Abstract
A 58-year-old woman with a history of systemic lupus erythematosus (SLE) who was taking prednisolone and mycophenolate mofetil presented with gait disturbances that progressively worsened over a period of 3 months. Her blood test and cerebrospinal fluid (CSF) examination results did not indicate active SLE. Initial brain magnetic resonance imaging (MRI) revealed a small spotty lesion in the left cerebellar peduncle. The clinical course was consistent with rapidly progressive cerebellar syndrome (RPCS), which sometimes involves neuronal antibodies. The line blot assay detected anti-Yo antibodies, but no malignancy was found. Immunohistological techniques using rat brain sections yielded a negative result for anti-Yo antibodies. The second MRI revealed a focal lesion and surrounding spotty lesion in the left cerebellar peduncle, which was consistent with the punctate pattern observed in progressive multifocal leukoencephalopathy (PML). The CSF JCV-DNA test indicated the presence of cerebellar PML. Immunosuppressants were reduced, and mefloquine and mirtazapine were initiated. After approximately 2 years and 1 month, the CSF JCV-DNA results became negative. Cerebellar PML may exhibit a clinical course that is consistent with RPCS. The punctate pattern should be recognized as an early manifestation of PML. The CSF JCV-DNA copy number may serve as a useful indicator of PML stabilization.
1. Introduction
Progressive multifocal leukoencephalopathy (PML) is a demyelinating central nervous system disease caused by John Cunningham virus (JCV) disease [1,2]. JCV is a widely spread virus, with a worldwide antibody prevalence of >50% of the adult population [2,3]. JCV is a double-stranded DNA virus classified within the Polyomavirus family. It consists of an archetype, which possesses a stable genome and is nonpathogenic, and a prototype, which exhibits mutations characterized by DNA sequence deletions and/or duplications in the noncoding control region (NCCR) [1,2,4]. The prototype JCV causes reactivation and replication in oligodendrocytes, causing central demyelination [2,4]. Immune responses against JCV infection involve antibody, CD8+ cytotoxic T cell, and CD4+ T lymphocyte production [5,6]. Therefore, PML develops with immunosuppression such as human immunodeficiency virus (HIV) infection, lymphoma, leukemia, systemic lupus erythematosus (SLE), and organ transplantation [1,2,3,4]. Some PML cases have been attributed to the use of antibody drugs such as natalizumab, rituximab, and efalizumab [1,2,4,7,8]. PML typically occurs after a subacute course and presents with various symptoms, including cognitive impairment, motor dysfunction, gait disturbance, language impairment, visual field disturbance, and sensory disturbance [3,4,9,10]. Specific symptoms vary depending on lesion location, emphasizing the significance of neuroimaging, particularly magnetic resonance imaging (MRI). PML lesions exhibit high signal intensity on T2 and fluid-attenuated inversion recovery (FLAIR) sequences, low signal intensity on T1, and occasional contrast enhancement, extending from white matter to subcortical white matter [3,11]. PML diagnosis relies on clinical symptomatology, imaging findings, and confirmation of the presence of JCV through JCV-DNA detection in cerebrospinal fluid and/or brain biopsy [3,4]. Therefore, PML should be considered a potential cause when immunosuppressed patients present with subacute central nervous system symptoms. PML more frequently appears in supra-tentorial lesions than in sub-tentorial lesions [11]. Cerebellar involvement in PML may present with typical cerebellar symptoms, including dizziness, vertigo, nausea, limb ataxia or clumsiness, gait disturbances, and speech difficulties [12,13,14,15,16,17,18,19,20].
A variety of factors other than PML, including immune-mediated, infectious, degenerative, and deficiency diseases, as well as drug or alcohol abuse, cause progressive cerebellar ataxia [21]. In particular, a cerebellar syndrome interfering with daily life within 3 months is known as rapidly progressive cerebellar syndrome (RPCS), which is sometimes associated with anti-Yo antibodies [22]. This case report describes a patient who initially tested positive for anti-Yo antibodies by line blot assay, but the immunohistological test was negative, ultimately indicating a cerebellar PML diagnosis. Distinguishing cerebellar PML from anti-Yo-antibody-associated RPCS was difficult based on the clinical symptoms. Here, we present the interpretation of anti-Yo-antibody-positive cases and the characteristics of MRI findings that should raise suspicion for early cerebellar PML.
