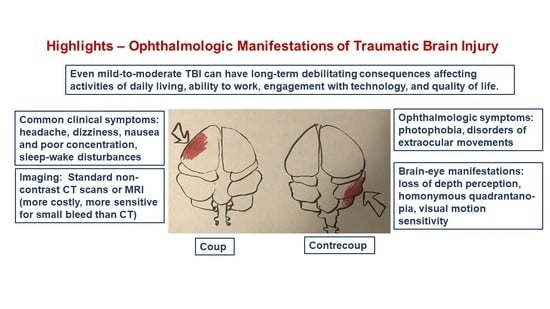
Mild-to-Moderate Traumatic Brain Injury: A Review with Focus on the Visual System
Abstract
1. Introduction
2. Methods
3. Basics of TBI
3.1. Detemining Severity
3.2. Initial Treatment
3.3. Diagnostic Issues in mTBI
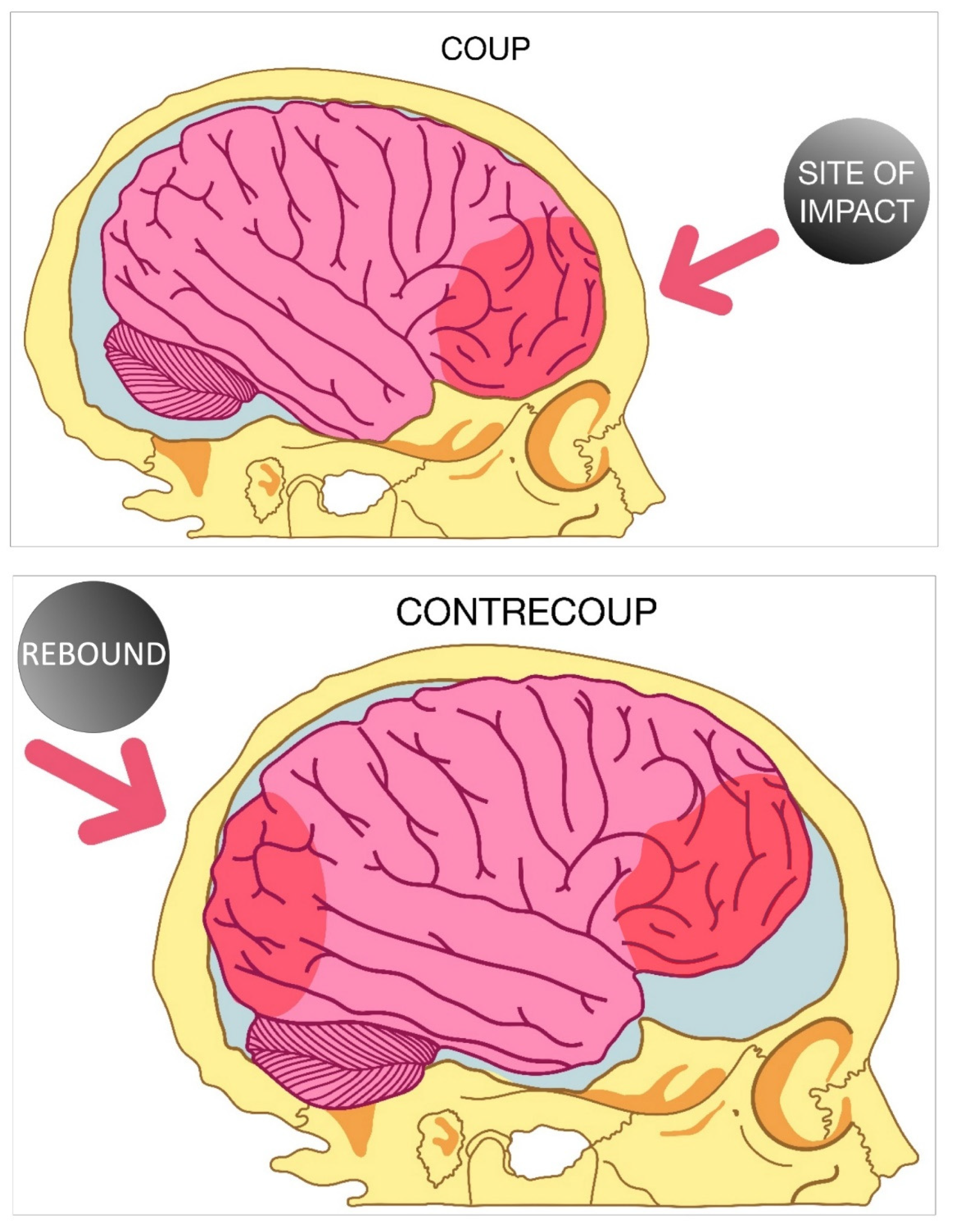
4. Focal and Diffuse Injury
5. Brain Imaging Techniques in TBI
6. Visual Symptoms of TBI
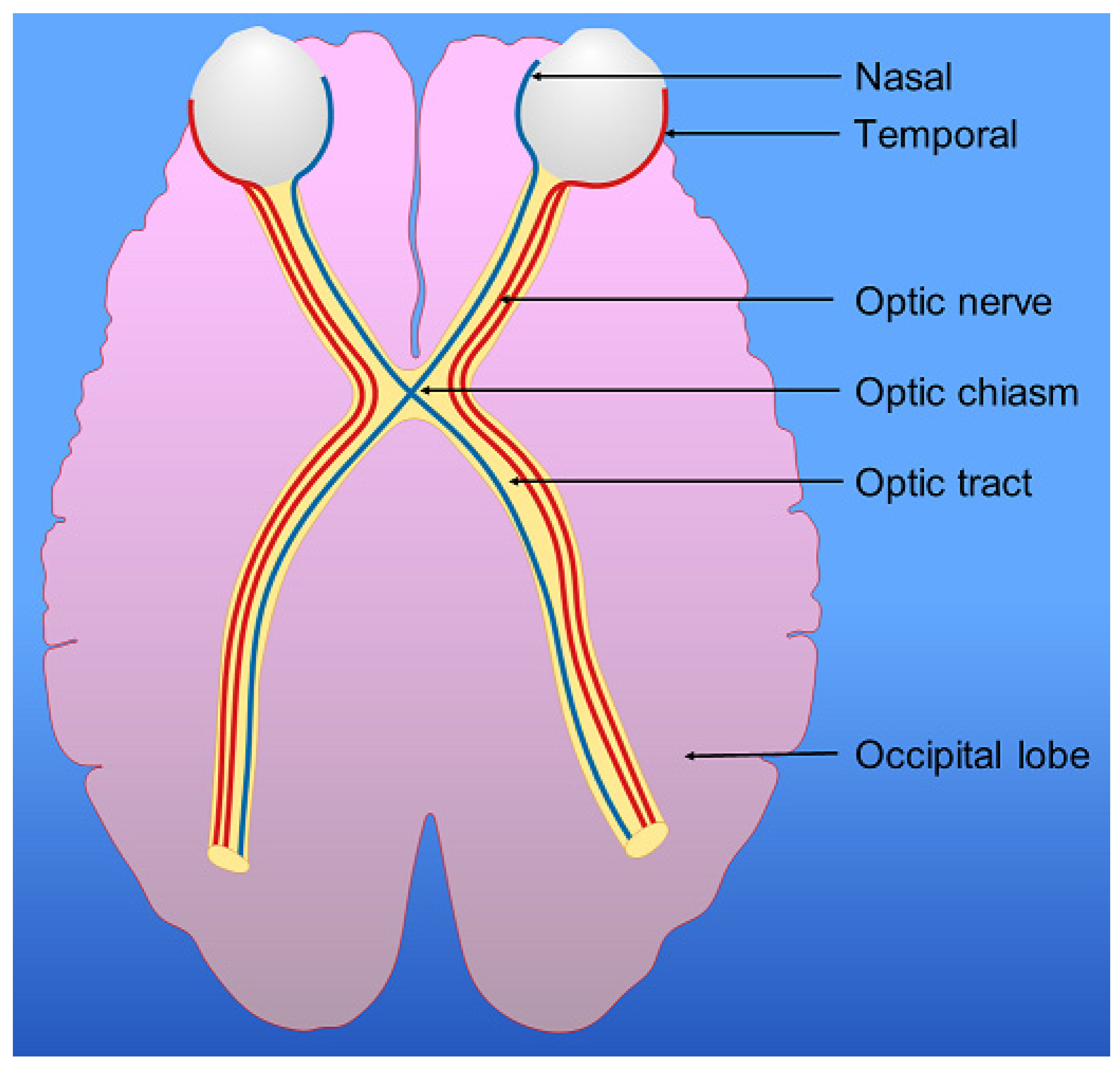
7. Visual Pathway, Parietal Lobes and Vision
8. TBI Affects the Parietal Lobes, Vestibular System and Visual Perception
8.1. Parietal Lobes
8.2. Vestibular System
8.3. Visuospatial Neglect
9. TBI, Insomnia, and the Eyes
10. The Ruptured Globe
11. An Ophthalmologist Clinical Perspective
11.1. Diagnostic Tools for Ophthalmologic Evaluation of TBI
11.2. Visual Consequences of TBI in Daily Life
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iaccarino, C.; Carretta, A.; Nicolosi, F.; Morselli, C. Epidemiology of severe traumatic brain injury. J. Neurosurg. Sci. 2018, 62, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. National Center for Health Statistics: Mortality Data on CDC WONDER. Available online: https://wonder.cdc.gov/mcd.html (accessed on 20 April 2022).
