A Rare Case of Posterior Fossa Tumor and Central Precocious Puberty: Case Presentation and Review of the Literature
Abstract
1. Introduction
2. Case Presentation (Material)
2.1. Methods
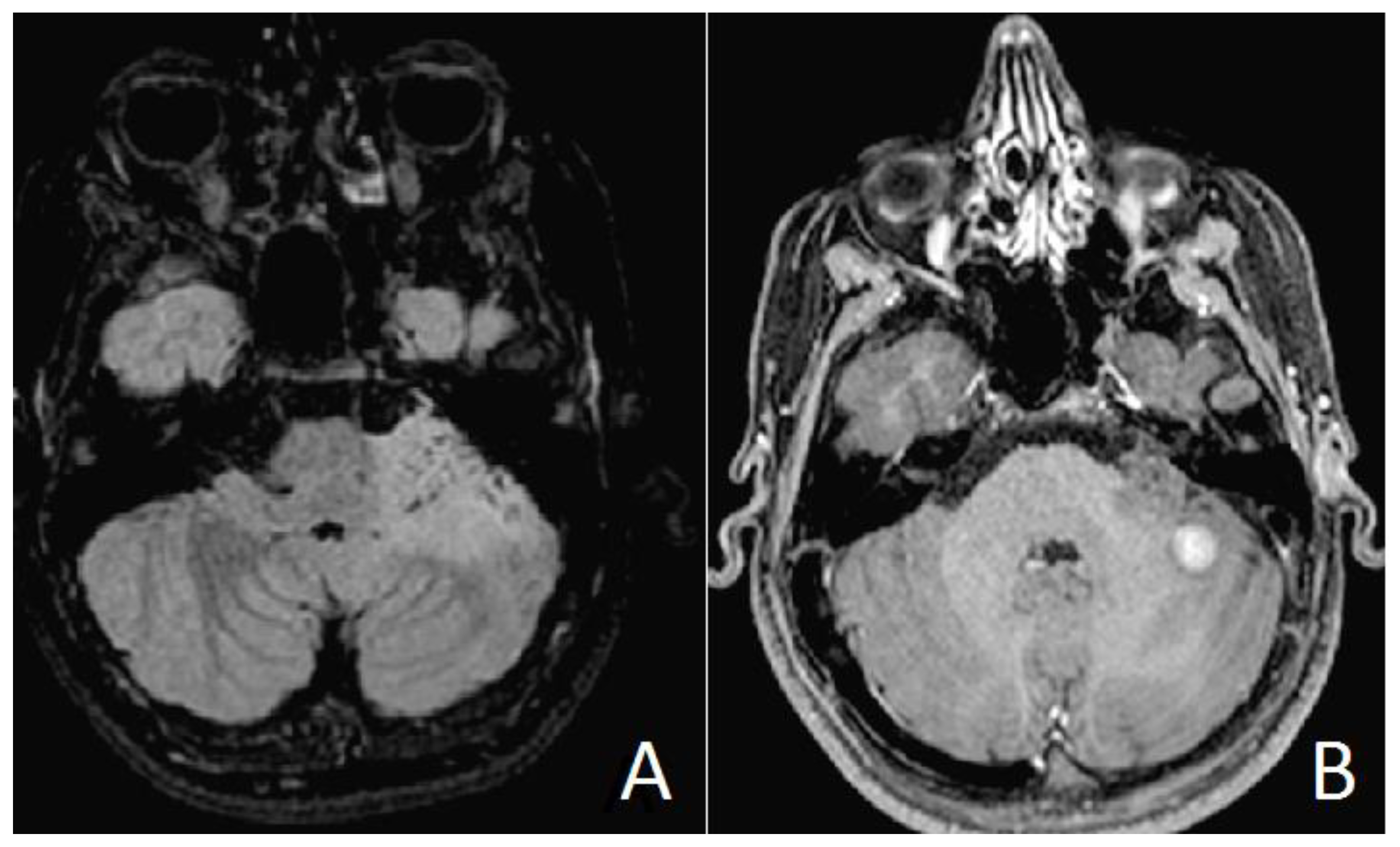
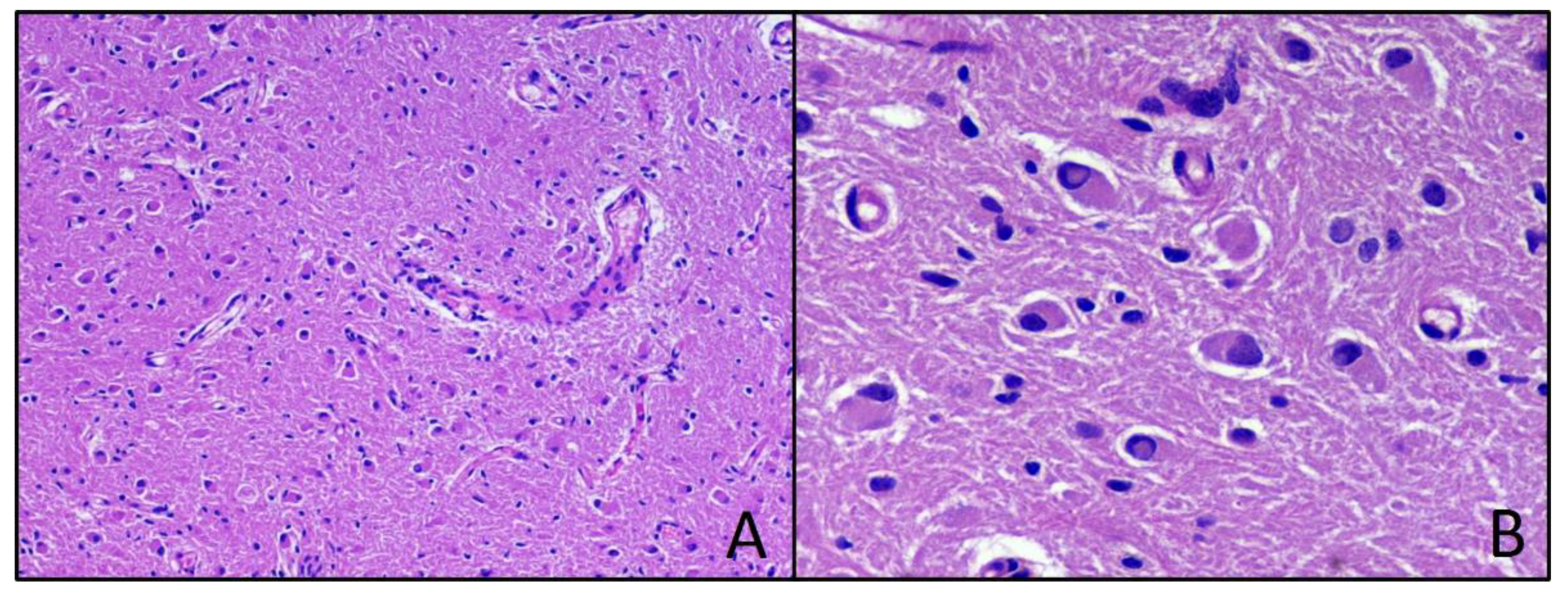
2.2. Results
3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Berberoglu, M. Precocious Puberty and Normal Variant Puberty: Definition, etiology, diagnosis and. J. Clin. Res. Pediatr. Endocrinol. 2009, 1, 164–174. [Google Scholar] [CrossRef]
- Carel, J.; Eugster, E.A.; Rogol, A.; Ghizzoni, L.; Mark, R.; Carel, J. Consensus Statement on the Use of Gonadotropin-Releasing Hormone Analogs in Children. Pediatrics 2009, 123, e752–e762. [Google Scholar] [CrossRef] [PubMed]
- Stephen, Z.; Waguespack, S.G. Gonadotropin-Dependent Precocious Puberty: Neoplastic Causes and Endocrine Considerations. Int. J. Pediatr. Endocrinol. 2011, 2011, 184502. [Google Scholar] [CrossRef]
- Chen, M.; Eugster, E.A. Central Precocious Puberty: Update on Diagnosis and Treatment. Paediatr. Drugs 2015, 17, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Faizah, M.Z.; Zuhanis, A.H.; Rahmah, R.; Raja, A.A.; Wu, L.L.; Dayang, A.A.; Zulfiqar, M.A. Precocious puberty in children: A review of imaging findings. Biomed. Imaging Interv. J 2012, 8, e6. [Google Scholar] [PubMed]
- Kaplowitz, P.B. Do 6-8 year old girls with central precocious puberty need routine brain imaging? Int. J. Pediatr. Endocrinol. 2016, 9, 10–13. [Google Scholar] [CrossRef]
- Choi, K.H.; Chung, S.J.; Kang, M.J.; Yoon, J.Y.; Lee, J.; Lee, Y.A.; Shin, C.H.; Yang, S.W. Boys with precocious or early puberty: Incidence of pathological brain magnetic resonance imaging findings and factors related to newly developed brain lesions. Ann. Pediatr. Endocrinol. Metab. 2013, 18, 183–190. [Google Scholar] [CrossRef]
- Medina, R.G.; Dempsher, D.P.; Gauvain, K.M.; Geller, T.J.; Elbabaa, S.K. Resolution of precocious puberty following resection of fourth ventricular medulloblastoma: Case report. J. Neurosurg. Pediatr. 2015, 16, 287–290. [Google Scholar] [CrossRef]