2. Case Presentation
A 57-year-old woman presented with dizziness and progressive gait disturbance for approximately three months. The patient was diagnosed with SLE eight years before the visit and received 5 mg/day of prednisolone (PSL) and 1500 mg/day of mycophenolate mofetil (MMF). The patient has received follow-up treatment by a rheumatologist since disease onset, and her SLE symptoms were well-controlled for eight years. Except for the SLE, the patient’s medical history was unremarkable. The patient was a homemaker with no exposure to toxins and no travel abroad and denied any dietary deviations, drinking, or smoking. There was no family history of neurological diseases, including neurodegenerative disorders.
Upon initial examination (day 1), her body temperature was 36.4 °C. Consciousness, blood pressure, heart rate, and oxygen saturation were normal. No scleritis, arthritis, or rash were observed. The lung and cardiac examinations were normal. The patient’s speech, eye movement, and facial movements and sensations were normal. Muscle strength, muscle tone, and tactile, pain, and vibratory sensations in the extremities were normal. The finger–nose–finger test was normal, but the heel-to-shin test revealed mild bilateral ataxia. Biceps and patellar tendon reflexes were bilaterally hyperactive, but pathological reflexes were unremarkable. The patient could stand, but exhibited a wide-base gait and was unable to walk alone, requiring assistance. The Romberg test was negative, and no signs of autonomic dysfunction or meningeal irritation were detected.
Blood tests revealed a high titer of antinuclear antibodies (1:640), which was similar to last year. Otherwise, no other findings indicated increased SLE activity. Her vitamin B1 levels were 22 ng/mL (normal range: 24 ng/mL and above), and her vitamin B12 levels were 114 pg/mL (normal range: 180 pg/mL and above). The HIV antibody was negative. Furthermore, glutamic acid decarboxylase, thyroid peroxidase, and thyroglobulin were negative. Evaluation of her CSF revealed no elevation in cell count (1/mm3), total protein level (25 mg/dL), IgG index (0.44), or interleukin-6 (3.8 pg/mL). Brain MRI on day 7 showed no cerebellar atrophy, and small spotty hyperintense lesions without mass effect were observed in the left cerebellar peduncle on diffusion-weighted and T2-weighted images (Figure 1). The hyperintense lesions did not show a low signal in the apparent diffusion coefficient map, and no gadolinium enhancement was observed.
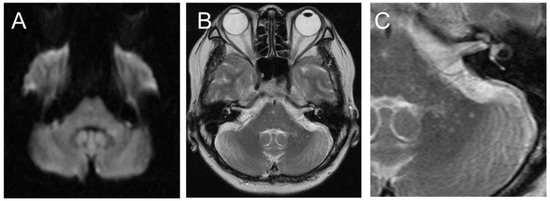
Figure 1.
Initial brain magnetic resonance imaging (MRI) showed a small, high-intensity lesion in the left cerebellar peduncle on diffusion-weighted imaging (A). On T2-weighted imaging (B), the lesion appeared to be a cluster of small spots ((C): enlarged image of (B)).
Vitamin B12 deficiency was suspected to play a role in the symptoms, and transvenous vitamin supplementation was initiated. Although the vitamin B12 levels sufficiently increased, the patient’s gait disturbance gradually worsened. A serum line blot assay test for 12 paraneoplastic antibodies (i.e., Hu, Yo, CV2, Ri, Ma2/Ta, GAD65, amphiphysin, recoverin, SOX1, titin, zic4, and Tr, EUROLINE, Euroimmun, Lübeck, Germany) was performed, and the anti-Yo antibody was positive. Contrast-enhanced computed tomography, pelvic MRI, mammary echocardiography, gallium scintigraphy, fecal occult blood test, and upper gastrointestinal endoscopy failed to detect any malignancies. After 3 weeks, the patient became bedridden due to dizziness and nausea and developed mild ataxia in the upper extremities. The patient had difficulty taking MMF capsules internally; thus, MMF was discontinued, and the PSL dose was increased to 15 mg/day. Brain MRI on day 39 showed a focal lesion and surrounding spotty lesions in the left cerebellar peduncle. The cerebellar atrophy was not remarkable (Figure 2).
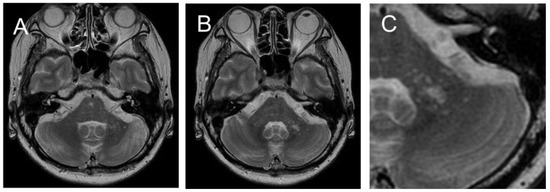
Figure 2.
MRI on day 39 revealed increasing spotty lesions in the left peduncle that became centrally clustered, with a spreading spotty lesion surrounding the central area (A,B). ((C): Enlarged image of (B)). No cerebellar cortex atrophy was observed.