- Savitsky, B.; Givon, A.; Rozenfeld, M.; Radomislensky, I.; Peleg, K. Traumatic brain injury: It is all about definition. Brain Inj. 2016, 30, 1194–1200. [Google Scholar] [CrossRef] [PubMed]
- Dewan, M.C.; Rattani, A.; Gupta, S.; Baticulon, R.E.; Hung, Y.C.; Punchak, M.; Agrawal, A.; Adeleye, A.O.; Shrime, M.G.; Rubiano, A.M.; et al. Estimating the global incidence of traumatic brain injury. J. Neurosurg. 2019, 130, 1080–1097. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.A.; Bell, J.M.; Breiding, M.J.; Xu, L. Traumatic brain injury–related emergency department visits, hospitalizations, and deaths—United States, 2007 and 2013. MMWR Surveill. Summ. 2017, 66, 1–16. [Google Scholar] [CrossRef]
- Khellaf, A.; Khan, D.Z.; Helmy, A. Recent advances in traumatic brain injury. J. Neurol. 2019, 266, 2878–2889. [Google Scholar] [CrossRef]
- Bonow, R.H.; Barber, J.; Temkin, N.R.; Videtta, W.; Rondina, C.; Petroni, G.; Lujan, S.; Alanis, V.; La Fuente, G.; Lavadenz, A.; et al. The outcome of severe traumatic brain injury in Latin America. World Neurosurg. 2018, 111, e82–e90. [Google Scholar] [CrossRef]
- Rubiano, A.M.; Carney, N.; Chesnut, R.; Puyana, J.C. Global neurotrauma research challenges and opportunities. Nature 2015, 527, S193–S197. [Google Scholar] [CrossRef]
- McAllister, T.W.; Flashman, L.A.; McDonald, B.C.; Saykin, A.J. Mechanisms of working memory dysfunction after mild and moderate TBI: Evidence from functional MRI and neurogenetics. J. Neurotrauma 2006, 23, 1450–1467. [Google Scholar] [CrossRef]
- Cassidy, J.D.; Cancelliere, C.; Carroll, L.J.; Côté, P.; Hincapié, C.A.; Holm, L.W.; Hartvigsen, J.; Donovan, J.; Boussard, C.N.-D.; Kristman, V.L.; et al. Systematic review of self-reported prognosis in adults after mild traumatic brain injury: Results of the international collaboration on mild traumatic brain injury prognosis. Arch. Phys. Med. Rehabil. 2014, 95, S132–S151. [Google Scholar] [CrossRef]
- Menon, D.K.; Schwab, K.; Wright, D.W.; Maas, A.I. Position statement: Definition of traumatic brain injury. Arch. Phys. Med. Rehabil. 2010, 91, 1637–1640. [Google Scholar] [CrossRef]
- Feddermann-Demont, N.; Echemendia, R.J.; Schneider, K.J.; Solomon, G.S.; Hayden, K.A.; Turner, M.; Dvořák, J.; Straumann, D.; Tarnutzer, A.A. What domains of clinical function should be assessed after sport-related concussion? A systematic review. Br. J. Sports Med. 2017, 51, 903–918. [Google Scholar] [CrossRef] [PubMed]
- Darshini, J.K.; Afsar, M.; Vandana, V.P.; Shukla, D.; Rajeswaran, J. The Triad of Cognition, Language, and Communication in Traumatic Brain Injury: A Correlational Study. J. Neurosci. Rural Pract. 2021, 12, 666–672. [Google Scholar] [CrossRef] [PubMed]
- Capizzi, A.; Woo, J.; Verduzco-Gutierrez, M. Traumatic Brain Injury: An Overview of Epidemiology, Pathophysiology, and Medical Management. Med. Clin. N. Am. 2020, 104, 213–238. [Google Scholar] [CrossRef]
- Teasdale, G.; Maas, A.; Lecky, F.; Manley, G.; Stocchetti, N.; Murray, G. The Glasgow coma scale at 40 years: Standing the test of time. Lancet Neurol. 2014, 13, 844–854. [Google Scholar] [CrossRef]
- Mena, J.H.; Sanchez, A.I.; Rubiano, A.M.; Peitzman, A.B.; Sperry, J.L.; Gutierrez, M.I.; Puyana, J.C. Effect of the modified Glasgow Coma Scale score criteria for mild traumatic brain injury on mortality prediction: Comparing classic and modified Glasgow Coma Scale score model scores of 13. J. Trauma 2011, 71, 1185–1193. [Google Scholar] [CrossRef]
- Chung, P.; Khan, F. Traumatic Brain Injury (TBI): Overview of Diagnosis and Treatment. J. Neurol. Neurophysiol. 2013, 5, 1. [Google Scholar] [CrossRef]
- Davanzo, J.R.; Sieg, E.P.; Timmons, S.D. Management of Traumatic Brain Injury. Surg. Clin. N. Am. 2017, 97, 1237–1253. [Google Scholar] [CrossRef] [PubMed]
- Abdelmalik, P.A.; Draghic, N.; Ling, G.S.F. Management of moderate and severe traumatic brain injury. Transfusion 2019, 59, 1529–1538. [Google Scholar] [CrossRef]
- Taran, S.; Pelosi, P.; Robba, C. Optimizing oxygen delivery to the injured brain. Curr. Opin. Crit. Care 2022, 28, 145–156. [Google Scholar] [CrossRef]
- Khan, R.; Alromaih, S.; Alshabanat, H.; Alshanqiti, N.; Aldhuwaihy, A.; Almohanna, S.A.; Alqasem, M.; Al-Dorzi, H. The Impact of Hyperoxia Treatment on Neurological Outcomes and Mortality in Moderate to Severe Traumatic Brain Injured Patients. J. Crit. Care Med. (Targu Mures) 2021, 7, 227–236. [Google Scholar] [CrossRef]
- Laskowski, R.A.; Creed, J.A.; Raghupathi, R. Pathophysiology of Mild TBI: Implications for Altered Signaling Pathways. In Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects; Kobeissy, F.H., Ed.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2015. [Google Scholar]
- Skandsen, T.; Nilsen, T.L.; Einarsen, C.; Normann, I.; McDonagh, D.; Haberg, A.K.; Vik, A. Incidence of Mild Traumatic Brain Injury: A Prospective Hospital, Emergency Room and General Practitioner-Based Study. Front. Neurol. 2019, 10, 638. [Google Scholar] [CrossRef] [PubMed]
- Forrest, R.H.; Henry, J.D.; McGarry, P.J.; Marshall, R.N. Mild traumatic brain injury in New Zealand: Factors influencing post-concussion symptom recovery time in a specialised concussion service. J. Prim. Health Care 2018, 10, 159–166. [Google Scholar] [CrossRef] [PubMed]
- McCrory, P.; Meeuwisse, W.H.; Aubry, M.; Cantu, R.C.; Dvořák, J.; Echemendia, R.J.; Engebretsen, L.; Johnston, K.M.; Kutcher, J.S.; Raftery, M.; et al. Consensus statement on concussion in sport—The 4th International Conference on Concussion in Sport held in Zurich, November 2012. PM R 2013, 5, 255–279. [Google Scholar] [CrossRef] [PubMed]
- Pavlovic, D.; Pekic, S.; Stojanovic, M.; Popovic, V. Traumatic brain injury: Neuropathological, neurocognitive and neurobehavioral sequelae. Pituitary 2019, 22, 270–282. [Google Scholar] [CrossRef]
- Shepherd, D.; Landon, J.; Kalloor, M.; Barker-Collo, S.; Starkey, N.; Jones, K.; Ameratunga, S.; Theadom, A.; BIONIC Research Group. The association between health-related quality of life and noise or light sensitivity in survivors of a mild traumatic brain injury. Qual. Life Res. 2020, 29, 665–672. [Google Scholar] [CrossRef]
