Evaluating Therapeutic Equivalence of Generic and Original Levetiracetam in Patients with Epilepsy: A Retrospective Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Population
2.3. Outcomes
2.4. Data Collection
2.5. Statistical Analysis
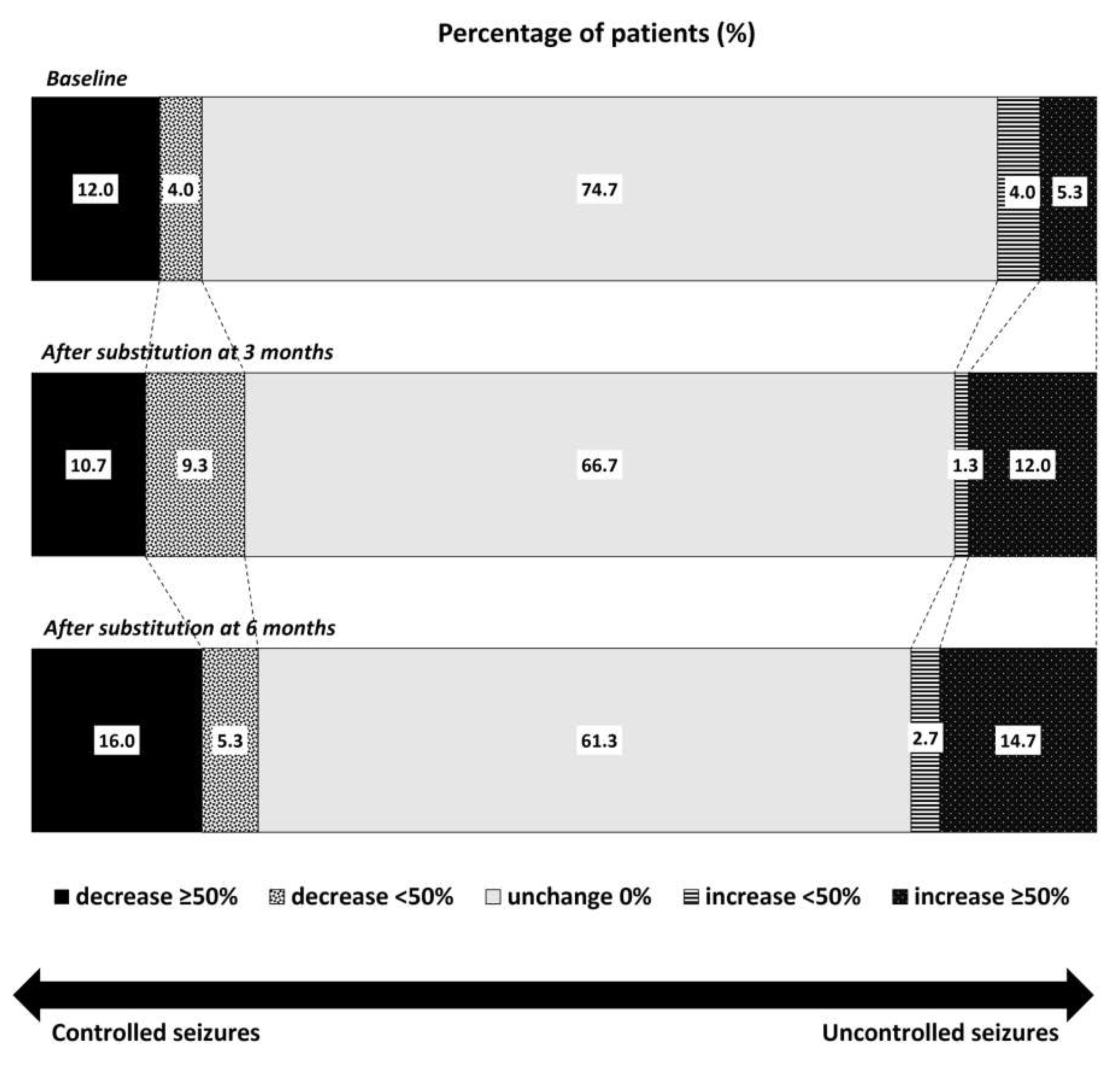
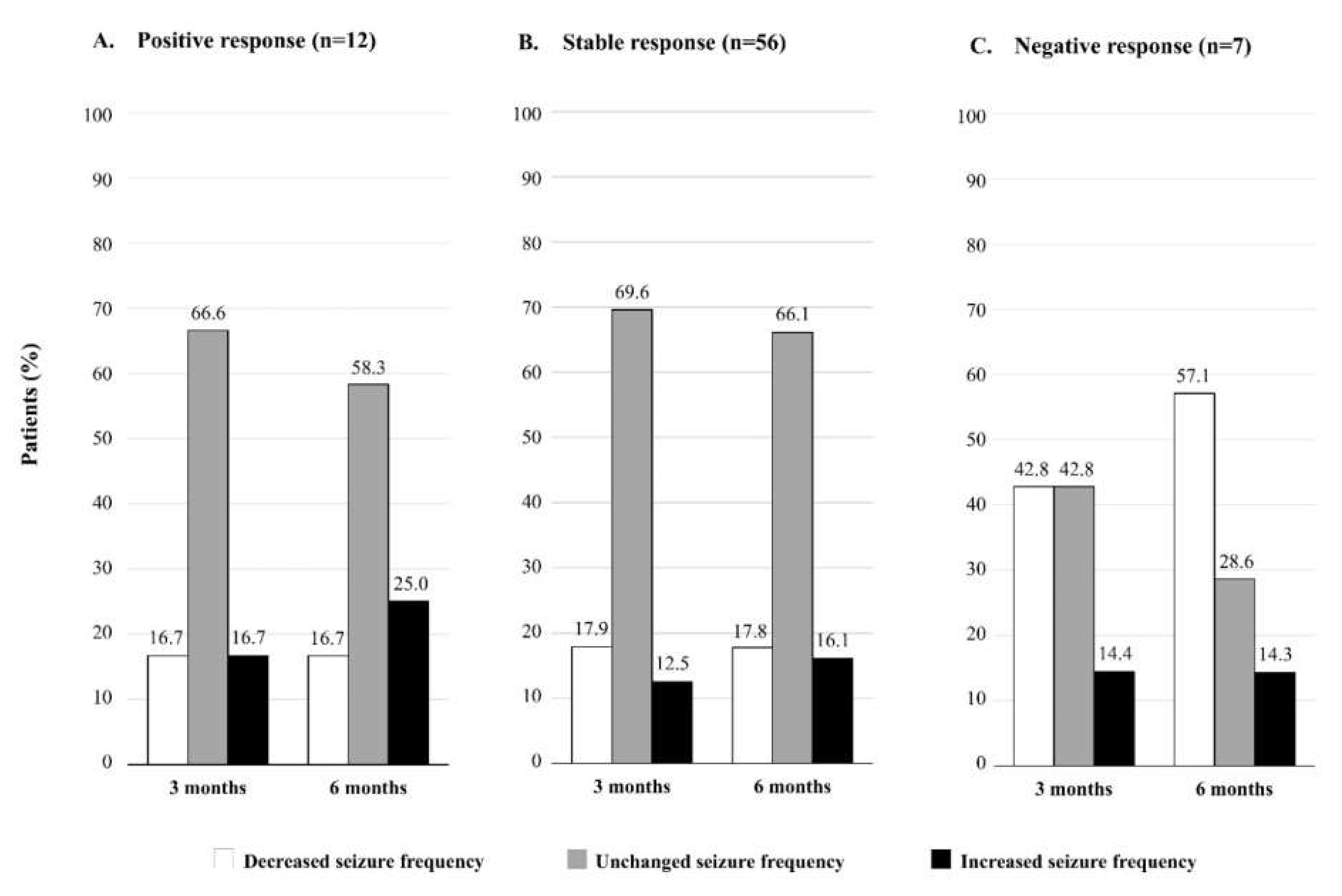
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Crepeau, A.Z.; Treiman, D.M. Levetiracetam: A comprehensive review. Expert Rev. Neurother. 2010, 10, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Perucca, E.; Gidal, B.E.; Baltès, E. Effects of antiepileptic comedication on levetiracetam pharmacokinetics: A pooled analysis of data from randomized adjunctive therapy trials. Epilepsy Res. 2003, 53, 47–56. [Google Scholar] [CrossRef]
- Patsalos, P.N. Pharmacokinetic profile of levetiracetam: Toward ideal characteristics. Pharmacol. Ther. 2000, 85, 77–85. [Google Scholar] [CrossRef]
- Mbizvo, G.K.; Dixon, P.; Hutton, J.L.; Marson, A.G. Levetiracetam add-on for drug-resistant focal epilepsy: An updated Cochrane Review. Cochrane Database Syst. Rev. 2012, 12, CD001901. [Google Scholar] [CrossRef] [PubMed]
- Rheinstein, P.H. Therapeutic Inequivalence. Drug Saf. 1990, 5, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Holtkamp, M.; Theodore, W.H. Generic antiepileptic drugs—Safe or harmful in patients with epilepsy? Epilepsia 2018, 59, 1273–1281. [Google Scholar] [CrossRef] [PubMed]
- Guidance for Industry Waiver of In Vivo Bioavailability and Bioequivalence Studies for Immediate-Release Solid Oral Dosage Forms Based on a Biopharmaceutics Classification System. Available online: http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/default.htm (accessed on 1 July 2018).