Progressive cerebellar ataxia which significantly affected daily life within three months was consistent with RPCS involving the anti-Yo antibody [22]. On day 67, positron emission computed tomography was performed in another hospital for advanced malignancy screening, but no evidence of malignancy was found. An in-house tissue-based assay (TBA) was performed using rat frozen cerebellar sections, which yielded a negative result for the anti-Yo antibody (Supplementary Figure S1).
In natalizumab-related and SLE-related PML, small punctate lesions occur as early PML imaging features, called the “punctate pattern” or “milky way” [11,23,24,25,26]. The spotty lesions observed in the left peduncle in our patient indicated PML. Real-time PCR was used to measure the CSF JCV-DNA [27]. While waiting for the examination results, ataxia of the extremities and trunk progressively worsened, causing the patient to remain bedridden. Although physical examination and laboratory results did not indicate active SLE, steroid pulse therapy and intravenous immunoglobulin therapy (IVIg) were started based on the possibility of central nervous system lupus from day 70, and PSL of 15 mg/day was continued.
On day 76, JC virus DNA at 15,870 copies/mL was detected in the patient’s CSF. Additionally, PCR typing was performed, targeting a DNA sequence within the viral genome (region D of the NCCR) retained in the archetype JCV but often missing in the prototype JCV [28]. The JCV detected in the patient’s CSF lacked this region and was identified as a prototype with a mutation. Therefore, the patient was diagnosed with cerebellar PML. The scale for the assessment and rating of the ataxia (SARA) score [29] was 25.5. In consultation with a rheumatologist, the PSL dose was reduced. In addition, 15 mg/day of mirtazapine and 275 mg/week of mefloquine were initiated; off-label use of these drugs was approved by the pharmaceutical affairs committee. On day 82, the T2 hyperintense lesion in the left cerebellar peduncle extended to the brainstem (Figure 3).
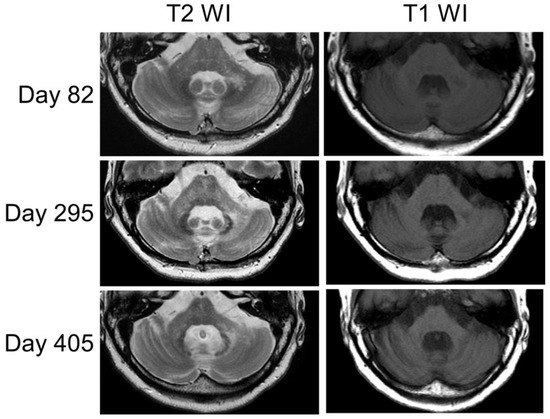
Figure 3.
The MRI findings changed over time (days 82, 295, and 405). The left peduncle lesion appeared enlarged on the T2-weighted images, and with this enlargement, atrophy became prominent in both the cerebellum and brainstem on the T1-weighted images.
On day 114, the SARA score was 27.0. Since that point, the patient’s clinical symptoms neither worsened nor improved (the SARA score remained at 27.0). On day 193, the CSF JCV-DNA was retested and was found to decrease to 30 copies/mL. On day 295, MRI revealed T2 high-intensity spreading to the pons and right peduncle, and the pontine and cerebellar cortex atrophy progressed (Figure 3). Despite the PSL decrease (6 mg/day), the JCV-DNA remained at 43 copies/mL on day 300. On day 358, the patient was diagnosed with an SLE flare-up based on generalized myalgia, arthralgia, decreased CH50, and increased anti-ds DNA; thus, the PSL dose was temporarily increased. On day 405, the MRI showed pontine and cerebellar cortex atrophy progression (Figure 3); however, no considerable change was observed in the patient’s clinical presentation.
On day 470, the patient was discharged home in a reclining wheelchair with sustained seating and was put on a soft meal. The patient continued to regularly visit our hospital in the reclining wheelchair. On day 778, the CSF JCV-DNA was below the detection limit, and mefloquine and mirtazapine were discontinued. MRI on day 834 did not indicate brainstem cerebellar atrophy progression, and there was no deterioration in the patient’s neurological findings.
3. Discussion
A middle-aged woman with a history of SLE presented to our hospital with a clinical course consistent with RPCS. Her MRI results revealed a punctate pattern, which increased suspicions of cerebellar PML. The diagnosis was confirmed by measuring the JCV-DNA in the patient’s CSF. The immunosuppressants were reduced, and mefloquine and mirtazapine were initiated. The CSF JCV-DNA decreased below the sensitivity level, cerebellar atrophy ceased, and long-term survival was achieved. Commercial line blot assays often give false-positive results in patients without tumors [20]; thus, we ruled out anti-Yo-antibody-associated RPCS in this case based on the long-term follow up and TBA-negative results. Based on the initial symptoms and clinical course, distinguishing cerebellar PML from anti-Yo-antibody-associated RPCS was difficult.