- Werner, C.; Engelhard, K. Pathophysiology of traumatic brain injury. Br. J. Anaesth. 2007, 99, 4–9. [Google Scholar] [CrossRef]
- Andriessen, T.M.; Jacobs, B.; Vos, P.E. Clinical characteristics and pathophysiological mechanisms of focal and diffuse traumatic brain injury. J. Cell. Mol. Med. 2010, 14, 2381–2392. [Google Scholar] [CrossRef]
- McGinn, M.J.; Povlishock, J.T. Pathophysiology of Traumatic Brain Injury. Neurosurg. Clin. N. Am. 2016, 27, 397–407. [Google Scholar] [CrossRef]
- Bigler, E.D. Anterior and middle cranial fossa in traumatic brain injury: Relevant neuroanatomy and neuropathology in the study of neuropsychological outcome. Neuropsychology 2007, 21, 515–531. [Google Scholar] [CrossRef]
- Yue, J.K.; Winkler, E.A.; Puffer, R.C.; Deng, H.; Phelps, R.; Wagle, S.; Morrissey, M.R.; Rivera, E.J.; Runyon, S.J.; Vassar, M.J.; et al. The Track-Tbi Investigators Temporal lobe contusions on computed tomography are associated with impaired 6-month functional recovery after mild traumatic brain injury: A TRACK-TBI study. Neurol. Res. 2018, 40, 972–981. [Google Scholar] [CrossRef]
- Cipolotti, L.; MacPherson, S.E.; Gharooni, S.; van-Harskamp, N.; Shallice, T.; Chan, E.; Nachev, P. Cognitive estimation: Performance of patients with focal frontal and posterior lesions. Neuropsychologia 2018, 115, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Korn, A.; Golan, H.; Melamed, I.; Pascual-Marqui, R.; Friedman, A. Focal cortical dysfunction and blood-brain barrier disruption in patients with Postconcussion syndrome. J. Clin. Neurophysiol. 2005, 22, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bar-Klein, G.; Lublinsky, S.; Kamintsky, L.; Noyman, I.; Veksler, R.; Dalipaj, H.; Senatorov, V.V., Jr.; Swissa, E.; Rosenbach, D.; Elazary, N.; et al. Imaging blood-brain barrier dysfunction as a biomarker for epileptogenesis. Brain 2017, 140, 1692–1705. [Google Scholar] [CrossRef] [PubMed]
- Drew, L.B.; Drew, W.E. The contrecoup-coup phenomenon: A new understanding of the mechanism of closed head injury. Neurocrit. Care 2004, 1, 385–390. [Google Scholar] [CrossRef]
- Ratnaike, T.E.; Hastie, H.; Gregson, B.; Mitchell, P. The geometry of brain contusion: Relationship between site of contusion and direction of injury. Br. J. Neurosurg. 2011, 25, 410–413. [Google Scholar] [CrossRef]
- Green, W.; Ciuffreda, K.J.; Thiagarajan, P.; Szymanowicz, D.; Ludlam, D.P.; Kapoor, N. Static and dynamic aspects of accommodation in mild traumatic brain injury: A review. Optometry 2010, 81, 129–136. [Google Scholar] [CrossRef]
- Humble, S.S.; Wilson, L.D.; Wang, L.; Long, D.A.; Smith, M.A.; Siktberg, J.C.; Mirhoseini, M.F.; Bhatia, A.; Pruthi, S.; Day, M.A.; et al. Prognosis of diffuse axonal injury with traumatic brain injury. J. Trauma Acute Care Surg. 2018, 85, 155–159. [Google Scholar] [CrossRef]
- Galgano, M.; Toshkezi, G.; Qiu, X.; Russel, T.; Chin, L.; Zhao, L.R. Traumatic Brain Injury: Current Treatment Strategies and Future Endeavors. Cell Transplant. 2017, 26, 1118–1130. [Google Scholar] [CrossRef]
- Adams, J.H.; Graham, D.I.; Murray, L.S.; Scott, G. Diffuse Axonal Injury Due to Nonmissile Head Injury in Humans: An Analysis of 45 Cases. Ann. Neurol. 1982, 12, 557–563. [Google Scholar] [CrossRef]
- Mutch, C.A.; Talbott, J.F.; Gean, A. Imaging Evaluation of Acute Traumatic Brain Injury. Neurosurg. Clin. N. Am. 2016, 27, 409–439. [Google Scholar] [CrossRef]
- Lobato, R.D.; Alen, J.F.; Perez-Nuñez, A.; Alday, R.; Gómez, P.A.; Pascual, B.; Lagares, A.; Miranda, P.; Arrese, I.; Kaen, A. Utilidad de la TAC secuencial y la monitorización de la presión intracraneal para detectar nuevo efecto masa intracraneal en pacientes con traumatismo craneal grave y lesión inicial Tipo I-II [Value of serial CT scanning and intracranial pressure monitoring for detecting new intracranial mass effect in severe head injury patients showing lesions type I-II in the initial CT scan]. Neurocirugia 2005, 16, 217–234. [Google Scholar] [PubMed]
- Brown, C.V.; Zada, G.; Salim, A.; Inaba, K.; Kasotakis, G.; Hadjizacharia, P.; Demetriades, D.; Rhee, P. Indications for routine repeat head computed tomography (CT) stratified by severity of traumatic brain injury. J. Trauma 2007, 62, 1339–1344; discussion 1344–1345. [Google Scholar] [CrossRef] [PubMed]
- Lindberg, D.M.; Stence, N.V.; Grubenhoff, J.A.; Lewis, T.; Mirsky, D.M.; Miller, A.L.; O’Neill, B.R.; Grice, K.; Mourani, P.M.; Runyan, D.K. Feasibility and Accuracy of Fast MRI Versus CT for Traumatic Brain Injury in Young Children. Pediatrics 2019, 144, e20190419. [Google Scholar] [CrossRef] [PubMed]
- Wilson, T.T.; Merck, L.H.; Zonfrillo, M.R.; Movson, J.S.; Merck, D. Efficacy of Computed Tomography Utilization in the Assessment of Acute Traumatic Brain Injury in Adult and Pediatric Emergency Department Patients. R. I. Med. J. 2019, 102, 33–35. [Google Scholar]
- Darlan, D.; Prasetya, G.B.; Ismail, A.; Pradana, A.; Fauza, J.; Dariansyah, A.D.; Wardana, G.A.; Apriawan, T.; Bajamal, A.H. Algorithm of Traumatic Brain Injury in Pregnancy (Perspective on Neurosurgery). Asian J. Neurosurg. 2021, 16, 249–257. [Google Scholar] [CrossRef]
- Smith, L.; Milliron, E.; Ho, M.L.; Hu, H.H.; Rusin, J.; Leonard, J.; Sribnick, E.A. Advanced neuroimaging in traumatic brain injury: An overview. Neurosurg. Focus 2019, 47, E17, Erratum in Neurosurg. Focus 2021, 50, E22. [Google Scholar] [CrossRef]
- Chastain, C.A.; Oyoyo, U.E.; Zipperman, M.; Joo, E.; Ashwal, S.; Shutter, L.A.; Tong, K.A. Predicting outcomes of traumatic brain injury by imaging modality and injury distribution. J. Neurotrauma 2009, 26, 1183–1196. [Google Scholar] [CrossRef]
- Audenaert, K.; Jansen, H.M.; Otte, A.; Peremans, K.; Vervaet, M.; Crombez, R.; de Ridder, L.; van Heeringen, C.; Thirot, J.; Dierckx, R.; et al. Imaging of mild traumatic brain injury using 57Co and 99mTc HMPAO SPECT as compared to other diagnostic procedures. Med. Sci. Monit. 2003, 9, MT112–MT117. [Google Scholar]