- Proposal to Waive In Vivo Bioequivalence Requirements for WHO Model List of Essential Medicines Immediate-Release, Solid Oral Dosage Forms. Available online: http://apps.who.int/medicinedocs/documents/s19640en/s19640en.pdf (accessed on 3 May 2019).
- Markoula, S.; Chatzistefanidis, D.; Gatzonis, S.; Siatouni, A.; Siarava, E.; Verentzioti, A.; Kyritsis, A.P.; Patsalos, P.N. Brand-to-generic levetiracetam switch in patients with epilepsy in a routine clinical setting. Seizure 2017, 48, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Gha-Hyun, L.; Dae, S.J. Brand name to generic substitution of levetiracetam in patients with epilepsy. Seizure 2018, 60, 127–131. [Google Scholar] [CrossRef] [Green Version]
- Vari, M.S.; Pinto, F.; Mencaroni, E.; Giudizioso, G.; Minetti, C.; La Neve, A.; Francavilla, T.; Piccioli, M.; Striano, S.; del Gaudio, L.; et al. Safety of overnight switch from brand-name to generic levetiracetam. Clin. Drug Investig. 2016, 36, 87–91. [Google Scholar] [CrossRef]
- Trimboli, M.; Russo, E.; Mumoli, L.; Tripepi, G.; Fortunato, F.; Mastroianni, G.; Abate, F.; De Sarro, G.; Gambardella, A.; Labate, A. Brand-to-generic levetiracetam switching: A 4-year prospective observational real-life study. Eur. J. Neurol. 2018, 25, 666–671. [Google Scholar] [CrossRef]
- Bosak, M.; Slowik, A.; Turaj, W. Safety of switching from brand-name to generic levetiracetam in patients with epilepsy. Drug Des. Devel. Ther. 2017, 11, 2287–2291. [Google Scholar] [CrossRef] [Green Version]
- Fanella, M.; Morano, A.; Fattouch, J.; Albini, M.; Basili, L.M.; Casciato, S.; Manfredi, M.; Giallonardo, A.T.; Di Bonaventura, C. Switch from originator to equivalent drug in the era of generic antiepileptic drugs: Study of Keppra versus Epitiram clinical equivalence. Clin. Neuropharmacol. 2017, 40, 239–242. [Google Scholar] [CrossRef]
- Chaluvadi, S.; Chiang, S.; Tran, L.; Goldsmith, C.E.; Friedman, D.E. Clinical experience with generic levetiracetam in people with epilepsy. Epilepsia 2011, 52, 810–815. [Google Scholar] [CrossRef] [Green Version]
- Vossler, D.G.; Anderson, G.D.; Bainbridge, J. AES position statement on generic substitution of antiepileptic drugs. Epilepsy Curr. 2016, 16, 209–211. [Google Scholar] [CrossRef] [Green Version]
- Perucca, E.; Albani, F.; Capovilla, G.; Bernardina, B.D.; Michelucci, R.; Zaccara, G. Recommendations of the Italian League against Epilepsy working group on generic products of antiepileptic drugs. Epilepsia 2006, 47 (Suppl. 5), 16–20. [Google Scholar] [CrossRef]
- Position Statement on the Substitution of Different Formulations of Antiepileptic Drugs for the Treatment of Epilepsy. Available online: https://www.ilae.org/files/ilaeGuideline/PositionStatementGenericAEDs-AES-2007.pdf (accessed on 1 March 2019).