Progressive cerebellar ataxia that interferes with daily life within 3 months is referred to as RPCS [22]. RPCS is associated with the anti-Yo antibody, anti-Tr antibody [22], anti-GAD antibody [30,31], or SLE [32,33]. In this case, the line blot assay was positive for anti-Yo antibodies, which was considered the cause of RPCS. However, commercial line blot assays for the anti-Yo antibody have low specificity [22,34]. These assays are targeted against CDR2; however, recent studies have demonstrated that the actual target of the anti-Yo antibody is CDR2L, which has only 45% homology with CDR2 [34,35]. Therefore, false-positive results may be obtained when only testing for CDR2 using line blot assays, which are not specific to RPCS. For anti-Yo-antibody-positive cases that do not conform to paraneoplastic neurological syndrome, the line blot assay and immunohistology results are concordant in only 8% (1/13) of cases [34]. Therefore, it is important to confirm the presence of positive anti-Yo antibodies through immunohistological tests such as TBA, especially in cases without malignancy [22,34].
There have been reported cases of SLE patients with a clinical course of RPCS [32,33]. In these reports of SLE-related RPCS, SLE activity was elevated when RPCS was present [32,33]. The pathogenesis of SLE-associated RPCS was suggested to be due to autoimmunity, cerebrovascular disease, or angiogenic edema, and the clinical symptoms were improved by immunotherapy [32,33]. In the present case, there were no signs of active SLE, and the patient’s clinical symptoms did not improve with steroid pulse therapy and IVIg. This suggests that SLE was unlikely to have contributed to the RPCS in this case. If SLE-associated RPCS is suspected, disease activity and response to immunotherapy should be confirmed.
SLE is considered as a risk factor of PML [36]; therapeutic glucocorticoids and MMF are also implicated in drug-related PML [8]. The neurological symptoms of PML are often cerebrum-related (cognitive dysfunction, language impairment, motor paralysis, etc.) [3], and a multicenter study of PML images reported that supra-tentorial lesions are more common than sub-tentorial lesions (supra-tentorial: 69.4%, sub-tentorial: 11.1%, both: 19.4%) [11]. Determining the imaging findings that should raise the suspicion of PML in PML-risk cases with a course of RPCS is important because the differentiation of RPCS varies widely. The MRI findings of progressed cerebellar PML were T2 and FLAIR hyperintense lesions extending from the cerebellar peduncle to the periventricular area of the fourth ventricle [12,14,15,16,19,20,37,38]. A punctate pattern characterizes natalizumab-associated PML [11,23,26], SLE-associated PML [24], and immune reconstitution inflammatory syndrome associated with PML [25,26]. In the present case, the focal lesion and surrounding spotty lesions in the left cerebellar peduncle were noticeable on day 39; however, the first MRI on day 7 also revealed a small spotty lesion. After the punctate pattern was observed, the lesion expanded along the fourth ventricle with time, and cerebellar and brainstem atrophy became evident. It is noteworthy that the punctate pattern in the MRI findings is an early manifestation of PML.
The treatment of PML often involves resolution of the immunosuppressive condition and discontinuation of the causative agent [4,12,14,15,16,39]. Combination antiretroviral therapy against HIV [4,39] is used for HIV-related PML, and plasma exchange is used for natalizumab-related PML [40]. However, SLE-related PML has no specific treatment. Mirtazapine, which is a noradrenergic and specific serotonergic antidepressant, has prevented the virus from binding to oligodendrocytes, and mefloquine, which is an antimalarial agent, has exhibited antiviral activity in vitro and may be used [4,12,16,19,41]. The PSL dose was reduced after consultation with a rheumatologist and after MMF discontinuation in the present case. However, it was difficult to assess the PML activity based on the time course of clinical symptoms. Mainly in HIV-associated PML, CSF JCV-DNA levels have been associated with PML stability [39,41]; therefore, the JCV-DNA copy number is thought to be an indicator of PML activity. Therefore, we measured the JCV-DNA copy number over time, and the JCV-DNA copy number decreased. Eventually, the JCV-DNA level decreased below the detection limit, and the patient achieved long-term survival.
