity in astrocytes. Biochem. Biophys. Res. Commun. 1988, 156, 275–281. [Google Scholar] [CrossRef]
- Sárközy, G.; Griesmaier, E.; He, X.; Kapelari, K.; Urbanek, M.; Simbruner, G.; Gressens, P.; Keller, M. T3 replacement does not prevent excitotoxic cell death but reduces developmental neuronal apoptosis in newborn mice. Eur. J. Paediatr. Neurol. 2007, 11, 129–135. [Google Scholar] [CrossRef]
- Abo-Zenah, H.A.; Shoeb, S.A.; Sabry, A.A.; Ismail, H.A. Relating circulating thyroid hormone concentrations to serum interleukins-6 and -10 in association with non-thyroidal illnesses including chronic renal insufficiency. BMC Endocr. Disord. 2008, 8, 1. [Google Scholar] [CrossRef]
- Jublanc, C.; Bruckert, E.; Giral, P.; Chapman, M.J.; Leenhardt, L.; Carreau, V.; Turpin, G. Relationship of circulating C-reactive protein levels to thyroid status and cardiovascular risk in hyperlipidemic euthyroid subjects: Low free thyroxine is associated with elevated hsCRP. Atherosclerosis 2004, 172, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Warner, M.H.; Beckett, G.J. Mechanisms behind the non-thyroidal illness syndrome: An update. J. Endocrinol. 2010, 205, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Van der Poll, T.; Romijn, J.A.; Wiersinga, W.M.; Sauerwein, H.P. Tumor necrosis factor: A putative mediator of the sick euthyroid syndrome in man. J. Clin. Endocrinol. Metab. 1990, 71, 1567–1572. [Google Scholar] [CrossRef] [PubMed]
- Boelen, A.; Kwakkel, J.; Chassande, O.; Fliers, E. Thyroid hormone receptor β mediates acute illness-induced alterations in central thyroid hormone metabolism. J. Neuroendocrinol. 2009, 21, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Sadana, P.; Coughlin, L.; Burke, J.; Woods, R.; Mdzinarishvili, A. Anti-edema action of thyroid hormone in MCAO model of ischemic brain stroke: Possible association with AQP4 modulation. J. Neurol. Sci. 2015, 354, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Genovese, T.; Impellizzeri, D.; Ahmad, A.; Cornelius, C.; Campolo, M.; Cuzzocrea, S.; Esposito, E. Post-ischaemic thyroid hormone treatment in a rat model of acute stroke. Brain Res. 2013, 1513, 92–102. [Google Scholar] [CrossRef]
- Fernández, V.; Tapia, G.; Varela, P.; Cornejo, P.; Videla, L.A. Upregulation of liver inducible nitric oxide synthase following thyroid hormone preconditioning: Suppression by N-acetylcysteine. Biol. Res. 2009, 42, 487–495. [Google Scholar] [CrossRef]
- Alevizaki, M.; Synetou, M.; Xynos, K.; Pappa, T.; Vemmos, K.N. Low triiodothyronine: A strong predictor of outcome in acute stroke patients. Eur. J. Clin. Investig. 2007, 37, 651–657. [Google Scholar] [CrossRef]
- Ambrosius, W.; Kazmierski, R.; Gupta, V.; Warot, A.W.; Adamczewska-Kociałkowska, D.; Błazejewska, A.; Ziemnicka, K.; Nowinski, W.L. Low free triiodothyronine levels are related to poor prognosis in acute ischemic stroke. Exp. Clin. Endocrinol. Diabetes 2011, 119, 139–143. [Google Scholar] [CrossRef]
- Suda, S.; Muraga, K.; Kanamaru, T.; Okubo, S.; Abe, A.; Aoki, J.; Suzuki, K.; Sakamoto, Y.; Shimoyama, T.; Nito, C.; et al. Low free triiodothyronine predicts poor functional outcome after acute ischemic stroke. J. Neurol. Sci. 2016, 368, 89–93. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, S.; Bao, J.; Pan, S.; Zhang, X. Low T3 levels as a predictor marker predict the prognosis of patients with acute ischemic stroke. Int. J. Neurosci. 2017, 127, 559–566. [Google Scholar] [CrossRef]