- Josan, V.A.; Timms, C.D.; Rickert, C.W.D. Cerebellar astrocytoma presenting with precocious puberty in a girl. J. Neurosurg. Pediatr. 2007, 107, 66–68. [Google Scholar] [CrossRef]
- Gass, D.; Dewire, M.; Chow, L.; Rose, S.R.; Lawson, S. Pediatric tectal plate gliomas: A review of clinical outcomes, endocrinopathies, and neuropsychological sequelae. J. Neuroonncol. 2015, 122, 169–177. [Google Scholar] [CrossRef]
- Rossfeld, Z.M. A Very Tall 7-year-old Boy with Medulloblastoma. Pediatr. Rev. 2018, 39, 41. [Google Scholar] [CrossRef]
- Wendt, S.; Shelso, J.; Wright, K.; Furman, W. Neoplastic Causes of Abnormal Puberty. Pediatr. Blood Cancer 2014, 61, 664–671. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.C.-W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [PubMed]
- Tong, A.; Flemming, K.; Mcinnes, E.; Oliver, S.; Craig, J. Enhancing transparency in reporting the synthesis of qualitative research: ENTREQ. BMC Med. Res. Methodol. 2012, 12, 181. [Google Scholar] [CrossRef] [PubMed]
- Tejada, A.R.Q.; Miranda-Lloret, P.; Ros, M.L.; Ramirez, E.P.; Pancucci, G.; Barber, A.R.; Simal-Julián, J.A.; Botella-Asunción, C. Gangliogliomas in the pediatric population. Child’s Nerv. Syst 2021, 37, 831–837. [Google Scholar] [CrossRef] [PubMed]
- Dudley, R.W.; Torok, M.R.; Gallegos, D.R.; Mulcahy-Levy, J.M.; Hoffman, L.M.; Liu, A.K.; Handler, M.H.; Hankinson, T.C. Pediatric Low Grade Ganglioglioma/Gangliocytoma: Epidemiology, treatments, and outcome analysis on 348 children from the SEER database. Neurosurgery 2015, 76, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Blümcke, I.W.O. Gangliogliomas: An Intriguing Tumor Entity Associated with Focal Epilepsies. J. Neuropathol. Exp. Neurol. 2002, 61, 575–584. [Google Scholar] [CrossRef]
- Weis, S.; Sonnberger, M.; Dunzinger, A.; Voglmayr, E.; Aichholzer, M.; Kleiser, R.; Strasser, P. Ganglioglioma/Gangliocytoma. In Imaging Brain Diseases: A Neuroradiology, Nuclear Medicine, Neurosurgery, Neuropathology and Molecular Biology-Based Approach; Springer: Vienna, Austria, 2019; pp. 1553–1565. [Google Scholar] [CrossRef]