The association between JCV-DNA copy number and background disease, treatment, and outcome was investigated by collecting case reports of SLE-associated cerebellar PML. Table 1 presents cases of SLE-associated cerebellar PML [12,13,14,15,16,17,18,19,20,37,38].

Table 1.
Systematic-lupus-erythematosus-associated cerebellar progressive multifocal leukoencephalopathy.
Of the 12 patients, 10, including ours, were women [12,13,14,15,17,18,19,38]. In addition to SLE, two patients had rheumatoid arthritis [14,15], and one had a history of lymphoma [19] and hemophagocytic syndrome [13]. Corticosteroids were used in all patients for whom information was available, and other immunosuppressants used concomitantly were methotrexate in four patients [13,14,16,38]; rituximab in three [13,14,19]; MMF [19], etanercept [14,15], and azathioprine [12,13] in two; and cyclophosphamide [13], cyclosporine [20], tacrolimus [16], bendamustine [19], hydroxychloroquine [15], tocilizumab [14], and thalidomide in one patient. Eight patients were diagnosed with PML by spinal fluid specimens [13,14,15,16,20,38], two by biopsy [17,37], one by spinal fluid and biopsy [19], and one by autopsy [18]. The CSF JCV copy number ranged from 700 to 67,000 copies/mL [12,15,38]. The treatments included mefloquine in five patients [12,14,16,19], mirtazapine in four [12,16,19], cidofovir in three [12,13,20], plasmapheresis (plasma exchange, double filtration plasmapheresis one each) in two [14,15], cytarabine in two [12,15], paroxetine in one [14], and interferon in one [18]. Additionally, the dose of immunosuppressants was reduced in all six patients [12,14,15,16,38], in which a change in SLE treatment was mentioned (not shown in Table 1). The JCV-DNA was retested in five patients and turned negative [14,16,20] in all except one [12]. The contribution of JCV-DNA negativity to survival demonstrated that three of the four negative cases (75%) [14,16,20] were alive. This rate is higher than the survival rate in the review [36], but the number of cases is small, and more cases need to be accumulated. Of the 11 patients for which an outcome was shown, 7 (63.6%) died during the follow-up period of 3 to 36 months [12,15,17,18,19,20,38]. In one of the patients who died, the JCV-DNA levels decreased below the detection limit [20]. In the case report, the cause of death was kidney failure with elevated urinary protein, suggesting the involvement of SLE flare-up in the death [20]. Therefore, in patients with SLE-associated PML, the dose of immunosuppressants should be carefully assessed and reduced while evaluating disease activity.
4. Conclusions
Cerebellar PML can present a clinical course consistent with RPCS. If positive anti-Yo antibodies are detected by line blot assay, additional immunohistological test is necessary, particularly in cases without malignancies. Early identification of PML, which is characterized by focal lesions and surrounding small spotty lesions called a “punctate pattern,” in MRI findings is necessary. Although cerebellar PML has poor prognosis, the JCV-DNA copy number may serve as an indicator of PML stability.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/neurolint15030059/s1. Figure S1: The result of an in-house tissue-based assay.
Author Contributions
T.A.: literature search, figures, data collection, and writing. S.H. and H.N.: drafting and revising the manuscript critically for important intellectual content. M.H. and K.N.: acquisition, analysis, and interpretation of the data, and drafting and revising the manuscript critically for important intellectual. All authors have read and agreed to the published version of the manuscript.
Funding
This work was partly supported by the Research Committee of Prion Disease and Slow Virus Infection, Research on Policy Planning and Evaluation for Rare and Intractable Diseases, Health and Labour Sciences Research Grants, the Ministry of Health, Labour and Welfare, Japan, and by JSPS KAKENHI (grant No. 21K07450) (Kazuo Nakamichi) and MHLW grant number 22HA1003 and JSPS KAKENHI (grant No. JP20K07875) (Makoto Hara).
Institutional Review Board Statement
This study was approved by the Nihon University Itabashi Hospital, Clinical Research Judging Committee (SH-0004, 23 June 2023).