- Suda, S.; Shimoyama, T.; Nagai, K.; Arakawa, M.; Aoki, J.; Kanamaru, T.; Suzuki, K.; Sakamoto, Y.; Takeshi, Y.; Matsumoto, N.; et al. Low Free Triiodothyronine Predicts 3-Month Poor Outcome after Acute Stroke. J. Stroke Cerebrovasc. Dis. 2018, 27, 2804–2809. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, X.; Xu, S.; Yuan, J.; Si, Z.; Yang, Y.; Qiao, S.; Xu, X.; Wang, A. Low free triiodothyronineis predicts worsen neurological outcome of patients with acute ischemic stroke: A retrospective study with bioinformatics analysis. BMC Neurol. 2019, 19, 272. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Yang, C.; Wang, H. Free Triiodothyronine Is Associated with Poor Outcomes after Acute Ischemic Stroke. Int. J. Clin. Pract. 2022, 2022, 1982193. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Meyer, M.A. Clinical analysis on alteration of thyroid hormones in the serum of patients with acute ischemic stroke. Stroke Res. Treat. 2010, 2010, 290678. [Google Scholar] [CrossRef]
- Huang, G.Q.; Zeng, Y.Y.; Cheng, Q.Q.; Cheng, H.R.; Ruan, Y.T.; Yuan, C.X.; Chen, Y.B.; He, W.L.; Chen, H.J.; He, J.C. Low triiodothyronine syndrome is associated with hemorrhagic transformation in patients with acute ischaemic stroke. Aging 2019, 11, 6385–6397. [Google Scholar] [CrossRef]
- Hama, S.; Kitaoka, T.; Shigenobu, M.; Watanabe, A.; Imura, I.; Seno, H.; Tominaga, A.; Arita, K.; Kurisu, K. Malnutrition and nonthyroidal illness syndrome after stroke. Metabolism 2005, 54, 699–704. [Google Scholar] [CrossRef]
- Forti, P.; Maioli, F.; Coveri, M.; Nativio, V.; Arnone, G.; Loreti, A.; Zoli, M.; Sacquegna, T.; Procaccianti, G. Thyroid function tests and early outcomes of acute ischemic stroke in older euthyroid patients. Exp. Gerontol. 2015, 61, 8–14. [Google Scholar] [CrossRef]
- Li, L.Q.; Xu, X.Y.; Li, W.Y.; Hu, X.Y.; Lv, W. The prognostic value of total T3 after acute cerebral infarction is age-dependent: A retrospective study on 768 patients. BMC Neurol. 2019, 19, 54. [Google Scholar] [CrossRef]
- Xu, X.Y.; Li, W.Y.; Hu, X.Y. Alteration of Thyroid-Related Hormones within Normal Ranges and Early Functional Outcomes in Patients with Acute Ischemic Stroke. Int. J. Endocrinol. 2016, 2016, 3470490. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Zhou, X.; Yu, F.; Liu, Z.; Wang, J.; Li, Z.; Zhan, Q.; Yang, Q.; Liu, Y.; Xia, J. Low-normal free triiodothyronine and high leukocyte levels in relation to stroke severity and poor outcome in acute ischemic stroke with intracranial atherosclerotic stenosis. Int. J. Neurosci. 2019, 129, 635–641. [Google Scholar] [CrossRef]
- Liu, J.; Wang, D.; Xiong, Y.; Yuan, R.; Tao, W.; Liu, M. Low free triiodothyronine levels are related to symptomatic intracranial hemorrhage and poor functional outcomes after intravenous thrombolysis in acute ischemic stroke patients. Neurol. Res. 2016, 38, 429–433. [Google Scholar] [CrossRef]
- Qiu, M.; Fang, M.; Liu, X. Low free triiodothyronine levels predict symptomatic intracranial hemorrhage and worse short-term outcome of thrombolysis in patients with acute ischemia stroke. Medicine 2017, 96, e8539. [Google Scholar] [CrossRef]
- Ma, L.; Zhu, D.; Jiang, Y.; Liu, Y.; Ma, X.; Liu, M.; Chen, X. Low triiodothyronine: A new facet of inflammation in acute ischemic stroke. Clin. Chim. Acta 2016, 458, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Suda, S.; Aoki, J.; Shimoyama, T.; Suzuki, K.; Sakamoto, Y.; Katano, T.; Okubo, S.; Nito, C.; Nishiyama, Y.; Mishina, M.; et al. Low Free Triiodothyronine at Admission Predicts Poststroke Infection. J. Stroke Cerebrovasc. Dis. 2018, 27, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Irimie, C.A.; Vârciu, M.; Irimie, M.; Ifteni, P.I.; Minea, D.I. C-Reactive Protein and T3: New Prognostic Factors in Acute Ischemic Stroke. J. Stroke Cerebrovasc. Dis. 2018, 27, 2731–2737. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wu, Y.; Huang, G.; He, W.; Lin, S.; Zhang, X.; He, J. Low Tri-iodothyronine Syndrome Is Associated with Cognitive Impairment in Patients with Acute Ischemic Stroke: A Prospective Cohort Study. Am. J. Geriatr. Psychiatry 2018, 26, 1222–1230. [Google Scholar] [CrossRef]
