Reversible Cerebral Vasoconstriction Syndrome in the Postpartum Period: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
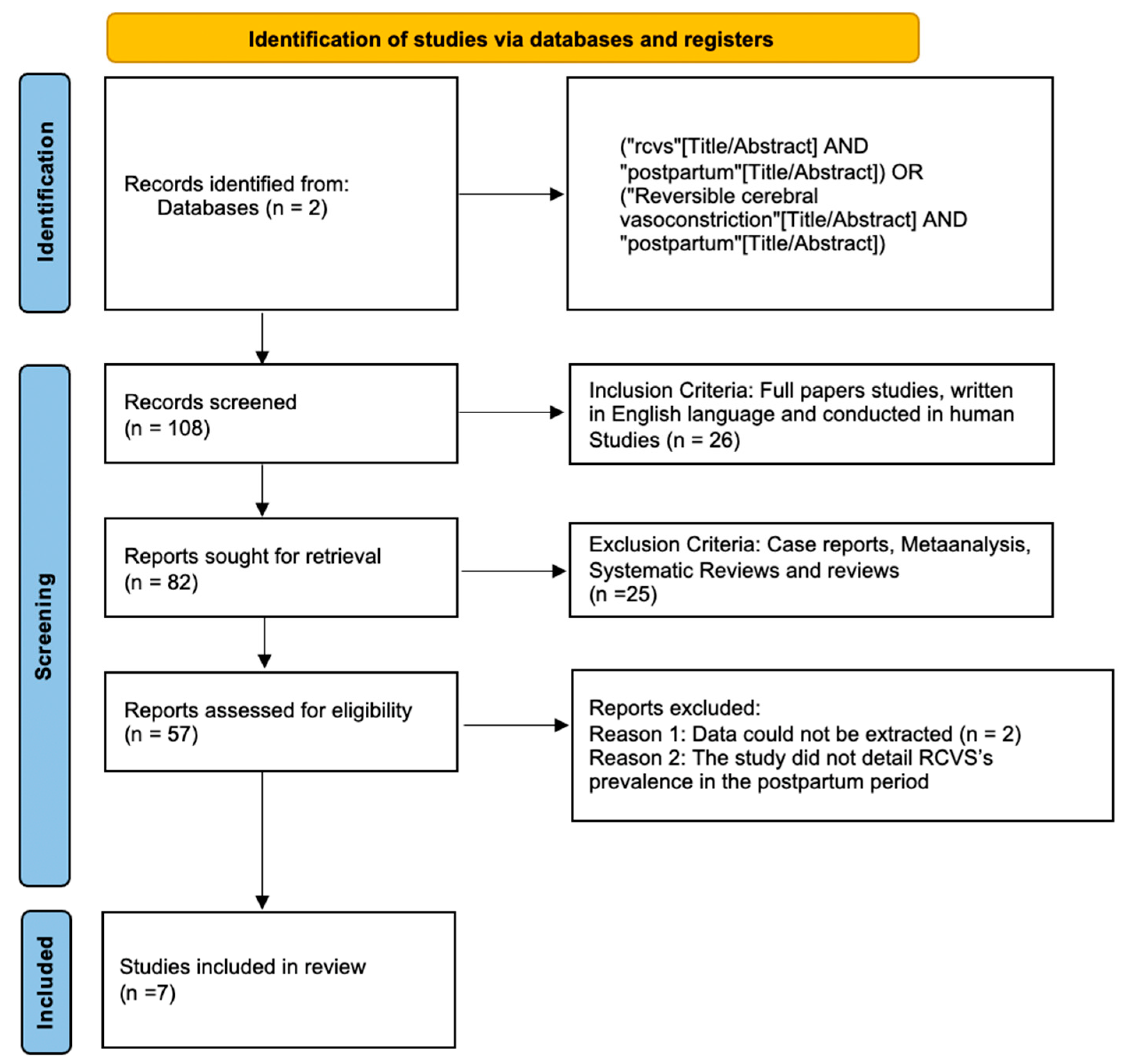
2. Materials and Methods
2.1. Protocol
2.1.1. Eligibility Criteria and Study Selection
2.1.2. Database and Search Strategy
2.1.3. Data Extraction and Analysis
2.1.4. Bias Assessment
2.1.5. Data Analysis
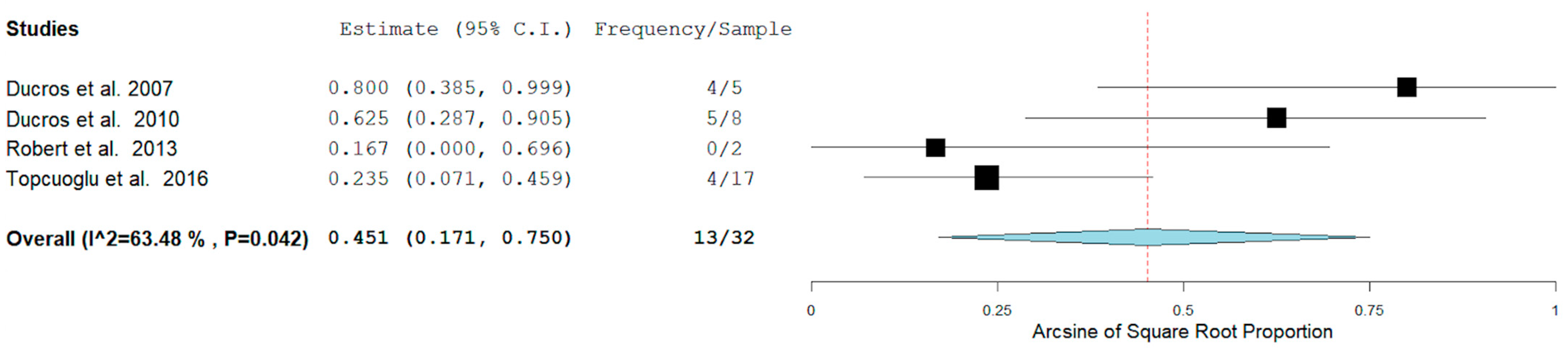
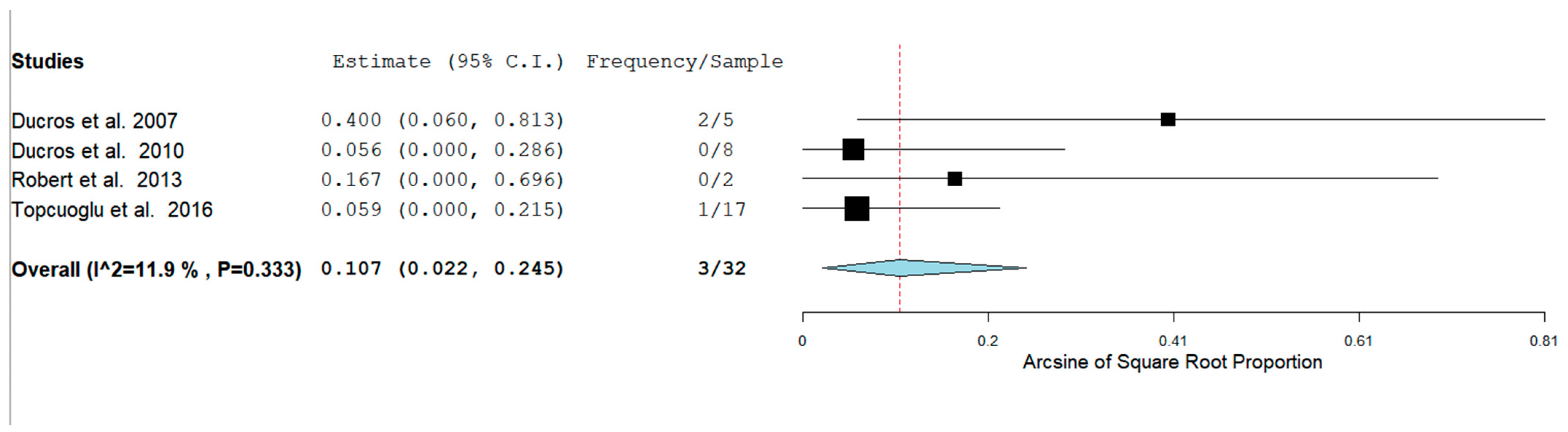
3. Results
Bias Analysis
4. Discussion
4.1. Role of Sex and Pregnancy
4.2. Hemorrhagic RCVS
4.3. Recurrence of RCVS
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Lemmens, R.; Smet, S.; Wilms, G.; Demaerel, P.; Thijs, V. Postpartum RCVS and PRES with normal initial imaging findings. Acta Neurol. Belg. 2012, 112, 189–192. [Google Scholar] [CrossRef] [PubMed]
- Burton, T.M.; Bushnell, C.D. Reversible Cerebral Vasoconstriction Syndrome. Stroke 2019, 50, 2253–2258. [Google Scholar] [CrossRef] [PubMed]
- Anzola, G.P.; Brighenti, R.; Cobelli, M.; Giossi, A.; Mazzucco, S.; Olivato, S.; Pari, E.; Piras, M.P.; Padovani, A.; Rinaldi, F.; et al. Reversible cerebral vasoconstriction syndrome in puerperium: A prospective study. J. Neurol. Sci. 2017, 375, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Pilato, F.; Distefano, M.; Calandrelli, R. Posterior Reversible Encephalopathy Syndrome and Reversible Cerebral Vasoconstriction Syndrome: Clinical and Radiological Considerations. Front. Neurol. 2020, 11, 34. [Google Scholar] [CrossRef] [PubMed]
- Cappelen-Smith, C.; Calic, Z.; Cordato, D. Reversible Cerebral Vasoconstriction Syndrome: Recognition and Treatment. Curr. Treat. Options Neurol. 2017, 19, 34. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, L.H.; Dodick, D.W.; Schwedt, T.; Singhal, A.B. Narrative Review: Reversible Cerebral Vasoconstriction Syndromes. Ann. Intern. Med. 2007, 146, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Cha, J.; Choi, H.A.; Woo, S.-Y.; Kim, S.; Wang, S.-J.; Chung, C.-S. Blood-brain barrier breakdown in reversible cerebral vasoconstriction syndrome: Implications for pathophysiology and diagnosis. Ann. Neurol. 2017, 81, 454–466. [Google Scholar] [CrossRef] [PubMed]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000, 283, 2008–2012. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fabara, S.P.; Ortiz, J.F.; Smith, D.W.; Parwani, J.; Srikanth, S.; Varghese, T.; Paez, M.; Desai, P.; Tirupathi, R. Crimean-Congo Hemorrhagic Fever Beyond Ribavirin. Cureus 2021, 13. [Google Scholar] [CrossRef] [PubMed]