Informed Consent Statement
Written informed consent was obtained from the patient to publish this paper.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Barth, H.; Solis, M.; Lepiller, Q.; Sueur, C.; Soulier, E.; Caillard, S.; Stoll-Keller, F.; Fafi-Kremer, S. 45 years after the discovery of human polyomaviruses BK and JC: Time to speed up the understanding of associated diseases and treatment approaches. Crit. Rev. Microbiol. 2017, 43, 178–195. [Google Scholar] [CrossRef]
- White, M.K.; Khalili, K. Pathogenesis of progressive multifocal leukoencephalopathy—Revisited. J. Infect. Dis. 2011, 203, 578–586. [Google Scholar] [CrossRef]
- Berger, J.R.; Aksamit, A.J.; Clifford, D.B.; Davis, L.; Koralnik, I.J.; Sejvar, J.J.; Bartt, R.; Major, E.O.; Nath, A. PML diagnostic criteria: Consensus statement from the AAN Neuroinfectious Disease Section. Neurology 2013, 80, 1430–1438. [Google Scholar] [CrossRef]
- Alstadhaug, K.B.; Myhr, K.M.; Rinaldo, C.H. Progressive multifocal leukoencephalopathy. Tidsskr. Nor. Laegeforen. 2017, 137, 23–24. [Google Scholar] [CrossRef][Green Version]
- Du Pasquier, R.A.; Corey, S.; Margolin, D.H.; Williams, K.; Pfister, L.A.; De Girolami, U.; Mac Key, J.J.; Wüthrich, C.; Joseph, J.T.; Koralnik, I.J. Productive infection of cerebellar granule cell neurons by JC virus in an HIV+ individual. Neurology 2003, 61, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Jelcic, I.; Jelcic, I.; Kempf, C.; Largey, F.; Planas, R.; Schippling, S.; Budka, H.; Sospedra, M.; Martin, R. Mechanisms of immune escape in central nervous system infection with neurotropic JC virus variant. Ann. Neurol. 2016, 79, 404–418. [Google Scholar] [CrossRef] [PubMed]
- Clifford, D.B.; De Luca, A.; Simpson, D.M.; Arendt, G.; Giovannoni, G.; Nath, A. Natalizumab-associated progressive multifocal leukoencephalopathy in patients with multiple sclerosis: Lessons from 28 cases. Lancet Neurol. 2010, 9, 438–446. [Google Scholar] [CrossRef]
- Yukitake, M. Drug-induced progressive multifocal leukoencephalopathy in multiple sclerosis: A comprehensive review. Clin. Exp. Neuroimmunol. 2018, 9, 37–47. [Google Scholar] [CrossRef]
- Berger, J.R.; Pall, L.; Lanska, D.; Whiteman, M. Progressive multifocal leukoencephalopathy in patients with HIV infection. J. Neurovirol. 1998, 4, 59–68. [Google Scholar] [CrossRef]
- Brooks, B.R.; Walker, D.L. Progressive multifocal leukoencephalopathy. Neurol. Clin. 1984, 2, 299–313. [Google Scholar] [CrossRef]
- Alleg, M.; Solis, M.; Baloglu, S.; Cotton, F.; Kerschen, P.; Bourre, B.; Ahle, G.; Pruvo, J.P.; Leclerc, X.; Vermersch, P.; et al. Progressive multifocal leukoencephalopathy: MRI findings in HIV-infected patients are closer to rituximab- than natalizumab-associated PML. Eur. Radiol. 2021, 31, 2944–2955. [Google Scholar] [CrossRef]
- Berntsson, S.G.; Katsarogiannis, E.; Lourenço, F.; Moraes-Fontes, M.F. Progressive Multifocal Leukoencephalopathy and Systemic Lupus Erythematosus: Focus on Etiology. Case Rep. Neurol. 2016, 8, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Brandão, M.; Damásio, J.; Marinho, A.; da Silva, A.M.; Vasconcelos, J.; Neves, E.; Almeida, I.; Farinha, F.; Vasconcelos, C. Systemic lupus erythematosus, progressive multifocal leukoencephalopathy, and T-CD4+ lymphopenia. Clin. Rev. Allergy Immunol. 2012, 43, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.F.; Su, J.J.; Chen, Y.F.; Lin, Y.C.; Huang, Y.M.; Li, K.J. Progressive multifocal leukoencephalopathy in a 27-year-old lady with systemic lupus erythematosus—Rheumatoid arthritis overlap syndrome. J. Formos. Med. Assoc. 2019, 118, 1560–1565. [Google Scholar] [CrossRef]