- Recommendations on the Use of Generics for the Treatment of Epilepsy. Available online: https://www.ilae.org/files/ilaeGuideline/PRESSRELEASEONGENERICAEDsFRENCHCHAPTEROFTHEILAE_000.pdf (accessed on 1 March 2019).
- Fong, J.K.; Chan, E.L.; Leung, H.; Chan, I.; Chang, R.S.; Fong, G.C.; Fung, E.L.; Lui, C.H.; Fung, B.B.; Poon, T.L.; et al. An update of the Hong Kong epilepsy guideline: Consensus statement on the use of antiepileptic drugs in Hong Kong. Hong Kong Med. J. 2017, 23, 74–88. [Google Scholar] [CrossRef] [Green Version]
- Thacker, A.K.; Misra, P.; Gupta, P.P. Exacerbations of seizures by levetiracetam. Epilepsia 2008, 49, 177. [Google Scholar] [CrossRef]
- Glauser, T.; Ben-Menachem, E.; Bourgeois, B.; Cnaan, A.; Chadwick, D.; Guerreiro, C.; Kalviainen, R.; Mattson, R.; Perucca, E.; Tomson, T. ILAE treatment guidelines: Evidence-based analysis of antiepileptic drug efficacy and effectiveness as initial monotherapy for epileptic seizures and syndromes. Epilepsia 2006, 47, 1094–1120. [Google Scholar] [CrossRef]
- Kinirons, P.; McCarthy, M.; Doherty, C.P.; Delanty, N. Predicting drug-resistant patients who respond to add-on therapy with levetiracetam. Seizure 2006, 15, 387–392. [Google Scholar] [CrossRef] [Green Version]
- Scheffer, I.E.; Berkovic, S.; Capovilla, G.; Connolly, M.B.; French, J.; Guilhoto, L.; Hirsch, E.; Jain, S.; Mathern, G.W.; Moshé, S.L.; et al. ILAE classification of the epilepsies: Position paper of the ILAE commission for classification and terminology. Epilepsia 2017, 58, 512–521. [Google Scholar] [CrossRef] [Green Version]
- Kwan, P.; Arzimanoglou, A.; Berg, A.T.; Brodie, M.J.; Allen Hauser, W.; Mathern, G.; Moshe, S.L.; Perucca, E.; Wiebe, S.; French, J. Definition of drug resistant epilepsy: Consensus proposal by the ad hoc task force of the ILAE commission on therapeutic strategies. Epilepsia 2010, 51, 1069–1077. [Google Scholar] [CrossRef]
- Whitehead, A.L.; Julious, S.A.; Cooper, C.L.; Campbell, M.J. Estimating the sample size for a pilot randomised trial to minimise the overall trial sample size for the external pilot and main trial for a continuous outcome variable. Stat. Methods Med. Res. 2016, 25, 1057–1073. [Google Scholar] [CrossRef]
- Levetiracetam Tablets. Available online: https://www.uspnf.com/sites/default/files/usp_pdf/EN/USPNF/revisions/levetiracetam_tablets.pdf (accessed on 1 May 2021).