- Ducros, A.; Boukobza, M.; Porcher, R.; Sarov, M.; Valade, D.; Bousser, M.-G. The clinical and radiological spectrum of reversible cerebral vasoconstriction syndrome. A prospective series of 67 patients. Brain 2007, 130, 3091–3101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ducros, A.; Fiedler, U.; Porcher, R.; Boukobza, M.; Stapf, C.; Bousser, M.-G. Hemorrhagic Manifestations of Reversible Cerebral Vasoconstriction Syndrome. Stroke 2010, 41, 2505–2511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robert, T.; Marchini, A.K.; Oumarou, G.; Uské, A. Reversible cerebral vasoconstriction syndrome identification of prognostic factors. Clin. Neurol. Neurosurg. 2013, 115, 2351–2357. [Google Scholar] [CrossRef] [PubMed]
- Topcuoglu, M.A.; Singhal, A.B. Hemorrhagic Reversible Cerebral Vasoconstriction Syndrome. Stroke 2016, 47, 1742–1747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Boysson, H.; Parienti, J.-J.; Mawet, J.; Arquizan, C.; Boulouis, G.; Burcin, C.; Naggara, O.; Zuber, M.; Touzé, E.; Aouba, A.; et al. Primary angiitis of the CNS and reversible cerebral vasoconstriction syndrome: A comparative study. Neurology 2018, 91, e1468–e1478. [Google Scholar] [CrossRef] [PubMed]
- Boitet, R.; De Gaalon, S.; Duflos, C.; Marin, G.; Mawet, J.; Burcin, C.; Roos, C.; Fiedler, U.; Bousser, M.-G.; Ducros, A. Long-Term Outcomes After Reversible Cerebral Vasoconstriction Syndrome. Stroke 2020, 51, 670–673. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.D.; Topiwala, K.; Saini, V.; Patel, N.; Pervez, M.; Al-Mufti, F.; Hassan, A.E.; Khandelwal, P.; Starke, R.M. Hemorrhagic reversible cerebral vasoconstriction syndrome: A retrospective observational study. J. Neurol. 2020, 268, 632–639. [Google Scholar] [CrossRef] [PubMed]
- Song, T.J.; Lee, K.H.; Li, H.; Kim, J.Y.; Chang, K.; Kim, S.H.; Smith, L. Reversible cerebral vasoconstriction syndrome: A comprehensive systematic review. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 3519–3529. [Google Scholar] [CrossRef] [PubMed]