- Graff-Radford, J.; Robinson, M.T.; Warsame, R.M.; Matteson, E.L.; Eggers, S.D.; Keegan, B.M. Progressive multifocal leukoencephalopathy in a patient treated with etanercept. Neurologist 2012, 18, 85–87. [Google Scholar] [CrossRef]
- Hamaguchi, M.; Suzuki, K.; Fujita, H.; Uzuka, T.; Matsuda, H.; Shishido-Hara, Y.; Arai, S.; Nakamura, T.; Kikuchi, S.; Nakamichi, K.; et al. Successful treatment of non-HIV progressive multifocal leukoencephalopathy: Case report and literature review. J. Neurol. 2020, 267, 731–738. [Google Scholar] [CrossRef]
- Jones, H.R., Jr.; Hedley-Whyte, E.T.; Freidberg, S.R.; Kelleher, J.E., Jr.; Krolikowski, J. Primary cerebellopontine progressive multifocal leukoencephalopathy diagnosed premortem by cerebellar biopsy. Ann. Neurol. 1982, 11, 199–202. [Google Scholar] [CrossRef]
- Kinoshita, M.; Iwana, K.; Shinoura, H.; Aotsuka, S.; Sumiya, M. Progressive multifocal leukoencephalopathy resembling central nervous system systemic lupus erythematosus. Clin. Exp. Rheumatol. 1998, 16, 313–315. [Google Scholar]
- Sakuraba, M.; Watanabe, S.; Nishiyama, Y.; Takahashi, K.; Nakamichi, K.; Suzuki, M.; Nawata, T.; Komai, K.; Gono, T.; Takeno, M.; et al. Infratentorial onset of progressive multifocal leukoencephalopathy in a patient with systematic lupus erythematosus complicated with lymphoma: A case report. Mod. Rheumatol. Case Rep. 2021, 5, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Salmaggi, A.; Maccagnano, E.; Castagna, A.; Zeni, S.; Fantini, F.; Cinque, P.; Savoiardo, M. Reversal of CSF positivity for JC virus genome by cidofovir in a patient with systemic lupus erythematosus and progressive multifocal leukoencephalopathy. Neurol. Sci. 2001, 22, 17–20. [Google Scholar] [CrossRef]
- de Silva, R.N.; Vallortigara, J.; Greenfield, J.; Hunt, B.; Giunti, P.; Hadjivassiliou, M. Diagnosis and management of progressive ataxia in adults. Pract. Neurol. 2019, 19, 196–207. [Google Scholar] [CrossRef]
- Graus, F.; Vogrig, A.; Muñiz-Castrillo, S.; Antoine, J.G.; Desestret, V.; Dubey, D.; Giometto, B.; Irani, S.R.; Joubert, B.; Leypoldt, F.; et al. Updated Diagnostic Criteria for Paraneoplastic Neurologic Syndromes. Neurol. R Neuroimmunol. Neuroinflammation 2021, 8, e1014. [Google Scholar] [CrossRef] [PubMed]
- Hodel, J.; Darchis, C.; Outteryck, O.; Verclytte, S.; Deramecourt, V.; Lacour, A.; Zins, M.; Pruvo, J.P.; Vermersch, P.; Leclerc, X. Punctate pattern: A promising imaging marker for the diagnosis of natalizumab-associated PML. Neurology 2016, 86, 1516–1523. [Google Scholar] [CrossRef]
- Ishii, J.; Shishido-Hara, Y.; Kawamoto, M.; Fujiwara, S.; Imai, Y.; Nakamichi, K.; Kohara, N. A Punctate Magnetic Resonance Imaging Pattern in a Patient with Systemic Lupus Erythematosus Is an Early Sign of Progressive Multifocal Leukoencephalopathy: A Clinicopathological Study. Intern. Med. 2018, 57, 2727–2734. [Google Scholar] [CrossRef] [PubMed]
- Nakahara, J.; Tomaske, L.; Kume, K.; Takata, T.; Kamada, M.; Deguchi, K.; Kufukihara, K.; Schneider, R.; Gold, R.; Ayzenberg, I. Three cases of non-carryover fingolimod-PML: Is the risk in Japan increased? Neurol. R Neuroimmunol. Neuroinflammation 2019, 6, e559. [Google Scholar] [CrossRef] [PubMed]
- Wattjes, M.P.; Verhoeff, L.; Zentjens, W.; Killestein, J.; van Munster, E.T.; Barkhof, F.; van Eijk, J.J. Punctate lesion pattern suggestive of perivascular inflammation in acute natalizumab-associated progressive multifocal leukoencephalopathy: Productive JC virus infection or preclinical PML-IRIS manifestation? J. Neurol. Neurosurg. Psychiatry 2013, 84, 1176–1177. [Google Scholar] [CrossRef]
- Nakamichi, K.; Kawamoto, M.; Ishii, J.; Saijo, M. Improving detection of JC virus by ultrafiltration of cerebrospinal fluid before polymerase chain reaction for the diagnosis of progressive multifocal leukoencephalopathy. BMC Neurol. 2019, 19, 252. [Google Scholar] [CrossRef]