- Welty, T.E.; Gidal, B.E.; Ficker, D.M.; Privitera, M.D. Levetiracetam: A different approach to the pharmacotherapy of epilepsy. Ann. Pharmacother. 2002, 36, 296–304. [Google Scholar] [CrossRef]
- Patsalos, P.N. Clinical pharmacokinetics of levetiracetam. Clin. Pharmacokinet. 2004, 43, 707–724. [Google Scholar] [CrossRef]
- Hovinga, C.A. Levetiracetam: A novel antiepileptic drug. Pharmacotherapy 2001, 21, 1375–1388. [Google Scholar] [CrossRef]
- Odi, R.; Franco, V.; Perucca, E.; Bialer, M. Bioequivalence and switchability of generic antiseizure medications (ASMs): A re-appraisal based on analysis of generic ASM products approved in Europe. Epilepsia 2021, 62, 285–302. [Google Scholar] [CrossRef]
- Petrusevska, M.; Berglez, S.; Krisch, I.; Legen, I.; Megusar, K.; Peternel, L.; Abrahamsson, B.; Cristofoletti, R.; Groot, D.W.; Kopp, S.; et al. Biowaiver Monographs for Immediate Release Solid Oral Dosage Forms: Levetiracetam. J. Pharm. Sci. 2015, 104, 2676–2687. [Google Scholar] [CrossRef]
- Stockis, A.; Sargentini-Maier, M.L.; Otoul, C.; Connor, A.; Wilding, I.; Wray, H. Assessment of levetiracetam bioavailability from targeted sites in the human intestine using remotely activated capsules and gamma scintigraphy: Open-label, single-dose, randomized, four-way crossover study in healthy male volunteers. Clin. Ther. 2010, 32, 1813–1821. [Google Scholar] [CrossRef]
- Buren, J.M.V.; Ajmone-Marsan, C. A Correlation of autonomic and EEG components in temporal lobe epilepsy. Arch. Neurol. 1960, 3, 683–703. [Google Scholar] [CrossRef]
- Freeman, R.; Schachter, S.C. Autonomic epilepsy. Semin. Neurol. 1995, 15, 158–166. [Google Scholar] [CrossRef]
- Gibaldi, M. Bioavailability. In Biopharmaceuticals and Clinical Pharmacokinetics, 4th ed.; Gibaldi, M., Ed.; Lea & Febiger: London, UK, 1991; pp. 146–175. [Google Scholar]
- Reimers, A.; Olsson, P.; Nilsson, J.; Hoff, E.; Reis, M.; Strandberg, M.; Lundgren, A.; Källén, K. Impact of generic substitution on levetiracetam serum concentration—A prospective study in an outpatient setting. Epilepsy Res. 2017, 134, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Patsalos, P.N.; Spencer, E.P.; Berry, D.J. Therapeutic Drug Monitoring of Antiepileptic Drugs in Epilepsy: A 2018 Update. Ther. Drug Monit. 2018, 40, 526–548. [Google Scholar] [CrossRef] [PubMed]
- Johannessen, S.I.; Battino, D.; Berry, D.J.; Bialer, M.; Krämer, G.; Tomson, T.; Patsalos, P.N. Therapeutic drug monitoring of the newer antiepileptic drugs. Ther. Drug Monit. 2003, 25, 347–363. [Google Scholar] [CrossRef] [PubMed]
- Lancelin, F.; Franchon, E.; Kraoul, L.; Garciau, I.; Brovedani, S.; Tabaouti, K.; Landré, E.; Chassoux, F.; Paubel, P.; Piketty, M.L. Therapeutic drug monitoring of levetiracetam by high-performance liquid chromatography with photodiode array ultraviolet detection: Preliminary observations on correlation between plasma concentration and clinical response in patients with refractory epilepsy. Ther. Drug Monit. 2007, 29, 576–583. [Google Scholar] [CrossRef]
- Lynch, B.A.; Lambeng, N.; Nocka, K.; Kensel-Hammes, P.; Bajjalieh, S.M.; Matagne, A.; Fuks, B. The synaptic vesicle protein SV2A is the binding site for the antiepileptic drug levetiracetam. Proc. Natl. Acad. Sci. USA 2004, 101, 9861–9866. [Google Scholar] [CrossRef] [Green Version]