- Topcuoglu, M.A.; McKee, K.E.; Singhal, A.B. Gender and hormonal influences in reversible cerebral vasoconstriction syndrome. Eur. Stroke J. 2016, 1, 199–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.-P.; Fuh, J.-L.; Lirng, J.-F.; Wang, Y.-F.; Wang, S.-J. Recurrence of reversible cerebral vasoconstriction syndrome: A long-term follow-up study. Neurology 2015, 84, 1552–1558. [Google Scholar] [CrossRef] [PubMed]


| Author and Year of Publication, Country | Study Design | No. of Patients | Mean Age | Associated Conditions | Prevalence of RCVS in the PP among Females | Hemorrhage among RCVS Patients in the PP | Recurrence among RCVS Patients in the PP |
|---|---|---|---|---|---|---|---|
| Ducrose et al. (2007) France [11] | Prospective single center observational study | N = 67 (F: 43 and M: 24) | 42.5 ± 11.8 (F: 46.9 ± 11.5) | Postpartum period (8%), vasoactive substance (55%), none (37%) | 5/43 | 5/5 (ICH 4/5 and SAH 2/5) | Not reported |
| Ducrose et al. (2010) France [12] | Prospective cohort | N = 89 (F: 61 and M: 28) | RCVS With ICH: 46.6 ± 11.0 RCVS Without ICH: 41.6 ± 11.6 | Postpartum period (8.98%), vasoactive substance (51.68), migraine (26.96%), HTA (11.23%) | 8/61 | 5/8 (ICH 5/8 and SAH 0/8) | Not reported |
| Robert et al. (2013) Switzerland [13] | Retrospective multi-center review | N = 10 (F: 7 and M: 3) | 46 | Postpartum period (29%), Migraine (28%), HTA (71%), vasoactive substances (57%) | 2/7 | 0/2 (ICH 0/2 and SAH 0/2) | Not reported |
| Topcuoglu et al. (2016) United States [14] | Single center restrospective study | N = 162 (F:126 and M: 36) | 44 ± 13 | Postpartum period (Hem: 9% no Hem: 11%), vasoconstrictive drugs (Hem: 61% no Hem: 59%), Physiological/Idiopathic (Hem: 31% no Hem: 29%), HTA (Hem: 42% no Hem: 3 | 17/126 | 6/17 (ICH 4/17 and SAH 1/17) | Not reported |
| De Boysson et al. (2018) France [15] | Comparative study | N = 173 (F: 122 and M: 51) | 44 | Postpartum period (8.09%), Migraine (32.37%), tobacco use (35.83%), HTA (15.02%), vasoactive substances (49.13%) | 14/122 | Not reported | Not reported |
| Boitet et al. (2019) France [16] | Prospective single center observational study | N = 173 (F: 122 and M: 51) | Not reported | Postpartum period (8.18%), vasoactive substance (48.53%), none (43.29%) | 14/122 | Not reported | 0/14 |