- Schweitzer, A.D.; Niogi, S.N.; Whitlow, C.T.; Tsiouris, A.J. Traumatic Brain Injury: Imaging Patterns and Complications. Radiographics 2019, 39, 1571–1595. [Google Scholar] [CrossRef]
- Shetty, V.S.; Reis, M.N.; Aulino, J.M.; Berger, K.L.; Broder, J.; Choudhri, A.F.; Kendi, A.T.; Kessler, M.M.; Kirsch, C.F.; Luttrull, M.D.; et al. ACR Appropriateness Criteria Head Trauma. J. Am. Coll. Radiol. 2016, 13, 668–679. [Google Scholar] [CrossRef]
- Marshall, L.F.; Marshall, S.B.; Klauber, M.R.; Van Berkum Clark, M.; Eisenberg, H.; Jane, J.A.; Luerssen, T.G.; Marmarou, A.; Foulkes, M.A. The diagnosis of head injury requires a classification based on computed axial tomography. J. Neurotrauma 1992, 9 (Suppl. S1), S287–S292. [Google Scholar] [PubMed]
- Maas, A.I.; Hukkelhoven, C.W.; Marshall, L.F.; Steyerberg, E.W. Prediction of outcome in traumatic brain injury with computed tomographic characteristics: A comparison between the computed tomographic classification and combinations of computed tomographic predictors. Neurosurgery 2006, 57, 1173–1182. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.W.; Nyström, H.; MacCallum, R.M.; Thornquist, B.; Lilja, A.; Bellander, B.M.; Rudehill, A.; Wanecek, M.; Weitzberg, E. Extended analysis of early computed tomography scans of traumatic brain injured patients and relations to outcome. J. Neurotrauma 2010, 27, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Raj, R.; Siironen, J.; Skrifvars, M.B.; Hernesniemi, J.; Kivisaari, R. Predicting outcome in traumatic brain injury: Development of a novel computerized tomography classification system (Helsinki computerized tomography score). Neurosurgery 2014, 75, 632–646; discussion 646–647. [Google Scholar] [CrossRef] [PubMed]
- Wintermark, M.; Li, Y.; Ding, V.Y.; Xu, Y.; Jiang, B.; Ball, R.L.; Zeineh, M.; Gean, A.; Sanelli, P. Neuroimaging Radiological Interpretation System for Acute Traumatic Brain Injury. J. Neurotrauma 2018, 35, 2665–2672. [Google Scholar] [CrossRef] [PubMed]
- Khaki, D.; Hietanen, V.; Corell, A.; Hergès, H.O.; Ljungqvist, J. Selection of CT variables and prognostic models for outcome prediction in patients with traumatic brain injury. Scand. J. Trauma Resusc. Emerg. Med. 2021, 29, 94. [Google Scholar] [CrossRef]
- Nedd, K.; Sfakianakis, G.; Ganz, W.; Uricchio, B.; Vernberg, D.; Villanueva, P.; Jabir, A.M.; Bartlett, J.; Keena, J. 99mTc-HMPAO SPECT of the brain in mild to moderate traumatic brain injury patients: Compared with CT—A prospective study. Brain Inj. 1993, 7, 469–479. [Google Scholar] [CrossRef]
- Lin, A.P.; Liao, H.J.; Merugumala, S.K.; Prabhu, S.P.; Meehan, W.P., 3rd; Ross, B.D. Metabolic imaging of mild traumatic brain injury. Brain Imaging Behav. 2012, 6, 208–223. [Google Scholar] [CrossRef]
- Raji, C.A.; Henderson, T.A. PET and Single-Photon Emission Computed Tomography in Brain Concussion. Neuroimaging Clin. N. Am. 2018, 28, 67–82. [Google Scholar] [CrossRef]
- Goffin, K.; van Laere, K. Single-photon emission tomography. Handb. Clin. Neurol. 2016, 135, 241–250. [Google Scholar] [CrossRef]
- Santra, A.; Kumar, R. Brain perfusion single photon emission computed tomography in major psychiatric disorders: From basics to clinical practice. Indian J. Nucl. Med. 2014, 29, 210–221. [Google Scholar] [CrossRef] [PubMed]
- Pavel, D.; Jobe, T.; Devore-Best, S.; Davis, G.; Epstein, P.; Sinha, S.; Kohn, R.; Craita, I.; Liu, P.; Chang, Y. Viewing the functional consequences of traumatic brain injury by using brain SPECT. Brain Cogn. 2006, 60, 211–213. [Google Scholar] [PubMed]
- Gowda, N.K.; Agrawal, D.; Bal, C.; Chandrashekar, N.; Tripati, M.; Bandopadhyaya, G.P.; Malhotra, A.; Mahapatra, A.K. Technetium Tc-99m ethyl cysteinate dimer brain single-photon emission CT in mild traumatic brain injury: A prospective study. AJNR Am. J. Neuroradiol. 2006, 27, 447–451. [Google Scholar] [PubMed]
- Sakas, D.E.; Bullock, M.R.; Patterson, J.; Hadley, D.; Wyper, D.J.; Teasdale, G.M. Focal cerebral hyperemia after focal head injury in humans: A benign phenomenon? J. Neurosurg. 1995, 83, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Khalili, H.; Rakhsha, A.; Ghaedian, T.; Niakan, A.; Masoudi, N. Application of Brain Perfusion SPECT in the Evaluation of Response to Zolpidem Therapy in Consciousness Disorder Due to Traumatic Brain Injury. Indian J. Nucl. Med. 2020, 35, 315–320. [Google Scholar] [CrossRef]
- Silverman, I.E.; Galetta, S.L.; Gray, L.G.; Moster, M.; Atlas, S.W.; Maurer, A.H.; Alavi, A. SPECT in patients with cortical visual loss. J. Nucl. Med. 1993, 34, 1447–1451. [Google Scholar]
- Laatsch, L.; Pavel, D.; Jobe, T.; Lin, Q.; Quintana, J.C. Incorporation of SPECT imaging in a longitudinal cognitive rehabilitation therapy programme. Brain Inj. 1999, 13, 555–570. [Google Scholar] [CrossRef]
- Digre, K.B.; Brennan, K.C. Shedding light on photophobia. J. Neuroophthalmol. 2012, 32, 68–81. [Google Scholar] [CrossRef]
- Merezhinskaya, N.; Mallia, R.K.; Park, D.; Millian-Morell, L.; Barker, F.M., 2nd. Photophobia Associated with Traumatic Brain Injury: A Systematic Review and Meta-analysis. Optom. Vis. Sci. 2021, 98, 891–900. [Google Scholar] [CrossRef]
- Armstrong, R.A. Visual problems associated with traumatic brain injury. Clin. Exp. Optom. 2018, 101, 716–726. [Google Scholar] [CrossRef]
- Mares, C.; Dagher, J.H.; Harissi-Dagher, M. Narrative Review of the Pathophysiology of Headaches and Photosensitivity in Mild Traumatic Brain Injury and Concussion. Can. J. Neurol. Sci. 2019, 46, 14–22. [Google Scholar] [CrossRef] [PubMed]
- DiCesare, C.A.; Kiefer, A.W.; Nalepka, P.; Myer, G.D. Quantification and analysis of saccadic and smooth pursuit eye movements and fixations to detect oculomotor deficits. Behav. Res. Methods 2017, 49, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Reddy, A.; Mani, R.; Selvakumar, A.; Hussaindeen, J.R. Reading eye movements in traumatic brain injury. J. Optom. 2020, 13, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Singman, E.; McCulley, T.; Wu, C.; Daphalapurkar, N. The Biomechanics of Indirect Traumatic Optic Neuropathy Using a Computational Head Model with a Biofidelic Orbit. Front. Neurol. 2020, 11, 346. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, S.M.; Van Stavern, G.P. Neuro-ophthalmic deficits after head trauma. Curr. Neurol. Neurosci. Rep. 2013, 13, 389. [Google Scholar] [CrossRef] [PubMed]