- Beghi, E.; Giussani, G. Aging and the Epidemiology of Epilepsy. Neuroepidemiology 2018, 51, 216–223. [Google Scholar] [CrossRef]
- Foldvary-Schaefer, N.; Falcone, T. Catamenial epilepsy: Pathophysiology, diagnosis, and management. Neurology 2003, 61 (Suppl. 2), S2–S15. [Google Scholar] [CrossRef]
- Yeh, W.C.; Lu, S.R.; Wu, M.N.; Lee, K.W.; Chien, C.F.; Fong, Y.O.; Li, K.Y.; Lai, Y.L.; Lin, C.J.; Li, Y.S.; et al. The impact of antiseizure medications on polysomnographic parameters: A systematic review and meta-analysis. Sleep Med. 2021, 81, 319–326. [Google Scholar] [CrossRef]
| Characteristics | Total Patients (n = 75) | Subgroup Patients | ||
|---|---|---|---|---|
| Controlled Seizures (n = 50) | Uncontrolled Seizures (n = 25) | p Value | ||
| Female, n (%) | 40 (53.3) | 26 (52.0) | 14 (56.0) | 0.743 |
| Age (years), mean ± SD | 40.3 ± 17.9 | 43.5 ± 18.9 | 33.9 ± 14.1 | 0.027 * |
| Weight (kg), mean ± SD | 59.4 ± 17.0 | 59.9 ± 17.2 | 58.4 ± 16.9 | 0.722 |
| Non-obese (BMI < 24.9 kg/m2), n (%) | 54 (72.0) | 36 (72.0) | 18 (72.0) | 1.000 |
| Renal dysfunction (creatinine clearance) a, n (%) | ||||
| No dysfunction (≥80 mL/min) | 53 (70.7) | 34 (68.0) | 19 (76.0) | 0.473 |
| Mild (50–79 mL/min) | 19 (25.3) | 14 (28.0) | 5 (20.0) | 0.453 |
| Moderate (30–49 mL/min) | 2 (2.7) | 1 (2.0) | 1 (4.0) | 1.000 |
| Severe (<30 mL/min) | 1 (1.3) | 1 (2.0) | 0 (0) | 1.000 |
| Age at onset of epilepsy (years), mean ± SD | 24.4 ± 18.8 | 28.7 ± 20.1 | 15.7 ± 12.1 | 0.001 * |
| Type of seizure, n (%) | ||||
| Focal onset | 64 (85.3) | 43 (86.0) | 21 (84.0) | 0.817 |
| Generalized onset | 7 (9.3) | 6 (12.0) | 1 (4.0) | 0.413 |
| Unknown onset | 4 (5.4) | 1 (2.0) | 3 (12.0) | 0.105 |
| Cause of epilepsy, n (%) | ||||
| Idiopathic | 10 (13.3) | 5 (10.0) | 5 (20.0) | 0.286 |
| Symptomatic | 57 (76.0) | 42 (84.0) | 15 (60.0) | 0.022 * |
| Cryptogenic | 8 (10.7) | 3 (6.0) | 5 (20.0) | 0.108 |
| Epilepsy duration ≥ 10 years, n (%) | 46 (61.3) | 27 (54.0) | 19 (76.0) | 0.065 |
| Refractory epilepsy, n (%) | 37 (49.3) | 16 (32.0) | 21 (84.0) | 0.000 * |
| Epilepsy surgery, n (%) | ||||
| Before drug transition | 5 (6.7) | 2 (4.0) | 3 (12.0) | 0.326 |
| After drug transition | 4 (5.3) | 0 (0) | 4 (16.0) | 0.005 * |
| Psychiatric disorders, n (%) | 12 (16.0) | 9 (18.0) | 3 (12.0) | 0.740 |
| Anxiety | 2 (2.7) | 2 (4.0) | 0 (0) | 0.550 |
| Depression | 3 (4.0) | 2 (4.0) | 1 (4.0) | 1.000 |
| Hallucinations | 1 (1.3) | 0 (0) | 1 (4.0) | 0.333 |
| Aggression | 2 (2.7) | 2 (4.0) | 0 (0) | 0.550 |
| Irritability | 1 (1.3) | 1 (2.0) | 0 (0) | 1.000 |
| Miscellaneous b | 3 (4.0) | 2 (4.0) | 1 (4.0) | 1.000 |
| Characteristics | Total Patients (n = 75) | Subgroup Patients | ||
|---|---|---|---|---|
| Controlled Seizures (n = 50) | Uncontrolled Seizures (n = 25) | p Value | ||
| AED use at the baseline | ||||
| Original levetiracetam dose (mg), median (Q1, Q3) | 1500 (1000, 2500) | 1000 (1000, 2000) | 2000 (1500, 3000) | 0.006 * |
| Polytherapy, n (%) | 58 (77.3) | 33 (66.0) | 25 (100.0) | 0.001 * |
| Concurrent AEDs (n), median (Q1, Q3) | 1.0 (1.0, 2.0) | 1.0 (0, 2.0) | 2.0 (2.0, 3.0) | 0.000 * |
| Concurrent AEDs by mode of action, n (%) | ||||