- Mao, L.; Chen, X.H.; Zhuang, J.H.; Li, P.; Xu, Y.X.; Zhao, Y.C.; Ma, Y.J.; He, B.; Yin, Y. Relationship between β-amyloid protein 1-42, thyroid hormone levels and the risk of cognitive impairment after ischemic stroke. World J. Clin. Cases 2020, 8, 76–87. [Google Scholar] [CrossRef]
- Wang, J.; Li, F.; Xiao, L.; Peng, F.; Sun, W.; Li, M.; Liu, D.; Jiang, Y.; Guo, R.; Li, H.; et al. Depressed TSH level as a predictor of poststroke fatigue in patients with acute ischemic stroke. Neurology 2018, 91, e1971–e1978. [Google Scholar] [CrossRef]
- Wollenweber, F.A.; Zietemann, V.; Gschwendtner, A.; Opherk, C.; Dichgans, M. Subclinical hyperthyroidism is a risk factor for poor functional outcome after ischemic stroke. Stroke 2013, 44, 1446–1448. [Google Scholar] [CrossRef]
- Lee, S.H.; Jang, M.U.; Kim, Y.; Park, S.Y.; Kim, C.; Kim, Y.J.; Sohn, J.H. Subclinical Hyperthyroidism Could Predict Poor Outcomes in Patients with Acute Ischemic Stroke Treated With Reperfusion Therapy. Front. Neurol. 2019, 10, 782. [Google Scholar] [CrossRef]
- Alevizaki, M.; Synetou, M.; Xynos, K.; Alevizaki, C.C.; Vemmos, K.N. Hypothyroidism as a protective factor in acute stroke patients. Clin. Endocrinol. 2006, 65, 369–372. [Google Scholar] [CrossRef]
- Baek, J.H.; Chung, P.W.; Kim, Y.B.; Moon, H.S.; Suh, B.C.; Jin, D.K.; Kim, B.M.; Rhee, E.J.; Lee, Y.T.; Park, K.Y. Favorable influence of subclinical hypothyroidism on the functional outcomes in stroke patients. Endocr. J. 2010, 57, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Akhoundi, F.H.; Ghorbani, A.; Soltani, A.; Meysamie, A. Favorable functional outcomes in acute ischemic stroke patients with subclinical hypothyroidism. Neurology 2011, 77, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Delpont, B.; Aboa-Eboulé, C.; Durier, J.; Petit, J.M.; Daumas, A.; Legris, N.; Daubail, B.; Giroud, M.; Béjot, Y. Associations between Thyroid Stimulating Hormone Levels and Both Severity and Early Outcome of Patients with Ischemic Stroke. Eur. Neurol. 2016, 76, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Neidert, S.; Katan, M.; Schuetz, P.; Fluri, F.; Ernst, A.; Bingisser, R.; Kappos, L.; Engelter, S.T.; Steck, A.; Müller, B.; et al. Anterior pituitary axis hormones and outcome in acute ischaemic stroke. J. Intern. Med. 2011, 269, 420–432. [Google Scholar] [CrossRef]
- O’Keefe, L.M.; Conway, S.E.; Czap, A.; Malchoff, C.D.; Benashski, S.; Fortunato, G.; Staff, I.; McCullough, L.D. Thyroid hormones and functional outcomes after ischemic stroke. Thyroid. Res. 2015, 8, 9. [Google Scholar] [CrossRef]
- Ciarambino, T.; Crispino, P.; Mastrolorenzo, E.; Viceconti, A.; Giordano, M. Stroke and Etiopathogenesis: What Is Known? Genes 2022, 13, 978. [Google Scholar] [CrossRef]
- Ciarambino, T.; Crispino, P.; Minervini, G.; Giordano, M. Cerebral Sinus Vein Thrombosis and Gender: A Not Entirely Casual Relationship. Biomedicines 2023, 11, 1280. [Google Scholar] [CrossRef]
| Authors, Year of Publication | Thyroid Biomarker | Type of Study | Type of Stroke | Number οf Participants/Mean Age | Time of Blood Sampling | Scale of Stroke Severity and Prognosis/Clinical Outcome | Follow-Up Time | Cut-Off Values; (Specificity); [Sensitivity] | Main Findings |
|---|---|---|---|---|---|---|---|---|---|
| T3, T4, TSH | Prospective | IS and HS | 737 patients/for low-T3: 69.9, for normal T3: 67.9 | Within 24 h of symptoms’ onset | SSS, mRS | 1 and 12 months after stroke onset | Not mentioned | Low-T3 syndrome may independently predict early and late mortality as well as disability at one year in patients with acute stroke |