- Rasiah, P.K.; Geier, B.; Jha, K.A.; Gangaraju, R. Visual deficits after traumatic brain injury. Histol. Histopathol. 2021, 36, 711–724. [Google Scholar] [CrossRef]
- Ellis, M.J.; Ritchie, L.; Cordingley, D.; Essig, M.; Mansouri, B. Traumatic Optic Neuropathy: A Potentially Unrecognized Diagnosis after Sports-Related Concussion. Curr. Sports Med. Rep. 2016, 15, 27–32. [Google Scholar] [CrossRef]
- Saliman, N.H.; Belli, A.; Blanch, R.J. Afferent Visual Manifestations of Traumatic Brain Injury. J. Neurotrauma 2021, 38, 2778–2789. [Google Scholar] [CrossRef]
- Chen, H.H.; Lee, M.C.; Tsai, C.H.; Pan, C.H.; Lin, Y.T.; Chen, C.T. Surgical Decompression or Corticosteroid Treatment of Indirect Traumatic Optic Neuropathy: A Randomized Controlled Trial. Ann. Plast. Surg. 2020, 84, S80–S83. [Google Scholar] [CrossRef]
- Yu-Wai-Man, P. Traumatic optic neuropathy-Clinical features and management issues. Taiwan J. Ophthalmol. 2015, 5, 3–8. [Google Scholar] [CrossRef]
- Volpe, N.J.; Levin, L.A. How should patients with indirect traumatic optic neuropathy be treated? J. Neuroophthalmol. 2011, 31, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Whitlock, J.R. Posterior parietal cortex. Curr. Biol. 2017, 27, R691–R695. [Google Scholar] [CrossRef] [PubMed]
- Caminiti, R.; Cafee, M.V.; Battaglia-Mayer, A.; Crowe, D.A.; Georgopoulos, A.P. Understanding the parietal lobe syndrome from a neurophysiological and evolutionary perspective. Eur. J. Neurosci. 2010, 31, 2320–2340. [Google Scholar] [CrossRef] [PubMed]
- Hadjidimitrakis, K.; Bakola, S.; Wong, Y.T.; Hagan, M.A. Mixed Spatial and Movement Representations in the Primate Posterior Parietal Cortex. Front. Neural Circuits 2019, 13, 15. [Google Scholar] [CrossRef]
- Medendorp, W.P.; Heed, T. State estimation in posterior parietal cortex: Distinct poles of environmental and bodily states. Prog. Neurobiol. 2019, 183, 101691. [Google Scholar] [CrossRef]
- Baltaretu, B.R.; Monaco, S.; Velji-Ibrahim, J.; Luabeya, G.N.; Crawford, J.D. Parietal Cortex Integrates Saccade and Object Orientation Signals to Update Grasp Plans. J. Neurosci. 2020, 40, 4525–4535. [Google Scholar] [CrossRef]
- Dziedzic, T.A.; Bala, A.; Marchel, A. Cortical and Subcortical Anatomy of the Parietal Lobe from the Neurosurgical Perspective. Front. Neurol. 2021, 12, 727055. [Google Scholar] [CrossRef]
- Husain, M.; Nachev, P. Space and the parietal cortex. Trends Cogn. Sci. 2001, 11, 30–36. [Google Scholar] [CrossRef]
- Sours, C.; Raghavan, P.; Medina, A.E.; Roys, S.; Jiang, L.; Zhuo, J.; Gullapalli, R.P. Structural and Functional Integrity of the Intraparietal Sulcus in Moderate and Severe Traumatic Brain Injury. J. Neurotrauma 2017, 34, 1473–1481. [Google Scholar] [CrossRef]
- Gottlieb, J. From thought to action: The parietal cortex as a bridge between perception, action, and cognition. Neuron 2007, 53, 9–16. [Google Scholar] [CrossRef]
- Breveglieri, R.; Bosco, A.; Borgomaneri, S.; Tessari, A.; Galletti, C.; Avenanti, A.; Fattori, P. Transcranial Magnetic Stimulation Over the Human Medial Posterior Parietal Cortex Disrupts Depth Encoding During Reach Planning. Cereb. Cortex 2021, 31, 267–280. [Google Scholar] [CrossRef] [PubMed]
- Marchette, S.A.; Vass, L.K.; Ryan, J.; Epstein, R.A. Anchoring the neural compass: Coding of local spatial reference frames in human medial parietal lobe. Nat. Neurosci. 2014, 17, 1598–1606. [Google Scholar] [CrossRef] [PubMed]
- Ozga-Hess, J.E.; Whirtley, C.; O’Hearn, C.; Pechacek, K.; Vonder Haar, C. Unilateral parietal brain injury increases risk-taking on a rat gambling task. Exp. Neurol. 2020, 327, 113217. [Google Scholar] [CrossRef] [PubMed]
- Tumati, S.; Martens, S.; de Jong, B.M.; Aleman, A. Lateral parietal cortex in the generation of behavior: Implications for apathy. Prog. Neurobiol. 2019, 175, 20–34. [Google Scholar] [CrossRef] [PubMed]
- Moretti, R.; Signori, R. Neural Correlates for Apathy: Frontal-Prefrontal and Parietal Cortical- Subcortical Circuits. Front. Aging Neurosci. 2016, 8, 289. [Google Scholar] [CrossRef]
- Suleiman, A.; Lithgow, B.J.; Anssari, N.; Ashiri, M.; Moussavi, Z.; Mansouri, B. Correlation between Ocular and Vestibular Abnormalities and Convergence Insufficiency in Post-Concussion Syndrome. Neuroophthalmology 2019, 44, 157–167. [Google Scholar] [CrossRef]
- Alvarez, T.L.; Kim, E.H.; Vicci, V.R.; Dhar, S.K.; Biswal, B.B.; Barrett, A.M. Concurrent vision dysfunctions in convergence insufficiency with traumatic brain injury. Optom. Vis. Sci. 2012, 89, 1740–1751. [Google Scholar] [CrossRef]
- Jacobson, D.M. The Localizing Value of a Quadrantanopia. Arch Neurol. 1997, 54, 401–404. [Google Scholar] [CrossRef]
- Suchoff, I.B.; Kapoor, N.; Ciuffreda, K.J.; Rutner, D.; Han, E.; Craig, S. The frequency of occurrence, types, and characteristics of visual field defects in acquired brain injury: A retrospective analysis. Optometry 2008, 79, 259–265. [Google Scholar] [CrossRef]
- Zhang, X.; Kedar, S.; Lynn, M.J.; Newman, N.J.; Biousse, V. Homonymous hemianopias: Clinical-anatomic correlations in 904 cases. Neurology 2006, 66, 906–910. [Google Scholar] [CrossRef]
- Monserrate, A.E.; De Jesus, O. Homonymous Superior Quadrantanopia; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Bowers, A.R. Driving with homonymous visual field loss: A review of the literature. Clin. Exp. Optom. 2016, 99, 402–418. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, E.; Hardiess, G.; Schaeffel, F.; Wiethoelter, H.; Karnath, H.O.; Mallot, H.; Schoenfisch, B.; Schiefer, U. Assessment of vision-related quality of life in patients with homonymous visual field defects. Graefes Arch. Clin. Exp. Ophthalmol. 2007, 245, 1749–1758. [Google Scholar] [CrossRef] [PubMed]
- Sahraie, A.; Smania, N.; Zihl, J. Use of NeuroEyeCoach™ to Improve Eye Movement Efficacy in Patients with Homonymous Visual Field Loss. Biomed. Res. Int. 2016, 2016, 5186461. [Google Scholar] [CrossRef] [PubMed]