| Patel et al. (2021) United States [17] | Retrospective observational study (data base) | N = 799 (F: 602 and M: 197) | 46.3 ± 0.8 | Postpartum period (11.5%), Migraine (22%), HTA (51.8%), Inflammatory disorders (18.2%), pregnancy (6.5%), pregnancy HTA (6.6%) | 69/602 | 35/69 | Not reported |
| Totales | 1083/1473 (73.5%) |
| Study | Newcastle-Ottawa Scale | Overall Risk of Bias | ||
|---|---|---|---|---|
| Selection (max 4) | Comparability (max 2) | Outcome/Exposure (max 3) | ||
| Ducrose et al. France, 2007 | *** | ** | ** | Moderate |
| Ducrose et al. France, 2010 | *** | ** | ** | Moderate |
| Robert et al. Switzerland, 2013 | * | * | * | High |
| Topcuoglu et al. United States, 2016 | *** | ** | ** | Moderate |
| De Boysson et al. France, 2018 | *** | ** | ** | Moderate |
| Boitet et al. France, 2019 | *** | ** | ** | Moderate |
| Patel et al. United States, 2021 | *** | ** | ** | Moderate |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pacheco, K.; Ortiz, J.F.; Parwani, J.; Cruz, C.; Yépez, M.; Buj, M.; Khurana, M.; Ojeda, D.; Iturburu, A.; Aguirre, A.S.; et al. Reversible Cerebral Vasoconstriction Syndrome in the Postpartum Period: A Systematic Review and Meta-Analysis. Neurol. Int. 2022, 14, 488-496. https://doi.org/10.3390/neurolint14020040
Pacheco K, Ortiz JF, Parwani J, Cruz C, Yépez M, Buj M, Khurana M, Ojeda D, Iturburu A, Aguirre AS, et al. Reversible Cerebral Vasoconstriction Syndrome in the Postpartum Period: A Systematic Review and Meta-Analysis. Neurology International. 2022; 14(2):488-496. https://doi.org/10.3390/neurolint14020040
Chicago/Turabian StylePacheco, Kimberly, Juan Fernando Ortiz, Jashank Parwani, Claudio Cruz, Mario Yépez, Maja Buj, Mahika Khurana, Diego Ojeda, Alisson Iturburu, Alex S. Aguirre, and et al. 2022. "Reversible Cerebral Vasoconstriction Syndrome in the Postpartum Period: A Systematic Review and Meta-Analysis" Neurology International 14, no. 2: 488-496. https://doi.org/10.3390/neurolint14020040
APA StylePacheco, K., Ortiz, J. F., Parwani, J., Cruz, C., Yépez, M., Buj, M., Khurana, M., Ojeda, D., Iturburu, A., Aguirre, A. S., Yuen, R., & Datta, S. (2022). Reversible Cerebral Vasoconstriction Syndrome in the Postpartum Period: A Systematic Review and Meta-Analysis. Neurology International, 14(2), 488-496. https://doi.org/10.3390/neurolint14020040