| fT3, fT4, TSH | Prospective | IS | 337 patients/first tertile: 72 (61–80), second tertile: 67 (56–76), third tertile: 65 (58.8–73.5) | Within 24 h of symptoms’ onset | NIHSS, mRS |
| Not mentioned | Low fT3 concentrations are associated with a poorer prognosis for stroke patients and may aid in the development of an IS outcome classification model |
| fT3, fT4, TSH | Retrospective | IS | 398 patients/73.3 ± 11.9 | Upon admission | NIHSS, mRS | No follow-up | For predicting poor functional outcome upon discharge: fT3 < 2.29 pg/mL; N.A. | A low fT3 value upon admission may be predictive of a poor functional outcome in patients with acute ischemic stroke |
| T3 | Prospective | IS | 359 patients/63.12 ± 11.3 | Upon admission | NIHSS, mRS | 1 and 3 months after stroke onset | T3: <1.34 nmol/L; (80%); [40%] | Low T3 levels can be utilized to predict the short-term prognosis of an ischemic stroke. A combined model (T3, age, and NIHSS score) can contribute substantial predictive information to the NIHSS |
| fT3, fT4, TSH | Retrospective | IS and HS | 702 patients/73 (64–81) | Upon admission | NIHSS, mRS | 3 months after stroke onset | fT3 < 2.00 pg/mL; N.A. | Low fT3 values at admission predict poor functional outcomes and three-month mortality in acute stroke patients |
| fT3 | Retrospective | IS | 221 patients, 182 non-IS cases/patients: 66.80 ± 7.78, non-IS cases: 63.13 ± 12.51 | Upon admission | NIHSS, mRS | 3 months after stroke onset | fT3: 4.30 pmol/L; (77%); [74%] | A low fT3 value was associated with an acute IS that was particularly severe. Moreover, it predicts poor neurological outcomes three months following acute IS |
| fT3, fT4, TSH | Retrospective | IS | 480 patients/N.A. | The following morning of the admission | NIHSS, mRS | 3 months after stroke onset | fT3 ≤ 3.69 pmol/L; (72.03%); [62.70%] | Decreased fT3 levels are a biomarker of a poor prognosis three months after an ischemic stroke |
| T3, T4, TSH | Retrospective | IS | 47 patients/67.4 ± 12.1 | The following morning of the admission | NIHSS, mRS | 2–4 weeks after discharge from the hospital | Not mentioned | NTIS is common in patients with acute ischemic stroke, and reduced T3 levels are associated with poor outcomes. Low T3 syndrome severity may predict functional recovery in acute ischemic stroke patients |
| T3, fT3 | Retrospective | IS | 208 participants with HT and 208 age- and gender-matched stroke patients without HT/with HT: 68.7 ± 11.6, without HT: 68.6 ± 11.5 | Upon admission | NIHSS | No follow-up | Not mentioned | Low T3 syndrome may predict severe HT independently in AIS patients. T3 monitoring may prevent HT in patients with ischemic stroke |
| fT3, fT4, TSH | Prospective | IS and HS | 51 patients/66.7 ± 10.2 | One day after admission | FIM | No follow-up (within one week of admission and two weeks prior to discharge, all patients were evaluated for disability using the FIM) | Not mentioned | During the recovery period following a stroke, it is essential to assess the presence of NTIS by measuring free T3 and to assist patients in regaining function through intensive rehabilitation and nutritional management |
| fT3, fT4, TSH | Prospective | IS | 775 patients/80.1 ± 8.7 | On the morning after SU admission | NIHSS, mRS | No follow-up | Not mentioned | TFT at the time of hospital admission can provide independent prognostic information regarding the early outcomes of acute IS in euthyroid geriatric patients |