- Lane, A.R.; Smith, D.T.; Schenk, T. Clinical treatment options for patients with homonymous visual field defects. Clin. Ophthalmol. 2008, 2, 93–102. [Google Scholar] [CrossRef][Green Version]
- Fox, S.M.; Koons, P.; Dang, S.H. Vision Rehabilitation after Traumatic Brain Injury. Phys. Med. Rehabil. Clin. N. Am. 2019, 30, 171–188. [Google Scholar] [CrossRef]
- Gilbert, C.D.; Li, W. Top-down influences on visual processing. Nat. Rev. Neurosci. 2013, 14, 350–363. [Google Scholar] [CrossRef] [PubMed]
- Yoder, R.M.; Taube, J.S. The vestibular contribution to the head direction signal and navigation. Front. Integr. Neurosci. 2014, 8, 32. [Google Scholar] [CrossRef]
- Britton, Z.; Arshad, Q. Vestibular and Multi-Sensory Influences Upon Self-Motion Perception and the Consequences for Human Behavior. Front. Neurol. 2019, 10, 63. [Google Scholar] [CrossRef]
- Trofimova, A.; Smith, J.L.; Ahluwalia, V.; Hurtado, J.; Gore, R.K.; Allen, J.W. Alterations in Resting-State Functional Brain Connectivity and Correlations with Vestibular/Ocular-Motor Screening Measures in Postconcussion Vestibular Dysfunction. J. Neuroimaging 2021, 31, 277–286. [Google Scholar] [CrossRef]
- Skóra, W.; Stańczyk, R.; Pajor, A.; Jozefowicz-Korczyńska, M. Vestibular system dysfunction in patients after mild traumatic brain injury. Ann. Agric. Environ. Med. 2018, 25, 665–668. [Google Scholar] [CrossRef]
- Davies, R.A.; Luxon, L.M. Dizziness following head injury: A neuro-otological study. J. Neurol. 1995, 242, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Basford, J.R.; Chou, L.S.; Kaufman, K.R.; Brey, R.H.; Walker, A.; Malec, J.F.; Moessner, A.M.; Brown, A.W. An assessment of gait and balance deficits after traumatic brain injury. Arch. Phys. Med. Rehabil. 2003, 84, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Glendon, K.; Desai, A.; Blenkinsop, G.; Belli, A.; Pain, M. Recovery of symptoms, neurocognitive and vestibular-ocular-motor function and academic ability after sports-related concussion (SRC) in university-aged student-athletes: A systematic review. Brain Inj. 2022; ahead of print. [Google Scholar] [CrossRef]
- Chamelian, L.; Feinstein, A. Outcome after mild to moderate traumatic brain injury: The role of dizziness. Arch. Phys. Med. Rehabil. 2004, 85, 1662–1666. [Google Scholar] [CrossRef] [PubMed]
- Leddy, J.J.; Haider, M.N.; Noble, J.M.; Rieger, B.; Flanagan, S.; McPherson, J.I.; Shubin-Stein, K.; Saleem, G.T.; Corsaro, L.; Willer, B. Clinical Assessment of Concussion and Persistent Post-Concussive Symptoms for Neurologists. Curr. Neurol. Neurosci. Rep. 2021, 21, 70. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.W.; Trofimova, A.; Ahluwalia, V.; Smith, J.L.; Abidi, S.A.; Peters, M.; Rajananda, S.; Hurtado, J.E.; Gore, R.K. Altered Processing of Complex Visual Stimuli in Patients with Postconcussive Visual Motion Sensitivity. AJNR Am. J. Neuroradiol. 2021, 42, 930–937. [Google Scholar] [CrossRef]
- Murray, D.A.; Meldrum, D.; Lennon, O. Can vestibular rehabilitation exercises help patients with concussion? A systematic review of efficacy, prescription and progression patterns. Br. J. Sports Med. 2017, 51, 442–451. [Google Scholar] [CrossRef]
- Karnath, H.O.; Rorden, C. The anatomy of spatial neglect. Neuropsychologia 2012, 50, 1010–1017. [Google Scholar] [CrossRef]
- Marotta, J.J.; McKeeff, T.J.; Behrmann, M. Hemispatial neglect: Its effects on visual perception and visually guided grasping. Neuropsychologia 2003, 41, 1262–1271. [Google Scholar] [CrossRef]
- Smania, N.; Fonte, C.; Picelli, A.; Gandolfi, M.; Varalta, V. Effect of eye patching in rehabilitation of hemispatial neglect. Front. Hum. Neurosci. 2013, 7, 527. [Google Scholar] [CrossRef]
- Rusconi, M.L.; Carelli, L. Long-term efficacy of prism adaptation on spatial neglect: Preliminary results on different spatial components. Sci. World J. 2012, 2012, 618528. [Google Scholar] [CrossRef]
- Mahmood, O.; Rapport, L.J.; Hanks, R.A.; Fichtenberg, N.L. Neuropsychological performance and sleep disturbance following traumatic brain injury. J. Head Trauma Rehabil. 2004, 19, 378–390. [Google Scholar] [CrossRef] [PubMed]
- Mathias, J.L.; Alvaro, P.K. Prevalence of sleep disturbances, disorders, and problems following traumatic brain injury: A meta-analysis. Sleep Med. 2012, 13, 898–905. [Google Scholar] [CrossRef] [PubMed]
- Crichton, T.; Singh, R.; Abosi-Appeadu, K.; Dennis, G. Excessive daytime sleepiness after traumatic brain injury. Brain Inj. 2020, 34, 1525–1531. [Google Scholar] [CrossRef] [PubMed]
- Ouellet, M.C.; Beaulieu-Bonneau, S.; Morin, C.M. Sleep-wake disturbances after traumatic brain injury. Lancet Neurol. 2015, 14, 746–757. [Google Scholar] [CrossRef]
- Leng, Y.; Byers, A.L.; Barnes, D.E.; Peltz, C.B.; Li, Y.; Yaffe, K. Traumatic Brain Injury and Incidence Risk of Sleep Disorders in Nearly 200,000 US Veterans. Neurology 2021, 96, e1792–e1799. [Google Scholar] [CrossRef]
- Theadom, A.; Rowland, V.; Levack, W.; Starkey, N.; Wilkinson-Meyers, L.; McPherson, K.; TBI Experiences Group. Exploring the experience of sleep and fatigue in male and female adults over the 2 years following traumatic brain injury: A qualitative descriptive study. BMJ Open 2016, 6, e010453. [Google Scholar] [CrossRef]
- Agtarap, S.D.; Campbell-Sills, L.; Jain, S.; Sun, X.; Dikmen, S.; Levin, H.; McCrea, M.A.; Mukherjee, P.; Nelson, L.D.; Temkin, N.; et al. TRACK-TBI Investigators Satisfaction with Life after Mild Traumatic Brain Injury: A TRACK-TBI Study. J. Neurotrauma 2021, 38, 546–554. [Google Scholar] [CrossRef]
- Manber, R.; Edinger, J.D.; Gress, J.L.; San Pedro-Salcedo, M.G.; Kuo, T.F.; Kalista, T. Cognitive behavioral therapy for insomnia enhances depression outcome in patients with comorbid major depressive disorder and insomnia. Sleep 2008, 31, 489–495. [Google Scholar] [CrossRef]
- Smith, M.T.; Haythornthwaite, J.A. How do sleep disturbance and chronic pain inter-relate? Insights from the longitudinal and cognitive-behavioral clinical trials literature. Sleep. Med. Rev. 2004, 8, 119–132. [Google Scholar] [CrossRef]