- Ventureyra, E.; Herder, S.; Mallya, B.K.; Keene, D. Temporal lobe gangliomas in children *. Child’s Nerv. Syst. 1986, 2, 63–66. [Google Scholar] [CrossRef]
- Adachi, Y.; Yagishita, A. Gangliogliomas: Characteristic imaging findings and role in the temporal lobe epilepsy. Neuroradiology 2008, 50, 829–834. [Google Scholar] [CrossRef]
- Bram, R.; Seidman, R.J.; Chesler, D. Atypical Presentation of a Pediatric Cerebellar Ganglioglioma. Pediatr. Neurosurg. 2018, 53, 43–48. [Google Scholar] [CrossRef]
- Safavi-Abbasi, S.; Di Rocco, F.; Chantra, K.; Feigl, G.C.; El-Shawarby, A.S.A. Posterior cranial fossa gangliogliomas. Skull Base 2007, 17, 253–264. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rana, R.; Perillo, T.; Santoro, N.; Ortolani, F.; Messina, R.; Resta, M.; Perrucci, I.; Ingravallo, G.; Cazzato, G.; Grassi, M.; et al. A Rare Case of Posterior Fossa Tumor and Central Precocious Puberty: Case Presentation and Review of the Literature. Neurol. Int. 2021, 13, 535-540. https://doi.org/10.3390/neurolint13040053
Rana R, Perillo T, Santoro N, Ortolani F, Messina R, Resta M, Perrucci I, Ingravallo G, Cazzato G, Grassi M, et al. A Rare Case of Posterior Fossa Tumor and Central Precocious Puberty: Case Presentation and Review of the Literature. Neurology International. 2021; 13(4):535-540. https://doi.org/10.3390/neurolint13040053
Chicago/Turabian StyleRana, Roberta, Teresa Perillo, Nicola Santoro, Federica Ortolani, Raffaella Messina, Mariachiara Resta, Ilenia Perrucci, Giuseppe Ingravallo, Gerardo Cazzato, Massimo Grassi, and et al. 2021. "A Rare Case of Posterior Fossa Tumor and Central Precocious Puberty: Case Presentation and Review of the Literature" Neurology International 13, no. 4: 535-540. https://doi.org/10.3390/neurolint13040053
APA StyleRana, R., Perillo, T., Santoro, N., Ortolani, F., Messina, R., Resta, M., Perrucci, I., Ingravallo, G., Cazzato, G., Grassi, M., Pesce, S., & Signorelli, F. (2021). A Rare Case of Posterior Fossa Tumor and Central Precocious Puberty: Case Presentation and Review of the Literature. Neurology International, 13(4), 535-540. https://doi.org/10.3390/neurolint13040053