| T3, T4, fT3, fT4, TSH | Retrospective | IS | 768 patients/Younger Age Group (<65): 57 (51–61), Older Age Group (≥65): 76 (70–80) | Upon admission | NIHSS, mRS | 2–4 weeks following hospital discharge | Not mentioned | Age affects total T3 level and functional prognosis in euthyroid acute ischemic stroke patients. A low total T3 concentration predicted poor functional outcomes after an ischemic stroke in adults 65 and older. None of the thyroid hormones, including total T3, independently predicted poor functional outcomes in individuals under 65 |
| T3, T4, fT3, fT4, TSH | Retrospective | IS | 722 patients/67 (IQR 59–76) | Upon admission | NIHSS, mRS | 2–4 weeks after discharge from the hospital | Not mentioned | Low total T3 levels predicted poor functional outcomes in ischemic stroke patients with normal thyroid-related hormone levels. Higher T4 and lower T3 values were related to greater clinical severity of stroke at admission |
| fT3 | Prospective | IS | 300 patients/48 (IQR 45-56) | Within 24 h after admission | NIHSS, mRS | No follow-up | Not mentioned | In early stroke, lower fT3 concentrations within normal limits are independently linked with sICAS severity and a poor prognosis |
| fT3, fT4, TSH | Retrospective | IS | 46 patients/63.6 ± 13.9 | The following morning of the admission | NIHSS, mRS | No follow-up | Not mentioned | Reduced free T3 levels are independently associated with post-IVT sICH and poor functional outcomes in patients with AIS who undergo IVT |
| T3, T4, fT3, fT4, TSH | Prospective | IS | 159 patients/65.36 ± 10.02 | The following morning of the admission | NIHSS, mRS | 3 and 6 months after stroke onset | The cut-off value of fT3 for sICH: 3.54 pg/mL; (83%); [83%] | Low fT3 levels at admission in AIS patients receiving rtPA thrombolytic therapy were associated with sICH and inferior outcomes at 3 months |
| fT3, fT4, TSH | Retrospective | IS | 117 patients/58.8 ± 13.7 | The following morning of the admission | NIHSS | No follow-up | fT3 ≤ 4.40 pmol/L; (65.2%); [68.8%] | Low fT3 levels may play a role in the pathogenic pathway connecting inflammation to stroke severity in AIS patients |
| fT3, fT4, TSH | Retrospective | IS | 520 patients/71.9 ± 13.2 | Upon admission | NIHSS, mRS | No follow-up | The cut-off value of fT3 for PSI occurrence, fT3 < 2.29 pg/mL; N.A. | Low fT3 levels at admission are independently related to the development of PSI |
| T3 | Prospective | IS | 120 patients/65.5 ± 9.88 | Upon admission | NIHSS, mRS, MMSE | At discharge, both functional and cognitive outcomes were assessed. No further follow-up | Cut-off value for T3 of 1.115 nmol/L to predict:
|
|
| T3, T4, TSH | Prospective | IS | 314 patients/62.97 ± 10.11 | Within 24 h of hospital admission | NIHSS, BI, MMSE | At 1 month | Not mentioned | Low T3 syndrome increased PSCI prevalence one month after an ischemic stroke |
| T3, fT4 | Prospective | IS | 195 patients/69.38 ± 10.05 | Within 24 h of hospital admission | NIHSS, MoCA | For cognitive functions at 1 week, 3 months, 6 months, and 1 year | Not mentioned | A1–42 and T3 can predict the development of PSCI |
| fT3, fT4, TSH | Prospective | IS | 634 patients/60.5 ± 13.1 | The second day after admission | MMSE, FSS, NIHSS | At 6 months | Not mentioned |
|
| fT3, fT4, TSH | Prospective | IS | 165 patients/70 (62–78) | In the morning within 3 days after symptom onset | BI, mRS, NIHSS | At 3 months | Not mentioned | Subclinical hyperthyroidism risk outcome three months after an ischemic stroke |
| T3, fT4, TSH | Prospective | IS | 156 patients/70.3 ± 11.6 | Within 18 h of stroke onset | NIHSS | No follow-up | Not mentioned |
|
| Authors, Year of Publication | Thyroid Biomarker | Type of Study | Type of Stroke | Number οf Participants/Mean Age | Time of Blood Sampling | Scale of Stroke Severity and Prognosis/Clinical Outcome | Follow-Up Time | Cut-Off Values; (Specificity); [Sensitivity] | Main Findings |