- Miller, M.; Williams, R.; Pagulayan, K.; Barber, J.; Ehde, D.M.; Hoffman, J. Correlates of sleep disturbance in Veterans with traumatic brain injury and chronic pain: A cross-sectional study. Disabil. Health J. 2022, 15, 101203. [Google Scholar] [CrossRef]
- Goel, N.; Rao, H.; Durmer, J.S.; Dinges, D.F. Neurocognitive consequences of sleep deprivation. Semin. Neurol. 2009, 29, 320–339. [Google Scholar] [CrossRef] [PubMed]
- Nakase-Richardson, R.; Sherer, M.; Barnett, S.D.; Yablon, S.A.; Evans, C.C.; Kretzmer, T.; Schwartz, D.J.; Modarres, M. Prospective evaluation of the nature, course, and impact of acute sleep abnormality after traumatic brain injury. Arch. Phys. Med. Rehabil. 2013, 94, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Lowe, A.; Neligan, A.; Greenwood, R. Sleep disturbance and recovery during rehabilitation after traumatic brain injury: A systematic review. Disabil. Rehabil. 2020, 42, 1041–1054. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Tang, L.; Zhou, J.; Anchouche, S.; Li, D.; Yang, Y.; Liu, Z.; Wu, J.; Hu, J.; Zhou, Y.; et al. Sleep deprivation induces corneal epithelial progenitor cell over-expansion through disruption of redox homeostasis in the tear film. Stem Cell Rep. 2022, 17, 1105–1119. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.T.; Li, H.; Dong, L.J. Role of glycolysis in retinal vascular endothelium, glia, pigment epithelium, and photoreceptor cells and as therapeutic targets for related retinal diseases. Int. J. Ophthalmol. 2021, 14, 1302–1309. [Google Scholar] [CrossRef]
- Malik, D.M.; Paschos, G.K.; Sehgal, A.; Weljie, A.M. Circadian and Sleep Metabolomics Across Species. J. Mol. Biol. 2020, 432, 3578–3610. [Google Scholar] [CrossRef]
- Quan, S.F.; Gillin, J.C. New definitions of sleep disordered breathing—Not yet a mandate in clinical practice. Sleep 1999, 22, 662. [Google Scholar] [CrossRef]
- O’Hara, R.; Luzon, A.; Hubbard, J.; Zeitzer, J.M. Sleep apnea, apolipoprotein epsilon 4 allele, and TBI: Mechanism for cognitive dysfunction and development of dementia. J. Rehabil. Res. Dev. 2009, 46, 837–850. [Google Scholar] [CrossRef]
- Coelho, J.; Ferreira, A.; Kuhn, F.; Meireles, A. Globe ruptures: Outcomes and prognostic analysis of severe ocular trauma. Ophthalmologica, 2022; ahead of print. [Google Scholar] [CrossRef]
- Fujikawa, A.; Mohamed, Y.H.; Kinoshita, H.; Matsumoto, M.; Uematsu, M.; Tsuiki, E.; Suzuma, K.; Kitaoka, T. Visual outcomes and prognostic factors in open-globe injuries. BMC Ophthalmol. 2018, 18, 138. [Google Scholar] [CrossRef]
- Schmidt, G.W.; Broman, A.T.; Hindman, H.B.; Grant, M.P. Vision survival after open globe injury predicted by classification and regression tree analysis. Ophthalmology 2008, 115, 202–209. [Google Scholar] [CrossRef]
- Agrawal, R.; Rao, G.; Naigaonkar, R.; Ou, X.; Desai, S. Prognostic factors for vision outcome after surgical repair of open globe injuries. Indian J. Ophthalmol. 2011, 59, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Bi, H.; Cui, Y.; Li, Y.; Wang, X.; Zhang, J. Clinical characteristics and surgical problems of ruptured globe injury. Curr. Ther. Res. Clin. Exp. 2013, 74, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Vingopoulos, F.; Wang, Y.; Grob, S.; Li, C.; Eliott, D.; Kim, L.A.; Vavvas, D.G.; Miller, J.B. Open Globe Injury with Intraocular Foreign Body. J. Vitreoretin. Dis. 2021, 5, 288–294. [Google Scholar] [CrossRef]
- Patel, S.N.; Langer, P.D.; Zarbin, M.A.; Bhagat, N. Diagnostic value of clinical examination and radiographic imaging in identification of intraocular foreign bodies in open globe injury. Eur. J. Ophthalmol. 2012, 22, 259–268. [Google Scholar] [CrossRef]
- Galor, A.; Davis, J.L.; Flynn, H.W., Jr.; Feuer, W.J.; Dubovy, S.R.; Setlur, V.; Kesen, M.R.; Goldstein, D.A.; Tessler, H.H.; Ganelis, I.B.; et al. Sympathetic ophthalmia: Incidence of ocular complications and vision loss in the sympathizing eye. Am. J. Ophthalmol. 2009, 148, 704–710. [Google Scholar] [CrossRef] [PubMed]
- Feng, K.; Hu, Y.T.; Ma, Z. Prognostic indicators for no light perception after open-globe injury: Eye injury vitrectomy study. Am. J. Ophthalmol. 2011, 152, 654–662.e2. [Google Scholar] [CrossRef]
- Heidari, E.; Taheri, N. Surgical treatment of severely traumatized eyes with no light perception. Retina 2010, 30, 294–299. [Google Scholar] [CrossRef]
- Zhao, X.; Zhao, Q.; Meng, L.; Zhang, W.; Chen, Y. Clinical and imaging features of sympathetic ophthalmia and efficacy of the current therapy. Acta Ophthalmol. 2022; ahead of print. [Google Scholar] [CrossRef]
- Hunt, A.W.; Mah, K.; Reed, N.; Engel, L.; Keightley, M. Oculomotor-Based Vision Assessment in Mild Traumatic Brain Injury: A Systematic Review. J. Head Trauma Rehabil. 2016, 31, 252–261. [Google Scholar] [CrossRef]
- Sussman, E.S.; Ho, A.L.; Pendharkar, A.V.; Ghajar, J. Clinical evaluation of concussion: The evolving role of oculomotor assessments. Neurosurg. Focus 2016, 40, E7. [Google Scholar] [CrossRef]
- Cochrane, G.D.; Christy, J.B.; Almutairi, A.; Busettini, C.; Swanson, M.W.; Weise, K.K. Visuo-oculomotor Function and Reaction Times in Athletes with and without Concussion. Optom. Vis. Sci. 2019, 96, 256–265. [Google Scholar] [CrossRef]
- Pillai, C.; Gittinger, J.W., Jr. Vision Testing in the Evaluation of Concussion. Semin. Ophthalmol. 2017, 32, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.A.; Wall, M.; Thompson, H.S. A history of perimetry and visual field testing. Optom. Vis. Sci. 2011, 88, E8–E15. [Google Scholar] [CrossRef] [PubMed]
- Guskiewicz, K.M.; Register-Mihalik, J.; McCrory, P.; McCrea, M.; Johnston, K.; Makdissi, M.; Dvořák, J.; Davis, G.; Meeuwisse, W. Evidence-based approach to revising the SCAT2: Introducing the SCAT3. Br. J. Sports Med. 2013, 47, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Le, R.K.; Ortega, J.D.; Chrisman, S.; Kontos, A.P.; Buckley, T.A.; Kaminski, T.W.; Meyer, B.P.; Clugston, J.R.; Goldman, J.; McAllister, T.W.; et al. King-Devick Sensitivity and Specificity to Concussion in Collegiate Athletes. J. Athl. Train. 2021; ahead of print. [Google Scholar] [CrossRef]