| Sodium-channel blocking | 38 (50.7) | 19 (38.0) | 19 (76.0) | 0.002 * |
| Calcium-channel blocking | 4 (5.3) | 2 (4.0) | 2 (8.0) | 0.597 |
| GABA-receptor modulating | 21 (28.0) | 9 (18.0) | 12 (48.0) | 0.006 * |
| Multiple (valproate, topiramate) | 32 (42.7) | 16 (32.0) | 16 (64.0) | 0.008 * |
| Duration of original levetiracetam (months) | ||||
| Mean ± SD | 48.2 ± 33.8 | 51.6 ± 33.6 | 41.2 ± 33.7 | 0.210 |
| Range | 1–127.8 | 2–127.8 | 1–108.3 | |
| AED use at six months after substitution | ||||
| Generic levetiracetam dose (mg), median (Q1, Q3) | 1500 (1000, 2500) | 1000 (1000, 2500) | 2000 (1000, 2500) | 0.113 |
| Polytherapy, n (%) | 54 (72.0) | 30 (60.0) | 24 (96.0) | 0.001 * |
| Concurrent AEDs (n), median (Q1, Q3) | 1.0 (0, 2.0) | 1.0 (0, 2.0) | 2.0 (2.0, 3.0) | 0.000 * |
| Concurrent AEDs by mode of action, n (%) | ||||
| Sodium-channel blocking | 39 (52.0) | 19 (38.0) | 20 (80.0) | 0.001 * |
| Calcium-channel blocking | 2 (2.7) | 2 (2.0) | 2 (4.0) | 1.000 |
| GABA-receptor modulating | 22 (29.3) | 10 (20.0) | 12 (48.0) | 0.012 * |
| Multiple (valproate, topiramate) | 33 (44.0) | 15 (30.0) | 18 (72.0) | 0.001 * |
| Time Point/Variable | Total Patients (n = 75) | Subgroup Patients | |
|---|---|---|---|
| Controlled Seizures (n = 50) | Uncontrolled Seizures (n = 25) | ||
| Baseline | |||
| Seizure frequency per month | |||
| Mean ± SD (range) | 2.77 ± 11.41 (0–90) | 0.03 ± 0.16 (0–1) | 8.26 ± 18.81 (0.5–90) |
| Median (Q1, Q3) | 0 (0, 1) | 0 (0, 0) | 2 (1, 4) |
| After three months | |||
| Seizure frequency per month | |||
| Mean ± SD (range) | 3.24 ± 14.49 (0–120) | 0.42 ± 1.56 (0–8) | 8.88 ± 24.35 (0–120) |
| Median (Q1, Q3) | 0 (0, 1) | 0 (0, 0) | 1 (0.25, 8) |
| p value (difference from baseline) | 0.806 | 0.063 | 0.443 |
| After six months | |||
| Seizure frequency per month, | |||
| Mean ± SD (range) | 3.15 ± 14.47 (0–120) | 0.56 ± 1.83 (0–10) | 8.34 ± 24.44 (0–120) |
| Median (Q1, Q3) | 0 (0, 1) | 0 (0, 0) | 1 (0, 3.5) |
| p value (difference from baseline) | 0.970 | 0.012 * | 0.173 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tharavichitkun, J.; Yadee, T.; Angkaow, P.; Suansanae, T. Evaluating Therapeutic Equivalence of Generic and Original Levetiracetam in Patients with Epilepsy: A Retrospective Study. Neurol. Int. 2022, 14, 271-283. https://doi.org/10.3390/neurolint14010022
Tharavichitkun J, Yadee T, Angkaow P, Suansanae T. Evaluating Therapeutic Equivalence of Generic and Original Levetiracetam in Patients with Epilepsy: A Retrospective Study. Neurology International. 2022; 14(1):271-283. https://doi.org/10.3390/neurolint14010022
Chicago/Turabian StyleTharavichitkun, Jannapas, Tinonkorn Yadee, Poomchai Angkaow, and Thanarat Suansanae. 2022. "Evaluating Therapeutic Equivalence of Generic and Original Levetiracetam in Patients with Epilepsy: A Retrospective Study" Neurology International 14, no. 1: 271-283. https://doi.org/10.3390/neurolint14010022
APA StyleTharavichitkun, J., Yadee, T., Angkaow, P., & Suansanae, T. (2022). Evaluating Therapeutic Equivalence of Generic and Original Levetiracetam in Patients with Epilepsy: A Retrospective Study. Neurology International, 14(1), 271-283. https://doi.org/10.3390/neurolint14010022