- Ryschkewitsch, C.F.; Jensen, P.N.; Major, E.O. Multiplex qPCR assay for ultra sensitive detection of JCV DNA with simultaneous identification of genotypes that discriminates non-virulent from virulent variants. J. Clin. Virol. 2013, 57, 243–248. [Google Scholar] [CrossRef]
- Schmitz-Hübsch, T.; du Montcel, S.T.; Baliko, L.; Berciano, J.; Boesch, S.; Depondt, C.; Giunti, P.; Globas, C.; Infante, J.; Kang, J.S.; et al. Scale for the assessment and rating of ataxia: Development of a new clinical scale. Neurology 2006, 66, 1717–1720. [Google Scholar] [CrossRef]
- Panegyres, P.K.; Graves, A. Anti-Yo and anti-glutamic acid decarboxylase antibodies presenting in carcinoma of the uterus with paraneoplastic cerebellar degeneration: A case report. J. Med. Case Rep. 2012, 6, 155. [Google Scholar] [CrossRef]
- Shargian-Alon, L.; Raanani, P.; Rozovski, U.; Siegal, T.; Yust-Katz, S.; Yeshurun, M. Immune Mediated Cerebellar Ataxia: An Unknown Manifestation of Graft-versus-Host Disease. Acta Haematol. 2019, 141, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Casciato, S.; Mascia, A.; Quarato, P.P.; D’Aniello, A.; Scoppetta, C.; Di Gennaro, G. Subacute cerebellar ataxia as presenting symptom of systemic lupus erythematosus. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 7401–7403. [Google Scholar] [CrossRef] [PubMed]
- Sy, M.C.C.; Reyes, N.G.D.; Zamora, G.T.; Fernandez, M.L.L. Cerebellar ataxia as a primary manifestation of neuropsychiatric systemic lupus erythematosus. BMJ Case Rep. 2021, 14, e236825. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-García, R.; Martínez-Hernández, E.; Saiz, A.; Dalmau, J.; Graus, F. The Diagnostic Value of Onconeural Antibodies Depends on How They Are Tested. Front. Immunol. 2020, 11, 1482. [Google Scholar] [CrossRef]
- Dalmau, J.; Dalakas, M.C.; Kolson, D.L.; Paul, F.; Sánchez-Valle, R.; Zamvil, S.S. N2 Year in Review. Neurol. R Neuroimmunol. Neuroinflammation 2023, 10, e200076. [Google Scholar] [CrossRef] [PubMed]
- Henegar, C.E.; Eudy, A.M.; Kharat, V.; Hill, D.D.; Bennett, D.; Haight, B. Progressive multifocal leukoencephalopathy in patients with systemic lupus erythematosus: A systematic literature review. Lupus 2016, 25, 617–626. [Google Scholar] [CrossRef]
- Svensson, P.A.; Larsson, E.M. Infratentorial progressive multifocal leucoencephalopathy (PML) in a patient with SLE (2008: 4b). Eur. Radiol. 2008, 18, 1526–1528. [Google Scholar] [CrossRef]
- Zhong, M.; Kempster, P.A.; Phan, T.G. John Cunningham virus granule cell neuronopathy in a mildly immunosuppressed patient with systemic lupus erythematosus. Intern. Med. J. 2019, 49, 804–805. [Google Scholar] [CrossRef]
- Bossolasco, S.; Calori, G.; Moretti, F.; Boschini, A.; Bertelli, D.; Mena, M.; Gerevini, S.; Bestetti, A.; Pedale, R.; Sala, S.; et al. Prognostic significance of JC virus DNA levels in cerebrospinal fluid of patients with HIV-associated progressive multifocal leukoencephalopathy. Clin. Infect. Dis. 2005, 40, 738–744. [Google Scholar] [CrossRef]
- Dong-Si, T.; Richman, S.; Wattjes, M.P.; Wenten, M.; Gheuens, S.; Philip, J.; Datta, S.; McIninch, J.; Bozic, C.; Bloomgren, G.; et al. Outcome and survival of asymptomatic PML in natalizumab-treated MS patients. Ann. Clin. Transl. Neurol. 2014, 1, 755–764. [Google Scholar] [CrossRef]
- Clifford, D.B.; Nath, A.; Cinque, P.; Brew, B.J.; Zivadinov, R.; Gorelik, L.; Zhao, Z.; Duda, P. A study of mefloquine treatment for progressive multifocal leukoencephalopathy: Results and exploration of predictors of PML outcomes. J. Neurovirol. 2013, 19, 351–358. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).