- Kaufman, M.W.; Su, C.A.; Trivedi, N.N.; Lee, M.K.; Nelson, G.B.; Cupp, S.A.; Voos, J.E. The Current Status of Concussion Assessment Scales: A Critical Analysis Review. JBJS Rev. 2021, 9, e20. [Google Scholar] [CrossRef]
- Laukkanen, H.; Scheiman, M.; Hayes, J.R. Brain Injury Vision Symptom Survey (BIVSS) Questionnaire. Optom. Vis. Sci. 2017, 94, 43–50. [Google Scholar] [CrossRef]
- Bigler, E.D. Structural neuroimaging in sport-related concussion. Int. J. Psychophysiol. 2018, 132, 105–123. [Google Scholar] [CrossRef]
- Smith, K. Traumatic brain injury: CT scan does not predict outcome of mild traumatic brain injury. Nat. Rev. Neurol. 2012, 8, 474. [Google Scholar] [CrossRef]
- Froment Tilikete, C. How to assess eye movements clinically. Neurol. Sci. 2022, 43, 2969–2981. [Google Scholar] [CrossRef]
- Yadav, S.; Tandon, R. Comprehensive eye examination: What does it mean? Community Eye Health 2019, 32, S1–S4. [Google Scholar]
- Schuett, S.; Heywood, C.A.; Kentridge, R.W.; Zihl, J. The significance of visual information processing in reading: Insights from hemianopic dyslexia. Neuropsychologia 2008, 46, 2445–2462. [Google Scholar] [CrossRef]
- Ciuffreda, K.J.; Ludlam, D.; Thiagarajan, P. Oculomotor diagnostic protocol for the mTBI population. Optom. J. Am. Optom. Assoc. 2011, 82, 61–63. [Google Scholar] [CrossRef] [PubMed]
- Rosenfield, M. Computer vision syndrome: A review of ocular causes and potential treatments. Ophthalmic Physiol. Opt. 2011, 31, 502–515. [Google Scholar] [CrossRef] [PubMed]
- Porcar, E.; Pons, A.M.; Lorente, A. Visual and ocular effects from the use of flat-panel displays. Int. J. Ophtalmol. 2016, 9, 881–885. [Google Scholar] [CrossRef]
- Beaton, M.D.; Hadly, G.; Babul, S. Stakeholder Recommendations to Increase the Accessibility of Online Health Information for Adults Experiencing Concussion Symptoms. Front. Public Health 2021, 8, 557814. [Google Scholar] [CrossRef]
- Callahan, M.L.; Lim, M.M. Sensory Sensitivity in TBI: Implications for Chronic Disability. Curr. Neurol. Neurosci. Rep. 2018, 18, 56. [Google Scholar] [CrossRef]
- Dyakova, O.; Rångtell, F.H.; Tan, X.; Nordström, K.; Benedict, C. Acute sleep loss induces signs of visual discomfort in young men. J. Sleep Res. 2019, 28, e12837. [Google Scholar] [CrossRef]
- Batuk, I.T.; Batuk, M.O.; Aksoy, S. Evaluation of the postural balance and visual perception in young adults with acute sleep deprivation. J. Vestib. Res. 2020, 30, 383–391. [Google Scholar] [CrossRef]
- Ciuffreda, K.J.; Yadav, N.K.; Ludlam, D.P. Binasal Occlusion (BNO), Visual Motion Sensitivity (VMS), and the Visually-Evoked Potential (VEP) in mild Traumatic Brain Injury and Traumatic Brain Injury (mTBI/TBI). Brain Sci. 2017, 7, 98. [Google Scholar] [CrossRef]
- Yadav, N.K.; Ciuffreda, K.J. Effect of binasal occlusion (BNO) and base-in prisms on the visual-evoked potential (VEP) in mild traumatic brain injury (mTBI). Brain Inj. 2014, 28, 1568–1580. [Google Scholar] [CrossRef]
- Padula, W.V.; Capo-Aponte, J.E.; Padula, W.V.; Singman, E.L.; Jenness, J. The consequence of spatial visual processing dysfunction caused by traumatic brain injury (TBI). Brain Inj. 2017, 31, 589–600. [Google Scholar] [CrossRef]
- Rytter, H.M.; Westenbaek, K.; Henriksen, H.; Christiansen, P.; Humle, F. Specialized interdisciplinary rehabilitation reduces persistent post-concussive symptoms: A randomized clinical trial. Brain Inj. 2019, 33, 266–281. [Google Scholar] [CrossRef] [PubMed]
| Classification | Scoring | Key Features |
|---|---|---|
| Marshall (1992) [53] | Diffuse Injury I to Diffuse Injury VI | Diffuse injury I—No visible intracranial pathology on CT. Progresses up to Diffuse Injury VI with high or mixed density lesion > 25 mL not surgically evacuated. Evaluates perimesencephalic cisterns, midline shift, and presence of a mass lesion. |
| Rotterdam (2006) [54] | 1 to 6 | 4 scored elements: basal cistern compression status; degree of midline shift; epidural hematomas, intraventricular and/or subarachnoid hemorrhage. Differentiates between types of mass lesions, recognizes more favorable prognosis for epidural hematomas. |
| Stockholm (2010) [55] | Traumatic subarachnoid hemorrhage score Range: (0 to 6) | Builds on Marshall and Rotterdam. Adds separate scoring for traumatic subarachnoid hemorrhage. Magnitude of midline shift used as a continuous variable (not dichotomous) for prediction of favorable or unfavorable outcome. Incorporates diffuse axonal injury. |
| Helsinki (2014) [56] | −3 to 14 | Refined to include type of mass lesion (subdural, intracerebral or epidural hematoma. Intraventricular hemorrhage as a predictor of outcome. Includes suprasellar cisterns status (normal, compressed, obliterated). |
| NeuroImaging Radiological Interpretation System (NIRIS) (2018) [57] | NIRIS 0 to NIRIS 4 | Score gives management guidance: NIRIS 0—patients typically discharged, NIRIS 1—follow-up neuroimaging and/or hospital admission, NIRIS 2—admission to an advanced care unit, NIRIS 3—neurosurgical intervention, NIRIS 4—high likelihood of fatal outcome from TBI. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rauchman, S.H.; Albert, J.; Pinkhasov, A.; Reiss, A.B. Mild-to-Moderate Traumatic Brain Injury: A Review with Focus on the Visual System. Neurol. Int. 2022, 14, 453-470. https://doi.org/10.3390/neurolint14020038
Rauchman SH, Albert J, Pinkhasov A, Reiss AB. Mild-to-Moderate Traumatic Brain Injury: A Review with Focus on the Visual System. Neurology International. 2022; 14(2):453-470. https://doi.org/10.3390/neurolint14020038
Chicago/Turabian StyleRauchman, Steven H., Jacqueline Albert, Aaron Pinkhasov, and Allison B. Reiss. 2022. "Mild-to-Moderate Traumatic Brain Injury: A Review with Focus on the Visual System" Neurology International 14, no. 2: 453-470. https://doi.org/10.3390/neurolint14020038
APA StyleRauchman, S. H., Albert, J., Pinkhasov, A., & Reiss, A. B. (2022). Mild-to-Moderate Traumatic Brain Injury: A Review with Focus on the Visual System. Neurology International, 14(2), 453-470. https://doi.org/10.3390/neurolint14020038