|---|---|---|---|---|---|---|---|---|---|
| T3, T4, TSH | Retrospective | IS and HS | 744 patients/70.0 | Within 24 h of stroke onset | GCS, SSS, mRS | 1 and 12 months | Not mentioned | Acute stroke patients with laboratory evidence of previous hypothyroidism have a better clinical presentation and prognosis |
| fT4, TSH | Retrospective | IS | 31 patients with SCH and 725 patients with normal thyroid function/patients: 66.3 ± 10.8, patients with normal thyroid function: 66.2 ± 12.1 | Within two days of admission, in the morning | NIHSS, mRS | 1 and 3 months | Not mentioned | Functional results were better for AIS patients with SCH at admission |
| T3, T4, TSH | Prospective | IS | 73 patients/66.7 | Upon admission | NIHSS, mRS, BI | 1 and 3 months | Not mentioned | Significant correlation between SCH and improved outcomes and decreased mortality after ischemic stroke |
| TSH | Prospective | IS | 731 patients/69.4 ± 15.4 | Within the 18 h following admission | NIHSS, mRS | No follow-up | Not mentioned | A higher TSH level was independently associated with a reduced severity score at admission and a better functional outcome at discharge in AIS patients |
| Authors, Year of Publication | Thyroid Biomarker | Type of Study | Type of Stroke | Number οf Participants/Mean Age | Time of Blood Sampling | Scale of Stroke Severity and Prognosis/Clinical Outcome | Follow-Up Time | Cut-Off Values; (Specificity); [Sensitivity] | Main Findings |
|---|---|---|---|---|---|---|---|---|---|
| T3, fT4, TSH | Prospective | IS | 281 patients/68 (IQR 63–82) | 1 day after admission | NIHSS, mRS | 3 and 12 months | Not mentioned |
|
| fT3, fT4, TSH | Prospective | IS | 129 patients/67.03 ± 14.474 | At 24 ± 6 h post-symptom onset | NIHSS, mRS, mBI | 3 and 12 months | Not mentioned | Lower fT3 levels were related to worse outcomes at hospital discharge, 3 months, and 12 months following stroke, although these correlations reduced when other well-established stroke outcome predictors were considered |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gkantzios, A.; Karapepera, V.; Tsiptsios, D.; Liaptsi, E.; Christidi, F.; Gkartzonika, E.; Karatzetzou, S.; Kokkotis, C.; Kyrtsopoulos, M.; Tsiakiri, A.; et al. Investigating the Predictive Value of Thyroid Hormone Levels for Stroke Prognosis. Neurol. Int. 2023, 15, 926-953. https://doi.org/10.3390/neurolint15030060
Gkantzios A, Karapepera V, Tsiptsios D, Liaptsi E, Christidi F, Gkartzonika E, Karatzetzou S, Kokkotis C, Kyrtsopoulos M, Tsiakiri A, et al. Investigating the Predictive Value of Thyroid Hormone Levels for Stroke Prognosis. Neurology International. 2023; 15(3):926-953. https://doi.org/10.3390/neurolint15030060
Chicago/Turabian StyleGkantzios, Aimilios, Vaia Karapepera, Dimitrios Tsiptsios, Eirini Liaptsi, Foteini Christidi, Elena Gkartzonika, Stella Karatzetzou, Christos Kokkotis, Mihail Kyrtsopoulos, Anna Tsiakiri, and et al. 2023. "Investigating the Predictive Value of Thyroid Hormone Levels for Stroke Prognosis" Neurology International 15, no. 3: 926-953. https://doi.org/10.3390/neurolint15030060
APA StyleGkantzios, A., Karapepera, V., Tsiptsios, D., Liaptsi, E., Christidi, F., Gkartzonika, E., Karatzetzou, S., Kokkotis, C., Kyrtsopoulos, M., Tsiakiri, A., Bebeletsi, P., Chaidemenou, S., Koutsokostas, C., Tsamakis, K., Baltzi, M., Mpalampanos, D., Aggelousis, N., & Vadikolias, K. (2023). Investigating the Predictive Value of Thyroid Hormone Levels for Stroke Prognosis. Neurology International, 15(3), 926-953. https://doi.org/10.3390/neurolint15030060








